Abstract
Carboxylesterases are important in the metabolism of cocaine, catalyzing the hydrolysis of cocaine to its two major metabolites, benzoylecgonine and ecgonine methyl ester. In the presence of ethanol, some cocaine undergoes transesterification with ethanol instead of hydrolysis with water producing the active metabolite, cocaethylene. The metabolic fate of cocaethylene is unknown, but given its structural similarity to cocaine, it was hypothesized that cocaethylene would also be metabolized by carboxylesterases and its elimination decreased in the presence of ethanol, as is cocaine's. Dogs were given cocaine alone, cocaethylene alone, cocaine and ethanol, cocaethylene and ethanol, and cocaine and cocaethylene on separate study days and sequential blood samples drawn. Plasma concentrations of cocaine, benzoylecgonine, and cocaethylene were determined by high-performance liquid chromatography. The pharmacokinetic dispositions of cocaine and cocaethylene were similar with clearance values of 0.91 ± 0.22 and 0.79 ± 0.16 l/min, and volumes of distribution of 2.6 ± 0.82 and 2.7 ± 0.47 l/kg, respectively. Both cocaine and cocaethylene clearances were decreased about 20% when given with ethanol. Following administration of cocaethylene alone, benzoylecgonine achieved similar plasma concentrations as those attained following cocaine alone, which indicates that benzoylecgonine is a major metabolite of cocaethylene. Carboxylesterases play an important role in the elimination of both cocaine and cocaethylene.
Most cocaine users also ingest ethanol (McCance-Katz et al., 1993). This combination results in a decrease in the clearance of cocaine and the formation of the pharmacologically active metabolite, cocaethylene (Perez-Reyes et al., 1994; Hart et al., 2000). These alterations in the metabolic disposition of cocaine are mediated through the effects of ethanol on carboxylesterase. In humans, two carboxylesterase enzymes have been identified, carboxylesterase 1 (hCE11) and carboxylesterase 2 (hCE2) that participate in the metabolism of cocaine (Dean et. al., 1991; Brzezinski et. al., 1997). Although present in many tissues including heart, stomach, kidney, colon, and others, they are most abundant in the liver (Riddles et al., 1991;Schwer et al., 1997). Carboxylesterases are located in the endoplasmic reticulum and catalyze the hydrolysis of lipophilic esters to their more water-soluble alcohol and acyl substituents. There is evidence for the involvement of carboxylesterases in the metabolism of endogenous substrates such as lipids and steroids, but their primary function seems to be protecting the body from foreign substances encountered through the diet and other routes (Satoh et al., 2002). The carboxylesterases hCE1 and hCE2 are low affinity, high capacity enzymes able to hydrolyze a wide variety of structurally dissimilar esters (Kroetz et al., 1993; Satoh et al., 2002).
Perhaps the best-known and most thoroughly studied substrate of hCE1 and hCE2 is cocaine. Cocaine is primarily eliminated by hydrolysis to benzoylecgonine by hCE1 and to ecgonine methyl ester by hCE2 with subsequent renal elimination. When ethanol is consumed with cocaine, a new metabolite is produced, cocaethylene (Rafla and Epstein, 1979;Boyer and Petersen, 1992; Bourland et al., 1997). Its formation results from hCE1 catalyzed transesterification between cocaine and ethanol as opposed to the normal reaction with water (hydrolysis) that produces the inactive metabolite, benzoylecgonine (Dean et al., 1991; Boyer and Petersen, 1992; Brzezinski et al., 1994; Bourland et al., 1997). Structurally, cocaine and cocaethylene differ only in the substitution of ethyl in place of the methyl ester [i.e., the ecgonine methyl ester (cocaine) is metabolized to ethyl ester (cocaethylene)]. Because of the similarity in chemical structure between cocaine and cocaethylene and the broad spectrum of activity of carboxylesterases, we hypothesized that cocaethylene would also be metabolized by carboxylesterases and that its elimination might also be inhibited in the presence of ethanol or cocaine.
Materials and Methods
Animal Model.
This study was conducted as part of a pharmacodynamic evaluation of the cardiovascular effects of cocaine, cocaethylene, and alcohol in the dog. Details of the animal model are described in previous publications (Parker et al., 1996, 1998). Briefly, six adult male mongrel dogs weighting 16.2 to 21.2 kg were given acepromazine (0.1 mg/kg i.m.) and atropine (0.05 mg/kg i.m.) and anesthetized with thiopental 25 mg/kg IV. A cuffed endotracheal tube was inserted and anesthesia maintained with isoflurane (1.5%) and oxygen. An indwelling catheter with V-A-P Access Port (model 6VP; Access Technologies, Skokie, IL) was implanted into the carotid artery. The catheter was tunneled to the back of the dog's neck and connected to a V-A-P Access Port that was sutured in place underneath the skin. The dogs were given postoperative antibiotics and allowed to recover from surgery for 7 days. During the recovery period, the dogs were trained to stand in a nylon sling. The catheter was flushed daily with heparinized saline (250U/ml) to maintain catheter patency. This study was approved by the University of Tennessee Animal Care and Use Committee and was performed according to the National Institutes of Health guidelines for the care and use of animals.
Experimental Procedures.
The dog was placed in the nylon sling and an i.v. catheter placed in a foreleg vein for the administration of study drugs. For all treatments the doses of cocaine, cocaethylene, and ethanol were 3 mg/kg, 2.6 mg/kg, and 1g/kg, respectively. The dose of cocaine was chosen to achieve plasma concentrations of approximately 2500 ng/ml, which is comparable to concentrations reported in human cases of cocaine toxicity (Bailey, 1993). The cocaethylene dose was chosen to achieve peak concentrations similar to cocaine and is based on our previous pharmacokinetic studies of cocaine and cocaethylene in the dog (Parker et al., 1998). The dose of ethanol was chosen to produce moderate ethanol intoxication. In our previous studies in dogs, this dose produced a peak ethanol blood concentration of 144 ± 28 mg/dl (n = 6) (Parker et al., 1996). Each dog received the following drug treatments on separate days with at least a 48-h hiatus between study days: 1) cocaine alone, 2) cocaine and ethanol, 3) cocaethylene alone, 4) cocaethylene and ethanol, and 5) cocaine and cocaethylene. Cocaine and cocaethylene were administered by a 5-min i.v. infusion, and ethanol was administered by a 40-min infusion prior to cocaine or cocaethylene administration.
Arterial blood samples (3–5 ml) were collected through the V-A-P Access Port at 0, 3, 5, 10, 15, 25, 35, 65, 125, 250, and 300 min after the start of the cocaine or cocaethylene infusion. Collected blood samples were transferred to a chilled vacutainer tube containing 30 mg of sodium fluoride, mixed, and put on ice until separation of plasma. Samples were centrifuged within 1 h for 10 min at 2000 rpm, and the plasma transferred to cryogenic storage vials and placed on ice. At the end of the study day all samples were stored at −70°C until assayed.
Cocaine, benzoylecgonine, and cocaethylene were extracted from plasma using 130 mg (3 ml) Bond Elut Certify solid-phase extraction columns (Varian Inc., Palo Alto, CA). After conditioning the columns with methanol and KH2PO4buffer, pH 6.0, the plasma sample containing 100 ng of added lidocaine (internal standard) was decanted onto the solid-phase extraction column and the columns washed with deionized water and 100 mM HCl. The compounds of interest were eluted with 2 ml of methanol/NH4OH (98:2), dried under nitrogen at 35°C, and reconstituted in 200 μl of mobile phase. A 170-μl aliquot was injected onto an isocratic, high-performance liquid chromatographic LC-ABZ (4.6 × 250 mm analytical) column (Supelco, Bellefont, PA) with a mobile phase of 50 mM KH2PO4 buffer, pH 5.5, and acetonitrile (84:16 v/v) at a flow rate of 1.4 ml/min with detection by UV absorbance at 230 nm. The within-day coefficient of variation at 400 ng/ml for cocaine, cocaethylene, and benzoylecgonine was 1.4, 1.9, and 1.1%, respectively; the between-day coefficient of variation at 400 ng/ml for cocaine, cocaethylene, and benzoylecgonine was 2.4, 1.5, and 3.7%, respectively.
Data Analysis.
Pharmacokinetic analysis
A first-order elimination model was fit to the cocaine and cocaethylene data using WinNonlin (version 3.1, Pharsight, Inc., Mountain View, CA) with the following model equation:
The model parameter estimates were subsequently used to calculate clearance (Cl), volume of distribution at steady state (Vss), and the half-life of elimination (t1/2). The clearance was calculated by
The area under the curve (AUC) from time 0 to 300 min was calculated for cocaine, cocaethylene, and benzoylecgonine (BE) using the trapezoidal rule.
Statistical analysis.
Two separate repeated measures analysis of variances were used to compare the Cl, Vss, andt½; between cocaine alone, cocaine with EtOH, and cocaine with cocaethylene, and between cocaethylene alone, cocaethylene with EtOH, and cocaethylene with cocaine. A posthoc Tukey's test was performed if a statistical difference (p < 0.05) was found. The AUC0–300 ratios were compared between parent drug given alone (cocaine or cocaethylene) and after ethanol using a paired t test.
Results
The model that best fit the cocaine and cocaethylene concentration-time data had two exponents indicating that both cocaine and cocaethylene have a significant distribution phase. Cocaine and cocaethylene pharmacokinetic parameter estimates are summarized in Table 1. There was a significant decrease in the clearance of cocaine and cocaethylene when ethanol was administered prior to their infusion (Table 1; Fig.1). When cocaine and cocaethylene were administered together, there was a significant decrease in the clearance of cocaine, but the clearance of cocaethylene, although lower, was not significantly decreased by cocaine.
Pharmacokinetic parameters of cocaine and cocaethylene
Concentration-time profile from a single dog after cocaine 3 mg/kg (top) or cocaethylene 2.6 mg/kg (bottom) with and without ethanol (EtOH) coadministration.
As shown in Table 2, benzoylecgonine seems to be a major metabolite of cocaethylene. The AUC ratio of benzoylecgonine to parent (i.e., cocaine or cocaethylene) is similar, and both significantly decrease after ethanol administration.
The ratio of parent drug to metabolite
Discussion
The present study has shown that cocaethylene elimination is decreased by ethanol, and that benzoylecgonine is formed from cocaethylene in the dog. Given the structural similarity between cocaine and cocaethylene, it is not surprising that carboxylesterases in the dog would hydrolyze both compounds resulting in the formation of benzoylecgonine. As seen in Table 2, the administration of cocaine 3 mg/kg or cocaethylene 2.6 mg/kg results in similar AUCs for the parent compounds, similar benzoylecgonine metabolite AUCs, and essentially identical decreases in the ratio of benzoylecgonine to parent (cocaine or cocaethylene) drug after ethanol administration. Since benzoylecgonine is a major metabolite of cocaine, and the pharmacokinetics of cocaine and cocaethylene are similar (see Table 1), it follows that the similarities in AUCs of benzoylecgonine after cocaine or cocaethylene administration indicate that benzoylecgonine is also a major metabolite of cocaethylene. The carboxylesterases have a broad spectrum of activity so it was not unexpected that they would be unable to distinguish cocaine from cocaethylene. This lack of specificity would suggest that cocaethylene, itself, could undergo transesterification with ethanol resulting in the formation of cocaethylene from cocaethylene. Bourland et al. demonstrated in S9 hepatic fractions from Sprague-Dawley rats that transesterification between cocaethylene and ethanol did occur. When deuterated ethanol and cocaethylene were incubated in hepatic fractions, deuterated cocaethylene was formed confirming that transesterification had occurred (Bourland et al., 1997). No deuterated cocaethylene was formed when the experiment was repeated in buffer, and the formation of deuterated cocaethylene was prevented by the addition of bis-(p-nitrophenyl)phosphate, a specific carboxylesterase inhibitor. This lack of enzyme specificity may also extend to the hydrolysis of the benzoyl group that results in the formation of ecgonine methyl ester suggesting the potential for the formation of ecgonine ethyl ester from cocaethylene.
When cocaine and cocaethylene were given together, the clearance of cocaine was significantly decreased, but the clearance of cocaethylene was not, although there was a slight decrease in the average clearance of cocaethylene. These results suggest cocaethylene may have a higher affinity for the active site of the enzyme than cocaine. This would explain why at similar concentrations of the two compounds that cocaine elimination is decreased, but cocaethylene clearance is unchanged. However, in the actual coabuse of ethanol and cocaine, the concentrations of cocaine most likely far exceed the concentrations of cocaethylene, and thus, under the assumption of competitive inhibition at the active site, it is likely that cocaine clearance is not affected and even possible that cocaine may inhibit the elimination of cocaethylene by carboxylesterases.
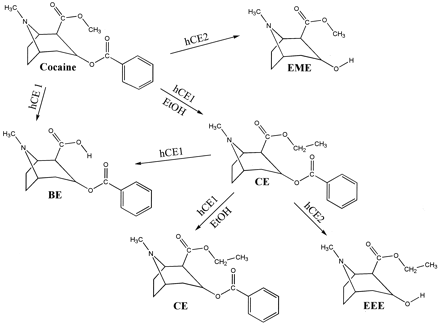
In humans, it has been shown that hCE1 catalyzes the hydrolysis of cocaine to benzoylecgonine and the transesterification between ethanol and cocaine resulting in the formation of cocaethylene (Dean et al., 1991; Brzezinski et al., 1994). The other major route of cocaine elimination is via hydrolytic conversion to ecgonine methyl ester by hCE2 and serum pseudocholinesterase. Neither hCE2 nor pseudocholinesterase catalyzes the formation of cocaethylene. It is hCE1 that is responsible for the formation of benzoylecgonine; thus, cocaethylene is formed at the expense of benzoylecgonine when cocaine is administered with ethanol. The metabolic fate of cocaethylene is less certain, but a growing body of evidence supports the view that it is subject to hydrolysis by carboxylesterases (Brzezinski et al., 1997; Bourland et al., 1997; Satoh et al., 2002). The metabolic scheme in Fig. 2 illustrates the likely fate of cocaethylene assuming hCE1 and hCE2 hydrolyze cocaethylene.
Carboxylesterase metabolism of cocaine and cocaethylene (CE) in the presence of ethanol. Cocaine is hydrolyzed to benzoylecgonine (BE) and ecgonine methyl ester (EME) by hCE1 and hCE2, respectively.
If ethanol is present, cocaine is also metabolized to CE by transesterification with ethanol catalyzed by hCE1. CE is hydrolyzed to BE by hCE1. When transesterification between CE and ethanol occurs, then the resulting product would be CE. The formation of ecgonine ethyl ester (EEE) is hypothesized based on the expected hydrolysis at the site of the benzoyl group of CE by hCE2.
The proposed metabolic scheme for cocaethylene has not been confirmed in humans. However, studies have shown that in both dogs and humans the two major metabolites of cocaine are benzoylecgonine and ecgonine methyl ester and that ethanol inhibits the elimination of cocaine (Dean et al., 1991, 1992; Boyer and Petersen, 1992; Roberts et al., 1993;Parker et al., 1996). In addition, in vitro formation of cocaethylene from cocaine and ethanol has been demonstrated in dog hepatic microsomal preparations (Song et al., 1999). This consistency in cocaine disposition is mirrored by the interspecies similarities in carboxylesterase activity. Hosokawa et al. (1990) reported that both the immunochemical properties and enzymatic activity of carboxylesterase isoenzymes in mouse, hamster, guinea pig, rabbit, dog, monkey, and humans were well conserved.
The transesterification with ethanol as opposed to hydrolysis with water catalyzed by carboxylesterase is not unique to cocaine. Recent studies indicate that methylphenidate also undergoes transesterification with ethanol producing the metabolite ethylphenidate, which, analogous to cocaethylene, is an active metabolite that retains pharmacologic activity similar to its parent compound (Markowitz et al., 2000). Numerous other drugs including heroin, flumazenil, irinotecan, lovastatin, and enalapril are metabolized by carboxylesterases and could be susceptible to an interaction with ethanol (Pang et al., 1991; Tang and Kalow, 1995;Kamendulis et al., 1996; Sai et al., 2001; Franssen et al., 2002).
Footnotes
-
This study was supported by a grant from the National Heart, Lung, and Blood Institute, Grant R15-HL54311.
- Abbreviations used are::
- hCE
- human carboxylesterase
- Cl
- clearance
- Vss
- steady state
- λz
- terminal elimination rate constant
- AUC
- area under the curve
- BE
- benzoylecgonine
- Received June 10, 2002.
- Accepted September 19, 2002.
- The American Society for Pharmacology and Experimental Therapeutics