Abstract
Opioids are important drugs used as analgesics, antitussives, antidiarrheals, and in the therapy of myocardial infarctions, and as antagonists of opioid intoxication. The glucuronidation of these compounds, catalyzed by UDP-glucuronosyltransferases (UGTs), is well known to be a primary step in their metabolism to hydrophilic products and in their ultimate excretion. The present study was designed to compare the reactivity and relative glucuronidation efficiencies of opioid agonists, antagonists, and partial agonists with two rat UGT isoforms; UGT1.1, which is generally considered the “bilirubin UGT,” and UGT2B1, which has previously been shown to catalyze the glucuronidation of testosterone, chloramphenicol, and (−)-morphine. Rat UGT2B1, stably expressed in HK293 cells, exhibited high glucuronidation rates and catalytic efficiencies for many opioids, although values for (−)-morphine and nalorphine were the highest. In contrast, these compounds were very poor substrates for expressed rat UGT1.1. Comparably high glucuronidation rates and efficiencies were found for buprenorphine and diprenorphine with both UGT isoforms. These results suggest that opioids with morphinan-based chemical structures similar to (−)-morphine interact with UGTs differently than those with oripavine-based chemical structures similar to buprenorphine. To investigate the contribution of rat UGT1.1 and UGT2B1 in the overall rate of glucuronidation of buprenorphine in the rat liver, hepatic microsomes from Gunn rats (where UGT1.1 activity is absent) and Wistar rats (where UGT1.1 activity is present) were studied. Buprenorphine glucuronidation activity in Gunn rat liver microsomes exhibit ∼25% of rates observed in Wistar rat liver microsomes, whereas (−)-morphine, naloxone, and naltrexone glucuronidation rates were not significantly different in microsomal preparations from Gunn and Wistar rats. These data suggest that UGT2B1 is the major hepatic enzyme involved in the glucuronidation of (−)-morphine and naloxone in livers from untreated rats, whereas buprenorphine glucuronidation is preferentially catalyzed by rat UGT1.1.
The conjugation of many xenobiotics and endobiotics with UDP-GlcUA1is catalyzed by UGTs. The glucuronide conjugates produced are generally less active and hydrophilic, and thus more readily excreted from the body via the liver or kidney. UGTs are intrinsic membrane proteins whose active sites are presumably localized on the luminal side of the endoplasmic reticulum. Substrates for UGTs include phenols, carboxylic acids, alcohols, and amines, including both xenobiotics and endobiotics (1).
Opioids are important therapeutic agents that undergo extensive metabolism via glucuronidation. Species differences in the glucuronidation of one such compound, (−)-morphine, are well known. Humans, guinea pigs, and rabbits form both the (−)-morphine-3-glucuronide and (−)-morphine-6-glucuronide, whereas rats and mice form only (−)-morphine-3-glucuronide (2). Identification and characterization of the specific UGT isoforms that catalyze opioid glucuronidation in rats has been investigated for many years. As early as 1975, del Villar et al. (3) separated and partially purified UGTs from rat liver microsomes that catalyzed the glucuronidation of (−)-morphine. Subsequently, Puig and Tephly (4) purified a phenobarbital-inducible morphine UGT isoform from rat liver. Recently, Coffman et al. (5) purified two UGTs to apparent homogeneity from rat liver microsomes that catalyzed the glucuronidation of opioid compounds and that were consistent with cloned and expressed UGT1.1 and UGT2B1. Pritchard et al. (6) showed that rat UGT2B1 catalyzed (−)-morphine glucuronidation at high rates, whereas rat UGT1.1 isolated, cloned, and stably expressed by our laboratory catalyzed the glucuronidation of buprenorphine and bilirubin at high glucuronidation rates with low rates of (−)-morphine glucuronidation (7). Thus, opioid glucuronide formation is conducted in rats by at least two different UGT isoforms. Although both rat UGT1.1 and UGT2B1 catalyze opioid glucuronidation, the purpose of the current study was to investigate the reactivity and relative enzymatic efficiencies of a number of opioid compounds with each of these UGT isoforms that were stably expressed in HK293 cells. Because opioid metabolism in vivo is highly dependent on glucuronidation, the current study further determined the relative contribution of each enzyme to the conjugation of opioids in vivo. Results suggest that UGT2B1 is the major enzyme involved in the glucuronidation of (−)-morphine and nalorphine in untreated rat liver, whereas buprenorphine is preferentially glucuronidated by UGT1.1.
Materials and Methods
Chemicals.
UDP-[U-14C]GlcUA (255 mCi/mmol) was purchased from ICN Pharmaceuticals (Irvine, CA), and [35S]methionine (1,000 Ci/mmol) was obtained from Amersham (Arlington Heights, IL). Aglycone substrates for glucuronidation assays were purchased from Aldrich Chemical Co. (Milwaukee, WI), Sigma Chemical Co. (St. Louis, MO), or Research Biochemicals International (Natick, MA). (−)-Morphine-3-glucuronide and (−)-morphine-6-glucuronide were obtained from Research Biochemicals International. Geneticin, dithiothreitol, saccharolactone, phosphatidylcholine (type XVI-E) and UDP-GlcUA were purchased from Sigma. The mammalian expression vector pcDNA3 was obtained from Invitrogen (San Diego, CA). Protein assay reagents were from Bio-Rad (Hercules, CA). All other reagents were of analytical grade.
Stable Expression of Rat UGT1.1 and UGT2B1.
The isolation and stable expression of a cDNA encoding rat UGT1.1 has been described previously by Coffman et al. (7). Isolation of UGT2B1 cDNA has been described previously (8). HK293 cells were transfected with a pREP9-UGT2B1 construct using the calcium phosphate transfection method (9). Forty-eight hours posttransfection, the cells were split and grown in media containing 700 μg geneticin/ml to establish stable transfectants.
Determination of Levels of Expression of Rat UGT1.1 and UGT2B1 in HK293 Cells.
HK293 cells expressing rat UGT1.1 and UGT2B1 proteins were labeled using [35S]methionine as described previously (10). UGT2B1 protein was immunoprecipitated using a sheep IgG raised against rabbit PNP UGT (11) and a mixture of Protein A- and Protein G-Sepharose (3.2 mg Protein A/G Sepharose/mg IgG). The Sepharose-bound immunocomplexes were washed extensively (12) and then released from the Sepharose beads by boiling in the presence of 2.4% (w/v) sodium dodecyl sulfate and 10% (v/v) mercaptoethanol. An aliquot of the supernatant was analyzed by liquid scintillation spectrometry (Packard Tricarb, Downers Grove, IL) to determine the amount of radioactivity specifically incorporated into UGT1.1 or UGT2B1. Similar samples of radiolabeled cellular proteins were incubated with preimmune sheep IgG as control samples. The amount of [35S]methionine incorporated into total cellular protein was determined using a modified Manns-Novelli procedure on glass fiber membranes. The amount of [35S]methionine incorporated into newly synthesized UGT1.1 was calculated by dividing the amount of radioactivity specifically immunoprecipitated by the anti-UGT IgG (i.e.counts immunoprecipitated by anti-UGT IgG minus counts immunoprecipitated by preimmune IgG) by the amount of [35S]methionine incorporated into total cellular protein.
Membrane Preparations of Rat UGT1.1 and UGT2B1.
Membrane preparations were made using a modification of the method of Battaglia et al. (13). Freshly harvested HK293 cells expressing either UGT1.1 or UGT2B1 were washed 3 times with phosphate-buffered saline. Cells were then resuspended (1:3, v/v) in 0.25 M sucrose, containing 5 mM HEPES (pH 7.4) and 0.5 mM dithiothreitol. A homogenate was obtained by the disruption of the cells using a tissue tearer (Bio Spec, Racine, WI) for 10 sec. Cell homogenates were then further diluted up to (1:4, v/v) with 0.25 M sucrose, containing 5 mM HEPES (pH 7.4) and sonicated 3 times in 10-sec bursts using a Sonifier Cell Disruptor (Ultrasonics, Plainview, NY). Then, cells were homogenized by hand with a Potter-Elvehjem homogenizer (10 strokes) before finally being centrifuged at 9,000g for 20 min. Supernatants were pooled and centrifuged at 100,000gfor 60 min to obtain membrane pellets. All steps were conducted at 4°C. The resulting pellets were overlaid with 0.5 ml of 0.25 M sucrose, containing 5 mM HEPES (pH 7.4) and frozen at −80°C.
UGT Assays.
Membrane preparations from HK293 cells that stably expressed rat UGT1.1 or UGT2B1 were suspended in 10 mM Tris-buffered saline (pH 7.4) containing 0.5 mM dithiothreitol. Opioid glucuronidation was determined using the method described by Puig and Tephly (4). The HPLC method of Svensson et al. (14) was used to identify (−)-morphine glucuronide products. Initial aglycone reactions with stably expressed rat UGT1.1 or UGT2B1 were conducted using 2 mM UDP-GlcUA (0.25 μCi/100 μl) and incubated at 37°C for 20 min to 1 hr. The standard enzyme assay mixture (100 μl total volume) contained 50 mM Tris-HCl, 10 mM MgCl2, 100 μg phosphatidylcholine/ml, and 8.5 mM saccharolactone. The limit of detection for glucuronide formation using 2.0 mM UDP-GlcUA was ∼2 pmol of glucuronide formed/min/mg protein. Membranes from nontransfected HK293 cells and pcDNA3-transfected HK293 cells showed no glucuronidation activity toward any of the opioids tested in the present study.
The apparent KM
for UDP-GlcUA (0.14 mM) in HK293 cells expressing rat UGT1.1 was previously determined by Coffmanet al. (7). The apparent KM
for UDP-GlcUA (0.22 mM) for UGT2B1 was previously determined by Pritchardet al. (6). For kinetic analysis of aglycones, the apparentKM
values were determined using the optimal pH (table 1), protein concentrations, and reaction times yielding linear product formation for each substrate. The apparentKM
for aglycone substrates was estimated by altering the aglycone concentration from ∼
Comparison of glucuronidation rates of opioids in membrane preparations from HK293 cells stably expressing rat UGT1.1 and UGT2B1
Results
Expression Levels of UGT1.1 and UGT2B1 in HK293 Cells.
Pulse-labeling experiments were conducted to estimate the relative steady-state levels of rat UGT1.1 and UGT2B1 proteins expressed in HK293 cells. The amount of [35S]methionine incorporated into newly synthesized rat UGT1.1 at steady-state was previously reported to be 0.51% of that incorporated into newly synthesized total cellular protein (15). [35S]Methionine incorporation into UGT2B1 was found to be 0.26%, corresponding to only ∼50% of the UGT1.1 protein expression level. These results probably reflect different numbers of copies of the pcDNA3-UGT1.1 or pREP9-UGT2B1 in the two cell populations that were stably incorporated into the HK293 cell genome.
Glucuronidation of Opioid Compounds Using Membrane Preparations from HK293 Cells Stably Expressing Rat UGT1.1 and UGT2B1.
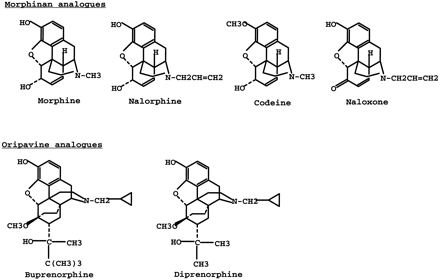
Using whole cell homogenates of HK293 cells expressing rat UGT1.1, our laboratory has previously shown that the expressed protein catalyzes the glucuronidation several opioid compounds (7, 15). Membrane preparations derived from HK293 cells stably expressing rat UGT1.1 (table 1) yielded results consistent with whole cell homogenate preparations, except that the glucuronidation rates observed for the membrane preparations were much higher than the whole cell homogenates. These data indicate that the UGT1.1 expressed in HK293 cells is membrane-bound. We extended our previous study by investigating the ability of expressed UGT1.1 to catalyze the glucuronidation of other oripavine-based and morphinan-based analogs. Of the opioids tested, buprenorphine showed the highest glucuronidation rate with rat UGT1.1. Buprenorphine analogs, such as diprenorphine and norbuprenorphine, also displayed high rates, whereas other opioid compounds, such as (−)-morphine, naloxone, nalorphine, and naltrexone were much lower. These data suggest that the oripavine-based analogs such as buprenorphine and diprenorphine are better substrates for rat UGT1.1, compared with (−)-morphine and its analogs (fig.1).
Chemical structures of morphinan and oripavine analogs.
In contrast to results obtained with expressed UGT1.1, expressed rat UGT2B1 catalyzed high rates of glucuronidation toward (−)-morphine and nalorphine. Naltrexone, naloxone, and buprenorphine also reacted with expressed UGT2B1, but their glucuronidation rates were considerably lower, compared with those of (−)-morphine and nalorphine. Other opioids studied, such as naltrindole, naltriben, and levorphanol, reacted at much lower rates, whereas codeine did not serve as a substrate for either UGT. Because codeine can only be glucuronidated at the 6-OH position, these data suggest that UGT2B1 and UGT1.1 catalyze the formation of opioid glucuronides only at the 3-OH group. Indeed, HPLC analysis showed that (−)-morphine-3-glucuronide was the only product formed for both UGT isoforms when (−)-morphine was the substrate (data not shown).
Kinetic Analysis of Glucuronidation Catalyzed by Expressed Rat UGT1.1 and UGT2B1.
Kinetic analysis for each substrate was performed after determination of optimal pH, and conditions providing linear rates of product formation with time and protein concentrations for both expressed UGT enzymes (table 2). The results, using membrane preparations, show that the glucuronidation efficiency for buprenorphine was the highest of the opioids tested for expressed rat UGT1.1. Comparison of the kinetic values for buprenorphine glucuronidation in whole cell homogenates (Vmax/KM = 730 μl/min/mg) and in membrane preparations (Vmax/KM = 475 μl/min/mg) are reasonably similar for the two UGT preparations with respect to efficiency of glucuronidation. Also, the apparentKM values for naltrexone and nalorphine in membrane preparations from HK293 cells expressing rat UGT1.1 (5,200 μM and 1,500 μM, respectively) were similar to those obtained using whole cell homogenates (5,100 μM and 1,350 μM, respectively) (15). The glucuronidation efficiencies of diprenorphine and norbuprenorphine for expressed rat UGT1.1 were also rather high. Of the opioid compounds tested, the glucuronidation efficiencies of buprenorphine and its derivatives were 10- to 1,000-fold higher than those obtained for (−)-morphine, naloxone, naltrexone, and nalorphine.
Comparison of kinetics of glucuronide formation in membrane preparations from HK293 cells stably expressing rat UGT1.1 and UGT2B1
Membrane preparations of expressed UGT2B1 yielded rates of glucuronidation for (−)-morphine and nalorphine with high efficiency, as might be predicted from the glucuronidation rates shown in table 1. The glucuronidation efficiencies of naloxone, naltrexone, buprenorphine, and diprenorphine were all similar. Of the opioids tested, norbuprenorphine displayed the lowest efficiency of glucuronidation with UGT2B1. Based on the data presented in table2, UGT2B1 seems to be an important enzyme involved in the glucuronidation of many of the opioid compounds tested. However, because UGT2B1 and UGT1.1 catalyze the glucuronidation of buprenorphine with similar efficiencies, the relative contribution of these two enzymes in metabolism of buprenorphine in microsomes of untreated rat liver was determined using a different experimental approach.
Glucuronidation of Opioid Compounds by Wistar and Gunn Rat Liver Microsomes.
To investigate the possible role of UGT1.1 and UGT2B1 in the glucuronidation of buprenorphine in vivo, the glucuronidation rates of buprenorphine, (−)-morphine, naloxone, naltrexone, and bilirubin were studied in Gunn and Wistar rat liver microsomes. Gunn rat liver microsomes were used because of their lack of UGT1.1 protein and inability to glucuronidate bilirubin and certain other compounds whose glucuronidation depends on this protein. Gunn rats do not express any UGT1 gene products due to a mutation in the common coding region of the UGT1 gene (16). As expected, bilirubin was not glucuronidated by Gunn rat liver microsomes (table3). Both Gunn and Wistar rat liver microsomes catalyzed the glucuronidation of (−)-morphine, naltrexone, and naloxone at similar rates. In contrast, buprenorphine glucuronidation activity in the Gunn rat liver microsomes was only about one-fourth of that found for the Wistar rat liver microsomes. These data support the idea that UGT1.1 preferentially catalyzes the glucuronidation of buprenorphine in untreated rat liver and that UGT2B1 is the main enzyme responsible for (−)-morphine glucuronidation.
Glucuronidation of opioids by Wistar and Gunn rat liver microsomes
Discussion
Despite the fact that rat UGT1.1 and UGT2B1 have <50% amino acid sequence identity, they do share a limited number of common substrates (e.g. coumarins and simple phenols) (6, 7, 15). However, compounds such as testosterone, chloramphenicol, and profen nonsteroidal antiinflammatory drugs are substrates for rat UGT2B1 but not for UGT1.1 (6, 15). Furthermore, compounds such as bilirubin and certain catechol estrogens serve as substrates for rat UGT1.1 but not for UGT2B1 (6, 15). Our results show that similarities and differences also exist in the glucuronidation of opioids catalyzed by expressed rat UGT2B1 and UGT1.1. With the exception of codeine, all opioids tested in the current study were substrates for both UGT isoforms. However, opioids with chemical structures similar to (−)-morphine (fig. 1) were far better substrates (100- to 1,000-fold) for UGT2B1, compared with UGT1.1. The fact that UGT2B1 was expressed at ∼50% of the levels of UGT1.1 would further magnify this difference if kinetic values were corrected based on the levels of UGT expression. On the other hand, buprenorphine analogs were glucuronidated with comparable catalytic efficiencies for both enzymes.
It is unclear why (−)-morphine analogs display such diverse reactivity with rat UGT2B1 and UGT1.1, whereas the kinetic values for buprenorphine are so similar between the enzymes. It is possible that the opioid binding site of the two UGT isoforms is highly lipophilic and buprenorphine is considered to be one of the most lipophilic opioids known. Thus, the low apparent KM of buprenorphine probably involves, to some degree, its lipophilicity. Even for UGT2B1, the apparent KM for buprenorphine is lower than for (−)-morphine, although the turnover number for (−)-morphine is higher. The main physiological substrate for rat UGT1.1, bilirubin, is also a very highly lipophilic compound, which further supports the hypothesis that the opioid binding site for this isoform is located in a lipophilic pocket of the molecule.
Expressed rat UGT2B1 catalyzed the glucuronidation of naloxone, naltrexone, and nalorphine at rates lower than that of (−)-morphine. However, the apparent KM values for these (−)-morphine analogs were lower than that of (−)-morphine. These data support the results of Sanchez et al. (17) who noted that, whereas the 3-OH position is important for the glucuronidation site, the presence and length of the N-alkyl side chain of opioids affect the ability of the compounds to interact with the opioid binding site. Thus, compounds that have low Ki values as inhibitors (17) also have low apparent KM values as substrates for rat UGT2B1.
In rat liver microsomes, (−)-morphine glucuronidation has been shown to be only at the 3-OH position (2). In this study, we determined that expressed rat UGT2B1 and UGT1.1 form only (−)-morphine-3-glucuronide. HPLC analysis did not detect (−)-morphine-6-glucuronide formation and, as expected, codeine is not a substrate for either of the expressed enzymes. In contrast, an expressed human enzyme (UGT2B7 variant) catalyzes the formation of both (−)-morphine-3-glucuronide and (−)-morphine-6-glucuronide.2
Expressed UGT2B1 and UGT1.1 show differences in the glucuronidation of (−)-morphine and buprenorphine, compared with the purified isoforms. Purified UGT2B1 (5, 18) catalyzes the glucuronidation of (−)-morphine at a high rate, but did not conjugate buprenorphine (5). In contrast, we have shown in the present study that expressed UGT2B1 glucuronidates buprenorphine. Similarly, differences in the glucuronidation of testosterone with expressed (6) and purified (18) UGT2B1 were observed. One explanation for these discrepancies may be due to the effect of detergent on the purified UGT2B1. Green and Tephly (19) have recently shown that some detergents can interfere with the glucuronidation of some compounds. Results using expressed UGT2B1 showed that buprenorphine is glucuronidated at only 4% the catalytic rate of (−)-morphine, and thus another explanation for the observation that purified UGT2B1 did not catalyze buprenorphine glucuronidation may be due to the limits of detection of the glucuronidation assay. A second discrepancy between purified UGT2B1 and UGT1.1 and the expressed isoforms is with the glucuronidation of (−)-morphine and nalorphine. (−)-Morphine and nalorphine displayed similar catalytic rates when compared with buprenorphine using purified UGT1.1 (5), but the glucuronidation rates of nalorphine and (−)-morphine are 15- to 50-fold lower than that of buprenorphine using expressed UGT1.1. The difference in the results using purified and expressed UGT1.1 may be due to an incomplete separation of the UGT2B1 isoform from the purified UGT1.1 fraction. Because UGT2B1 catalyzes the glucuronidation of (−)-morphine at a high rate, even a 1% contamination of purified UGT1.1 with UGT2B1 could result in an apparent increase in the rate of (−)-morphine glucuronidation.
Buprenorphine is a unique opioid in that it has several potential uses both as an opioid and in the treatment for substance abuse in which it is currently under clinical testing. To examine the contributions of UGT1.1 and UGT2B1 in the glucuronidation of buprenorphine in vivo, liver microsomes from untreated Gunn and Wistar rats were used. Interestingly, buprenorphine glucuronidation in Gunn rat liver microsomes was only 25% of the glucuronidation in Wistar rat liver microsomes. This suggests that rat UGT1.1 could be the major enzyme involved in buprenorphine glucuronidation and could lead to important clinical implications. Recently, our laboratory demonstrated that human UGT1.1 also catalyzes the glucuronidation of opioids (15) and that human UGT1.1 glucuronidated buprenorphine with similar rates and catalytic efficiencies as rat UGT1.1. Buprenorphine glucuronidation in Crigler-Najjar type I liver microsomes, interestingly, is also 25% that of normal human liver microsomes, indicating the importance of human UGT1.1 in metabolizing this opioid. The human Crigler-Najjar type I patients we examined were deficient in UGT1.1 only, whereas Gunn rats are devoid of all UGT1 gene products. Thus, in humans, UGT1.1 seems to be the only UGT gene product with a significant ability to glucuronidate buprenorphine. In rats though, bilirubin is also glucuronidated by UGT1.0 (20), but this protein is encoded by a pseudogene in humans and thus no protein is expressed. UGT1.1 and UGT1.0 share ∼70% amino acid identity, and it is possible that in rats UGT1.0 could also glucuronidate buprenorphine. Thus, the Gunn rat could be used as a clinical model for Crigler-Najjar type I patients in buprenorphine-type opioid metabolism in vivo.
In summary, we have investigated membrane preparations from HK293 cells stably expressing two rat UGT isoforms, UGT1.1 or UGT2B1, that were known to catalyze the glucuronidation of (−)-morphine (6, 7). A wide variety of buprenorphine and (−)-morphine derivatives were investigated for their reactivity and efficiency of glucuronidation with each of these UGT isoforms. UGT1.1 seemed to be the major UGT isoform involved in the glucuronidation of buprenorphine, whereas (−)-morphine and nalorphine are the best substrates for UGT2B1 isoform.
Acknowledgments
The compound (E)-7-benzylidinenaltrexone HCl (MG-B-139; lot no. WY-IV-80) was provided by Research Biochemicals International as part of the Chemical Synthesis Program of the National Institute of Mental Health (Contract N01MH30003). We also thank Birgit Coffman for her helpful suggestions in the preparation of this manuscript.
Footnotes
-
Send reprint requests to: Dr. Thomas R. Tephly, Department of Pharmacology, University of Iowa, 2-452 Bowen Science Building, Iowa City, IA 52242.
-
This research was supported by the National Institutes of Health Grant GM26221.
-
↵2 B. L. Coffman et al., manuscript in preparation.
- Abbreviations used are::
- UDP-GlcUA
- UDP-glucuronic acid
- UGT
- UDP-glucuronosyltransferase
- HK293 cell
- human embryonic kidney 293 cell
- IgG
- immunoglobulin G
- OH
- hydroxy
- Received August 19, 1996.
- Accepted November 7, 1996.
- The American Society for Pharmacology and Experimental Therapeutics