Abstract
3′-Azido-3′-deoxythymidine (AZT) is currently prescribed to pregnant women infected with human immunodeficiency virus to reduce the risk of vertical transmission of the virus to the fetus. Consequently, more information is needed concerning the placental transfer and tissue distribution of AZT and its metabolites. In the present study, the placental transfer and fetal accumulation of AZT, its glucuronide metabolite [3′-azido-3′-deoxythymidine-β-d-glucuronide (AZTG)], and phosphorylated metabolites were examined at steady-state in near-term rhesus macaques. One to 2 weeks before a chronic infusion, an intravenous bolus of 8 mg/kg AZT was administered to pregnant animals to determine the dose of AZT needed to reach steady-state plasma concentrations. On the day of hysterotomy, the mother was administered an intravenous loading dose of AZT, followed by a 3-hr steady-state intravenous infusion that also included a trace of [3H]AZT. After 3 hr of infusion, the mother was anesthetized, and the fetus was delivered. Plasma and amniotic fluid were analyzed for AZT and AZTG by HPLC, and tissue samples were analyzed for AZT, AZTG, and phosphorylated metabolites by strong anion exchange HPLC. Maternal steady-state plasma concentrations were 1.3–2.2 μg/ml for AZT and 2.3–8.0 μg/ml for AZTG. Fetal AZT and AZTG plasma concentrations were both lower (0.98–2.3 μg/ml and 1.3–5.4 μg/ml, respectively) than maternal concentrations, with fetal-to-maternal plasma ratios of 0.63–1.0 for AZT. Fetal tissue distribution of tritium was highest in the kidney and lowest in the brain. Although the active triphosphorylated metabolite was not detected in the fetus, the AZT-monophosphate was detected in almost all fetal tissues examined. Our data indicate that AZT is rapidly converted to the glucuronide and monophosphate metabolites in the fetus after maternal infusion.
One of the fastest growing populations suffering from HIV1 infection is women in their reproductive years (1). The number of HIV infections in the pediatric population is also increasing at an alarming rate (2). HIV infection in children can occur in several ways, but the dominant route is by vertical transmission from an HIV-infected mother to her infant (3). This vertical viral transmission may occur early or late in pregnancy, during birth, or postnatally through breast feeding (4). Although the fetus can be infected with the HIV virus during gestation, only ∼30% of children born to HIV-infected mothers are documented HIV-positive in the first several years of life (5). Treatment of HIV-infected pregnant women with anti-HIV therapeutics may, therefore, inadvertently expose many uninfected and healthy fetuses to these maternally administered and potentially toxic drugs.
The majority of agents approved for the treatment of AIDS and infection with HIV are nucleoside analog reverse-transcriptase inhibitors, which include AZT. AZT has recently been shown to reduce the vertical transmission of HIV from mother to fetus (6), but little is known about its fetal tissue distribution and metabolism. AZT is a dideoxynucleoside with structural similarities to endogenous nucleosides. The mechanism of anti-HIV activity of AZT is believed to involve the inhibition of HIV reverse transcriptase and DNA chain elongation by incorporation of AZT-TP into the viral DNA (7). The toxicity of AZT may also be due to the interaction of AZT-TP with mammalian DNA polymerases β and γ (mitochondrial) (8, 9). These triphosphates are potent inhibitors of reverse transcriptase, and differences in potency are primarily due to differences in metabolism to the active form (10). Although these compounds are efficacious in both adult and pediatric HIV-infected populations (11, 12), and reduce the risk of vertical transmission during pregnancy (6), they are associated with serious dose-limiting side effects. The major dose-limiting toxicity of AZT is the inhibition of the development of bone marrow cells resulting in anemia and neutropenia (13).
Our laboratory has previously demonstrated that the anti-HIV nucleosides ddC and 2′,3′-dideoxyinosine reach the fetal circulation after maternal intravenous administration, but the active triphosphates of these agents were not detectable in fetal tissues 3 hr after maternal dosing (14). The rhesus monkey’s placental structure and function, as well as pharmacokinetic similarities to humans, make this an appropriate model for examining AZT distribution during pregnancy (15, 16). The purpose of the present study was to examine fetal exposure to AZT by determining the placental transfer and fetal distribution of the parent compound and its glucuronidated and phosphorylated metabolites in the late-term rhesus monkey. This knowledge will provide a foundation for subsequent estimations concerning both the efficacy and the potential toxicity of these agents.
Methods
Animals.
Four pregnant rhesus macaques (Macaca mulatta) were obtained from the NCTR nonhuman primate colony. Animals were maintained in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and experimental procedures were approved by the NCTR’s Institutional Animal Care and Use Committee. Food (Purina Hi Protein Monkey Chow, Ralston Purina, St. Louis, MO) supplemented with fresh fruit and chewable multiple vitamins with iron (Arkansas Cooperative Assoc., Inc., North Little Rock, AR) was provided daily, and water was available ad libitum. The gestational age at the time of surgery was 150 ± 5 days (term = 165 days).
Chemicals.
AZT, AZTG, and AZddU were purchased from Sigma Chemical Co. (St. Louis, MO). AZT-MP, AZT-DP, AZT-TP, [3H]AZT, [methyl-3H]3′-azido-3′-deoxythymidine 5′-monophosphate (diammonium salt) and [methyl-3H]3′-azido-3′-deoxythymidine 5′-triphosphate (tetraammonium salt) were purchased from Moravek Biochemicals (Brea, CA).
Pharmacokinetics.
Two weeks before surgery, a pharmacokinetic study was performed to determine the dose of AZT needed to reach steady-state plasma concentrations in the mother. Approximately 2 weeks before this kinetic study (1 month before surgery), animals were acclimated to sitting in a restraint chair. On the day of the kinetic study, an intravenous bolus of 8 mg/kg AZT was administered to the awake animal while chair-restrained. Venous blood samples were collected beginning 2 min after the bolus dose for up to 3.5 hr, and immediately processed and analyzed for AZT and AZTG using reversed-phase HPLC.
Infusion and Surgery.
On the day of the surgery, the awake mother was administered an intravenous loading dose of AZT (1.2–2.3 mg/kg), including 40–60 μCi of [3H]AZT, followed by at least a 3-hr steady-state infusion (via maternal radial vein) of AZT (30–50 μg/min/kg, 15 ml/hr; Sage Instruments syringe pump model 351) and an additional 200 μCi of [3H]AZT. Maternal venous blood samples were collected every 30 min after the start of the infusion. Approximately 2.5 hr after infusion, anesthesia was induced with 10 mg/kg in ketamine hydrochloride (Ketaset, Fort Dodge Laboratories, Fort Dodge, IA), and at 3 hr a mixture of 1% halothane, 20–30% NO2, and oxygen was delivered via an endotracheal tube to maintain general anesthesia. When stable surgical anesthesia was achieved, the fetus was delivered viahysterotomy and exsanguinated. Amniotic fluid and maternal blood (via uterine vein) were also collected. The mother was allowed to recover and returned to the colony. Plasma, amniotic fluid, and fetal tissues were frozen at −70°C until analysis by HPLC.
Sample Analysis.
Plasma and amniotic fluid were analyzed for AZT and AZTG by solid-phase extraction, followed by reversed-phase HPLC according to a modification of the method of Qian et al. (17). AZddU (750 ng) and water (200 μl) were added to 100 μl plasma and the mixture applied to a BondElut column (1 ml column/50 mg solid phase; Varian, Harbor City, CA). The column was rinsed with 1 ml of 0.01 M phosphate buffer (pH 2.0) and the compounds eluted with 300 μl methanol. Methanol was evaporated to dryness and the compounds reconstituted in 200 μl of mobile phase. An aliquot of the samples were injected onto a Hypersil ODS column (5 μm, 150 × 4.6 mm; Alltech Associates, Inc., Deerfield, IL) using a Waters 717plus Autosampler (Waters Corp., Milford, MA). Elution of the compounds was achieved by using a mobile phase that consisted of a mixture of acetonitrile:water (7:93) adjusted to pH 2.5 with phosphoric acid and a flow rate of 2 ml/min. Compounds were analyzed and quantitated at 267 nm using a Waters 486 Tunable Absorbance Detector linked to a computer workstation with Millennium 2010 software (Waters Corp.). The approximate retention times for the compounds quantitated were 5.3 min for AZddU, 8.2 min for AZTG, and 10 min for AZT.
Tissues (250–500 mg) were digested in 1 ml of 1 N NaOH at 45°C in a shaking water bath. When all of the tissue was dissolved, the solution was neutralized with 1 ml of 1 N HCl and added to 10 ml UltimaGold (Packard Instrument Co., Meriden, CT) scintillation fluid for estimation of radioactivity by LSS on a Tracor Analytic Mark III 6881 liquid scintillation system (Tracor Analytical, Atlanta, GA). Similarly, aliquots of plasma (100 μl) were also added to 10 ml scintillation fluid for estimation of radioactivity by LSS.
Quantitation of phosphorylated metabolites in tissue was accomplished using SAX HPLC (14). Briefly, fetal tissue samples were prepared by adding tissue to a mixture of dichloromethane, methanol, and buffer (30 mM NaHPO3; pH 7.0). After homogenization, additional dichloromethane and buffer were added to each tube. Samples were centrifuged, and the supernatant was injected onto a Partisil-10 SAX column (250 × 4.6 mm; Whatman, Clifton, NJ). Resolution of the nucleotides was achieved using a linear gradient from 5 mM NH4H2PO4 to 750 mM NH4H2PO4 (10% methanol), with a flow rate of 2 ml/min and an absorbance of 267 nm (Waters 486 Tunable Absorbance Detector). Fractions were collected at 1-min intervals by an ISCO model 328 fraction collector (ISCO, Omaha, NE) and quantitated using LSS. The limit of detection for AZT-TP in tissue was 0.4 nmol/g (0.4 μM).
Pharmacokinetic Analysis.
Noncompartmental pharmacokinetic analysis of the plasma data from the initial kinetic study was performed using AUC-RPP software (18).
Results
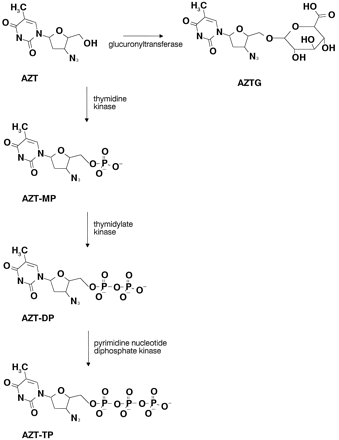
The metabolic pathway of AZT is diagrammed in fig.1. AZT is converted to its major metabolite, AZTG, by glucuronyltransferase, and converted to the phosphorylated metabolites by various kinases.
Structure of AZT and its major metabolites.
The maternal plasma concentration-time profiles of AZT and AZTG after an intravenous bolus dose of 8 mg/kg AZT are shown in fig.2. Plasma concentrations of AZTG peaked 15–30 min (16.39 μg/ml) after the administration of AZT. AZT and AZTG plasma concentrations decayed in parallel for all four pregnant macaques. Pharmacokinetic parameters for AZT (CL,Vdss , and t1/2) are listed in table 1.
Plasma concentration-time profiles for AZT and AZTG decay in parallel in pregnant rhesus macaques after an intravenous bolus dose of 8 mg/kg AZT.
Venous blood samples were collected beginning 2 min after the dose for up to 3.5 hr (see table 1). Values are expressed as μg AZT or AZTG/ml plasma (means ± SD) for four animals.
AZT pharmacokinetic parameters in the late-term maternal rhesus monkey
Using data from table 1, an infusion rate and loading dose were calculated for each animal (table 2). AZT and AZTG steady-state concentration-time profiles for two pregnant rhesus macaques (animals R4912 and R21) and their fetuses are shown in fig.3. In all four pregnant macaques, plasma AZT concentrations remained relatively constant from ∼30 min after the start of the infusion until the hysterotomy, indicating that steady-state had been reached. A slight rise in AZT and AZTG concentrations was noted in all four animals after initiation of anesthesia. Infusion pharmacokinetic parameters for both AZT and AZTG have been summarized in tables 2 and 3, respectively. The range of maternal Cpss for AZT was 1.3–2.15 μg/ml (table 2). Fetal-to-maternal plasma ratios (mean ± SD) at steady-state were 0.845 ± 0.152 for AZT and 0.628 ± 0.107 for AZTG. Fetal plasma-to-amniotic fluid ratios (mean ± SD) at steady-state were 1.187 ± 0.192 for AZT and 1.02 ± 0.424 for AZTG.
AZT infusion pharmacokinetic parameters
Plasma AZT and AZTG concentration-time profiles in two representative pregnant rhesus macaques before and during the hysterotomy.
Concentrations of AZT and AZTG in both the fetal plasma and amniotic fluid at the time of the hysterotomy are also included. Ketamine was administered at ∼2.5 hr, and gaseous anesthesia began at ∼3 hr. Values are expressed as μg AZT or AZTG/ml plasma.
AZTG concentrations in plasma and amniotic fluid
Radioactivity recovered from the fetal tissues, plasma, and amniotic fluid after administration of ∼200 μCi [3H]AZT to the four pregnant rhesus macaques is shown as AZT equivalents in fig.4. Radioactivity was highest in the kidney, followed closely by fetal plasma and amniotic fluid. Peripheral organs all contained similar amounts of radioactivity, with radioactivity the lowest, but relatively homogeneous, throughout all brain regions.
Total radioactivity recovered from fetal tissues, plasma, and amniotic fluid after administration of ∼200 μCi [3H]AZT to pregnant rhesus macaques.
Values are expressed as AZT equivalents ng/g or ng/ml (means ± SD; n = 4). AF, amniotic fluid; Sm Int, small intestine; Sk Musc, skeletal muscle;CB, cerebellum; Thal, thalamus; CN, caudate nucleus; Hypo, hypothalamus; BS, brainstem; Hip, hippocampus; PaPCG, parietal cortex plus precentral gyrus; PrCPG, premotor cortex plus postcentral gyrus; FC, frontal cortex; ROB, rest of brain; OC, occipital cortex.
Radioactivity associated with AZT and its metabolites is shown in a representative SAX HPLC chromatogram (fig. 5). Using the specific activity of the infusate and the radioactivity obtained from the fractions of the chromatographic runs, the concentrations of AZT and its metabolites in fetal tissue, plasma, and amniotic fluid were calculated (fig. 6). The majority of the radioactivity in the kidney (62%) and plasma (54%) was due to the glucuronidated metabolite AZTG, and yielded estimates of 14.34 nmol/g and 6.78 nmol/ml, respectively. In all of the other tissues, the majority of radioactivity (48–100%) was associated with the parent compound (yielding estimates 2.35–7.53 nmol/g). AZT-MP was the only phosphorylated metabolite detected in any of the tissues examined (0.613–5.913 nmol/g) and was the major metabolite (88% of total metabolites) in the spleen (5.913 nmol/g). No significant radioactivity eluted near AZT-DP or AZT-TP (fig. 5). The plasma did not contain any measurable amounts of AZT-MP. Brain regions examined contained only parent compound, except for the cerebellum, which consistently contained a small amount (0.613 nmol/g) of AZT-MP and one frontal cortex sample that also contained AZT-MP (0.3 nmol/g).
Representative SAX HPLC chromatogram of fetal kidney (top) and spleen (bottom) showing radioactivity associated with AZT and its metabolites.
Arrows indicate approximate retention times for the compounds of interest. AZT-MP was the only phosphorylated metabolite detected. Values are expressed as dpm. AZT-DP and AZT-TP indicate where the diphosphorylated and triphosphorylated metabolites, respectively, would elute if they were present.
Concentration of AZT (top), its glucuronidated metabolite—AZTG (middle), and AZT-MP (bottom) in fetal tissues, fetal plasma, and amniotic fluid after maternal constant-rate infusion of [3H]AZT.
Values are expressed as either nmol/g tissue, nmol/ml plasma, or nmol/ml amniotic fluid (mean ± SD; N = 4) for all tissues except the small intestine, frontal cortex, and thalamus, whereN = 3. Sm intest, small intestine;CB, cerebellum; FC, frontal cortex;PaPCG, parietal cortex plus precentral gyrus;Thal, thalamus.
Discussion
Pharmacokinetic parameters determined from either the single dose intravenous administration or the steady-state infusion of AZT were similar to data for humans and other nonhuman primates reported by others. The values for clearance (21 ml/min/kg) and volume of distribution (0.8 liters/kg) in the present study compare favorably with those for the pregnant pigtailed macaque (Macaca nemestrina) (CL: 23 ml/min/kg;Vdss : 0.8 liters/kg) (19) and in pregnant women (CL: 26 ml/min/kg; Vdss : 1.2 liters/kg) (20). The current recommended intrapartum AZT regimen for HIV-positive pregnant women is a 2 mg/kg/hr (33 μg/min/kg) loading dose during the first hour of labor, followed by 1 mg/kg/hr (17 μg/min/kg) for the duration of labor and delivery (6). In the present study, steady-state infusions of 30–50 μg/min/kg in pregnant rhesus monkeys resulted in maternal steady-state concentrations approaching 1.7 μg/ml. Plasma concentrations achieved herein are, therefore, at or slightly above those predicted by the pharmacokinetics and dosing for pregnant women during labor and delivery (1–1.5 μg/ml).
Very few studies to date have examined fetal-to-maternal plasma concentration ratios for AZT in humans at steady-state. A single case study in which AZT was administered via continuous intravenous infusion (0.12 mg/kg/hr) for 24 hr before delivery reported a cord blood-to-maternal blood ratio of 1.68 (21). Clinical fetal-to-maternal blood ratios of 0.76–2.50 have, however, been reported after single intravenous doses of AZT with single time point collections at delivery (20). In the present study, fetal-to-maternal AZT plasma concentration ratio averaged 0.85, which is nearly identical to the ratio reported for the pigtailed macaque at steady-state (mean of 0.83) (22). It has been suggested that AZT does not accumulate in the fetus and that placental transfer of AZT occurs rapidly by passive diffusion (22, 23). The fetal plasma-to-amniotic fluid concentration ratio of AZT was near unity. This ratio was expected because AZT concentrations in the amniotic fluid and fetal plasma should achieve equilibrium relatively quickly because the fetus swallows amniotic fluid; AZT is readily absorbed through the gastrointestinal tract (24), after which it is excreted in the urine.
It should be noted that an increase in the maternal concentration of AZT and AZTG was observed toward the end of the infusion. This was likely due to the induction of the anesthesia. A nearly 2-fold increase in the maternal levels of AZT and AZTG was observed in the pigtailed macaque after continuous AZT infusion, within the first hour after the initiation of anesthesia (22). Halothane and other volatile anesthetics have been shown to inhibit metabolism of drugs (25, 26), which may have affected the clearance of AZT and AZTG. In the present study, the hysterotomy was typically performed in <1 hr after the initiation of anesthesia, so the increase in maternal plasma AZT and AZTG levels was minimal. Also, the magnitude of the effect of the anesthesia may be smaller in the rhesus than in the pigtailed macaque.
Similar to studies that examined AZT pharmacokinetics during human pregnancy (27-29), AZTG was also found to be the major metabolite of AZT in the rhesus monkey. A primary difference between the findings in this study and those from a study in pigtailed macaques (19) was the relative amount of AZT glucuronidation. At steady-state, the AZTG-Cpss:AZT-Cpss ratio was always greater than 1.0 in both rhesus mothers (2.8) and fetuses (2.1), whereas these values were much lower in pigtailed macaques (maternal and fetal = 0.8). In the human literature (27-29), as well as in a baboon study (30), plasma AZTG concentrations are consistently higher than AZT concentrations. The AZTG:AZT plasma ratios range from ∼2.5 to 6.5 in maternal blood and from 1.8 to 6.5 in fetal blood, for both humans (27-29) and the baboon (30). These differences in the glucuronidation of AZT could be due to species differences, and suggest that the metabolism of AZT in the pregnant rhesus monkey more closely resembles that seen in humans than it does that of the pigtailed macaque.
The degree to which AZT and its metabolites accumulate in fetal tissues may be important in the evaluation of the risk-to-benefit ratio of AZT treatment during pregnancy. Although other studies have examined plasma levels of AZT and AZTG, this is the first study to examine in detail the fetal tissue distribution of AZT and its metabolites. AZT and/or its metabolites were found in every fetal tissue examined, including plasma and amniotic fluid. The greatest amount of AZT-derived compound was found in the fetal kidney, where most was in the form of AZTG (the AZTG:AZT ratio was nearly 2 to 1). Because AZTG is rapidly excreted in the urine (31), this was not an unexpected finding. AZT and AZTG levels in the fetal liver were 6.65 and 3.29 nmol/g, respectively. These levels were significantly greater than those reported in a human case study, wherein AZT and AZTG fetal liver levels of 0.14 and 0.16 nmol/g, respectively, were noted (29). However, lower levels would be expected, because these human fetal tissue levels were determined ∼4 hr after a 100 mg oral dose to the mother who had been chronically treated with AZT for 6 weeks (100 mg, 4 times/day).
The AZTG found in the fetus is likely due to fetal production, as well as maternal transfer. Although the placenta has not been previously shown to constitute a barrier to the movement of AZTG from the mother to the fetus or vice versa, generally, glucuronidated compounds exhibit limited placental transfer compared with their nonconjugates. In vitro perfusion studies using human placenta have demonstrated that AZTG does transfer to the fetal compartment with a much slower rate than AZT (23).
AZT has been shown to improve neurodevelopmental outcomes in HIV-infected children with encephalopathy (12). HIV has also been isolated from the CNS of infected adults (32). This suggests that examining the distribution of AZT within the CNS may prove important in predicting the efficacy of this compound in preventing neuropsychological deterioration. Brain levels of AZT ranged from 2.35 to 3.24 nmol/g in the present study, and these are significantly higher than the AZT level reported in a human case study, wherein a fetal CNS level of 0.01 nmol/g was observed (29). The fetal brain-to-plasma ratio of radioactivity derived from labeled AZT was 0.20–0.28, depending on the brain region examined. The CSF-to-plasma ratio at steady-state was 0.24 in children after a continuous intravenous administration of AZT (12). In the present study, the cerebellum-to-plasma ratio of AZT was 0.56, a value not much different than the CSF-to-plasma ratio observed in children.
It is important to determine the distribution of the phosphorylated metabolites of AZT to predict both efficacy and toxicity. In a recent study examining AZT metabolism in various cultured human T-lymphoblastoid CEM cells, it was suggested that AZT-TP concentrations correlated with anti-HIV activity, but that the monophosphate levels correlated with growth inhibitor effects (33). Therefore, it is possible that the presence of AZT-MP, even in the absence of significant triphosphate, may put the developing conceptus at risk. AZT-TP has been shown to inhibit HIV reverse transcriptase with aKi of 0.1 μM (10). In the present study, only AZT-MP was detected in the fetal tissues. Because AZT-MP was not found in the fetal plasma (fig. 6), we must assume that it was produced in the fetal tissues. This finding is similar to what was observed in rhesus fetal tissue after a single maternal intravenous dose of ddC (14). AZT-MP was the predominant compound found in peripheral tissues, especially the spleen; however, it was detected consistently in only one brain region: the cerebellum. In human placental trophoblasts and Hofbauer cells, it has been found that AZT-MP is the primary phosphorylated metabolite of AZT (17). The Qian et al. (17) study, as well as cell culture data from our laboratory (unpublished observations) and that of others (33), indicate that the AZT-TP metabolite constitutes <5% of the total intracellular phosphorylated AZT pool. This would indicate that, in the present study, a much greater amount of AZT-MP would need to be present to generate enough AZT-TP to allow detection. Because in the placenta and fetal spleen AZT-MP levels ranged from 1 to 10 nmol/g (μM), AZT-TP should be present at or above the Ki for HIV reverse transcriptase (0.1 μM) in these tissues, if it constituted 5% of the AZT-MP. However, the specific activity of the radiolabel used in the present study would not permit this low concentration of AZT-TP to be detected.
Because significant toxicity has occurred in adults during the course of AZT treatment (13, 34), the possibility for long-term toxicity in children exposed prenatally to AZT is of concern. There are indications from other animal studies that AZT exposure before or early in pregnancy could negatively impact pregnancy outcome. In mice, AZT exposure during gestation was associated with a reduction in viable offspring (35, 36). Pigtailed macaques, prenatally exposed to AZT, exhibited a lower postnatal weight gain and slower acquisition of a Black-White Learning discrimination task (37), and rhesus macaques treated with AZT during the second and third trimesters had increased numbers of abortions, fetal/neonatal deaths, and premature deliveries (38). These could result from both the direct and indirect effects of AZT during development. Infants in the AIDS Clinical Trials Group (protocol 076) study tolerated acute AZT treatment rather well (6), with a mild, transient anemia being the most frequent adverse effect. No AZT exposures occurred, however, before gestational week 14.
In summary, the intravenous kinetics of AZT in Macaca mulatta are similar to those reported for humans and other nonhuman primates. AZT, when administered intravenously was rapidly converted to its major metabolite, the glucuronide AZTG. When infused intravenously over a 3-hr period, AZT readily crossed the placenta and was found in fetal plasma, and in all fetal tissues examined as either parent compound, the glucuronide, or monophosphate metabolite. The AZT fetal-to-maternal plasma concentration ratio of 0.85 suggests that AZT does not accumulate in the fetus. In placenta and spleen, AZT-MP concentrations equaled or exceeded AZTG concentrations. Although fetal plasma AZT concentrations obtained in the present study were similar to peak plasma AZT concentrations observed clinically, the putative active antiviral metabolite, AZT-TP, was not detected in any monkey fetal tissue. However, based on the amount of the monophosphorylated metabolite observed in some tissues, clinically relevant concentrations of the triphosphate metabolite may still have been present.
Acknowledgments
We thank John R. Johnson for his technical assistance during surgery. We also thank Mrs. Betty White and the primate colony staff at the NCTR for their excellent care and handling of the monkeys, and for their assistance during the study.
Footnotes
-
Send reprint requests to: Dr. Tucker A. Patterson, Division of Neurotoxicology, HFT-132, National Center for Toxicological Research/Food and Drug Administration, 3900 NCTR Road, Jefferson, AR 72079-9502.
-
T. A. Patterson and J. A. Sandberg were supported in part by appointment to the Postgraduate Research Program in the Division of Neurotoxicology at the NCTR administered by Oak Ridge Institute for Science Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration. This work was supported in part by an interagency agreement between the NCTR and the National Institute for Environmental Health Sciences (IAG Y01-ES-10187).
- Abbreviations used are::
- HIV
- human immunodeficiency virus
- AIDS
- acquired immunodeficiency syndrome
- AZT
- 3′-azido-3′-deoxythymidine
- AZT-TP
- 3′-azido-3′-deoxythymidine 5′-triphosphate (tetratriethylammonium salt)
- ddC
- 2′,3′-dideoxycytidine
- NCTR
- National Center for Toxicological Research
- AZTG
- 3′-azido-3′-deoxythymidine-β-d-glucuronide
- AZddU
- 3′-azido-2′,3′-dideoxyuridine
- AZT-MP
- 3′-azido-3′-deoxythymidine 5′-monophosphate (diammonium salt)
- AZT-DP
- 3′-azido-3′-deoxythymidine 5′-diphosphate triethylammonium salt
- [3H]AZT
- [methyl-3H]3′-azido-3′-deoxythymidine
- LSS
- liquid scintillation spectroscopy
- SAX
- strong anion exchange
- CL
- total plasma clearance
- Vdss
- volume of distribution at steady-state
- t1/2
- terminal half-life
- Cpss
- steady-state plasma concentration
- CNS
- central nervous system
- CSF
- cerebrospinal fluid
- Received August 26, 1996.
- Accepted January 13, 1997.
- The American Society for Pharmacology and Experimental Therapeutics