1996 ASPET N-Glucuronidation of Xenobiotics Symposium
Abstract
Glucuronidation of either an aliphatic or aromatic tertiary amine group in a molecule results in a quaternary ammonium–linked glucuronide metabolite (i.e. N+-glucuronide). The development of sound information onN+-glucuronide metabolites, including their characterization, has been slow. In part, this is because the presence of both the carboxylic acid group and cationic center in their structure imparts physiochemical properties such that procedures used in their analysis, including extraction, require judicious selection. The techniques used in the identification ofN+-glucuronide metabolites and those metabolites identified in human urine are the focus of this review. Especially useful in their identification are the availability of an authentic synthetic sample and the use of mass spectrometry and nuclear magnetic resonance (NMR) techniques that, in the first instance, involve atmospheric pressure ionization or fast atom bombardment modes of ionization and high-resolution 1H NMR. More than 30N+-glucuronide metabolites of xenobiotics have been identified in human urine. In particular,N+-glucuronidation is a common phenomenon in the metabolism of H1 antihistamine and antidepressant drugs with an aliphatic tertiary amine group. Those marketed drugs in which the reported N+-glucuronide mean urinary excretion of the orally administered dose exceeds 10% include cyclizine, cyclobenzaprine, cyproheptadine, dothiepin, doxepin, ketotifen, lamotrigine, mianserin, and tioconazole. The pharmacological importance of N+-glucuronidation has not been clarified.
The phenomenon of glucuronidation occurs with a vast number of substrates at a diverse array of functional groups. The resultant glucuronide metabolites can have linkage of glucuronic acid through O, S, N, or C atoms. Far more research has been conducted with respect to O-glucuronides than the others. In the case of N-glucuronides, their formation occurs at various functional groups that include alkylamines, arylamines, hydroxylamines, carbamates, ureas, thioureas, and sulfonamides (Dutton, 1980; Caldwell, 1985).
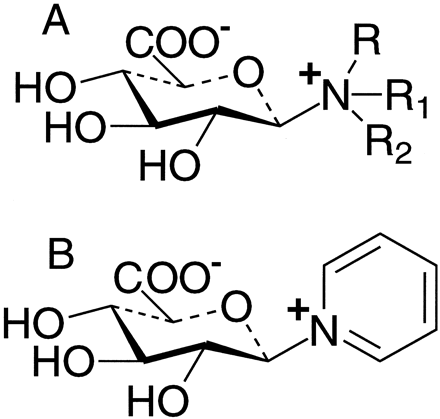
Glucuronidation of alkylamines and arylamines can occur at a primary, secondary, or tertiary amine group. In the case of tertiary amines,N-glucuronidation is a relatively recently discovered phenomenon in that the first reports appeared in the mid 1970s (Porteret al., 1975; Chaudhuri et al., 1976), and up to the late 1980s, only about ten substrates were known. The resultant products are referred to as quaternary ammonium–linked glucuronide metabolites, or N+-glucuronides. There are two types of N+-glucuronide metabolites in that glucuronidation can occur at a saturated or unsaturated nitrogen atom. In the case of the latter, all documented examples occur at an aromatic tertiary amine group. Therefore, the two types of metabolites can be described as aliphatic N+-glucuronides (fig.1A) and aromaticN+-glucuronides (fig. 1B). The purpose of this article is to review the known occurrences ofN+-glucuronidation in humans and the techniques used in metabolite identification. The techniques that have been utilized in the analysis of the known aliphatic and aromatic types of N+-glucuronide metabolites of xenobiotics are summarized in tables1 and 2, respectively.
General chemical structures ofN+-glucuronide metabolites.
A, Aliphatic N+-glucuronide, where R, R1, and R2 are alkyl groups; B,aromatic N+-glucuronide, where specifically pyridine N+-glucuronide is shown.
Aliphatic-type quaternary ammonium–linked glucuronide metabolites of drugs identified in human urine
Aromatic-type quaternary ammonium–linked glucuronide metabolites of drugs identified in human urine
Characterization of N+-Glucuronide Metabolites
Indirect Analysis.
The difficulties encountered in the analysis of glucuronide metabolites because of their high polarity and low volatility are well recognized. In the case of N+-glucuronide metabolites, these difficulties may be compounded in that they contain not only an ionizable carboxylic acid group but also a permanent positive charge. Under many conditions, including in biological milieu, they occur as the inner salts (fig. 1).
One approach to avoid difficulties such as the extraction of polar glucuronide metabolites from aqueous matrices is to conduct indirect analysis, i.e. analysis of the aglycone subsequent to differential enzymatic or chemical hydrolysis of the glucuronide metabolite.
The various types of N-glucuronide metabolites differ markedly in their susceptibility to chemical hydrolysis. In general, the N-glucuronides of primary and secondary amines are very unstable in solution below pH 7 (Dutton, 1980). In contrast,N+-glucuronides resemble ether glucuronides in that they are relatively stable at neutral pH and hydrolyze in basic but not acidic media. Protonation is essential for acidic hydrolysis and ether glucuronides are not readily protonated, while there is no site for protonation of the N-C linkage ofN+-glucuronides. The regeneration of the aglycone by treatment with hot alkali has been used infrequently in the qualitative and quantitative analysis ofN+-glucuronide metabolites (Chaudhuri et al., 1976; Dahl-Puustinen and Bertilsson, 1987; Dahl-Puustinenet al., 1989).
Few N+-glucuronide metabolites have been thoroughly studied with respect to stability and hydrolytic conditions. In our laboratories, doxepin N+-glucuronide and clozapine N+-glucuronide were stored for 3 months at room temperature (18°C–22°C) in aqueous buffers at each nominal pH value over the range of 1–11 (I. Kowalczyk et al., unpublished observations, 1996). DoxepinN+-glucuronide was stable over the pH range of 1–10 for 3 months, whereas significant degradation occurred at pH 11 after 1 month of storage (half-life time, 142 days). In contrast, the results for clozapine N+-glucuronide did not fit the general pattern of greater stability at acidic pH than alkaline pH. There was no significant change in the concentration of clozapineN+-glucuronide over the pH range of 4–11; however, significant degradation occurred at pH 1, pH 2, and pH 3 after 3 weeks of storage (half-life times 70, 51, and 86 days, respectively). Two N+-glucuronide metabolites have been reported to be very unstable in alkaline solution. LamotrigineN+-glucuronide underwent complete degradation after 5 hr in pH 11 buffer at room temperature (Sinz and Remmel, 1991b), and about 40% degradation of cryproheptadineN+-glucuronide occurred after 24 hr in pH 8.5 buffer at room temperature (Dulik and Fenselau, 1987). Studies are lacking with respect to the stability ofN+-glucuronide metabolites in biological media. Also, other than the aglycone, the degradation products ofN+-glucuronide metabolites have not been identified. In the case of lamotrigineN+-glucuronide, the degradation products were resistant to hydrolysis, which is similar in behavior to the rearrangement products of carboxylic acid glucuronides that are formed readily under basic conditions (Sinz and Remmel, 1991b).
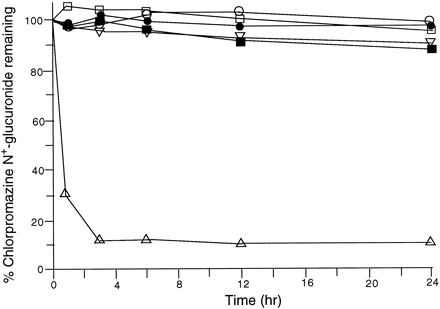
In contrast to chemical hydrolysis, enzymatic hydrolysis has been utilized frequently in the indirect analysis ofN+-glucuronide metabolites, both qualitative and quantitative (most cases cited in tables 1 and 2). β-Glucuronidase preparations from all three usual sources of the enzyme—namely, bacteria, liver, and mollusks—have been used in these enzymatic hydrolysis. In only a few instances is there indication that the hydrolytic conditions were optimized. For example, in the case of cyproheptadine N+-glucuronide, using Helix pomatia as the enzyme source, the optimal incubation conditions in aqueous buffer at 37.5°C were reported to be pH 6.5 for 16 hr (Beckett and Ali, 1978). In these laboratories, the enzymatic conditions for hydrolysis were studied for four aliphatic-typeN+-glucuronide metabolites in which quaternization had occurred in two cases at an acyclic tertiary amine (chlorpromazine, doxepin) and in the other two cases at a cyclic tertiary amine (clozapine, cyclizine) (I. Kowalczyk et al.,unpublished observations, 1996). Enzymatic preparations from bacteria (Escherichia coli), bovine liver, and mollusks (H. pomatia) at a concentration of ≈2 units per μg of substrate were studied in aqueous buffers of pH 5 and pH 7.4 at 37°C over 48 hr. Three of the four N+-glucuronide metabolites were not affected under these conditions by β-glucuronidase preparations from bovine liver and H. pomatia (fig.2 shows data obtained for chlorpromazineN+-glucuronide). Only clozapineN+-glucuronide was readily hydrolyzed by all three preparations of β-glucuronidase examined. Subsequent investigations of E. coli as an enzyme source showed that the optimal pH for hydrolysis of all fourN+-glucuronides examined was in the pH range of 6.5–8.0. These observations are surprising in view of the conditions most commonly reported in the hydrolysis of both aliphatic (Kennedyet al., 1977; Hucker et al., 1978b;Dahl-Puustinen and Bertilsson, 1987; Nishikata et al., 1992,1993) and aromatic (Janssen et al., 1982; Beider et al., 1983; McKillop et al., 1990; Rush et al., 1990, 1992; Sinz and Remmel, 1991b; Byrd et al.,1992; Caldwell et al., 1992) typeN+-glucuronide metabolites—namely, either bovine liver or H. pomatia as an enzyme source and a buffer in the pH range of 4.7–5.2 (usually for 16–24 hr at 37°C). Such type of conditions are commonly utilized in the hydrolysis ofO-glucuronides and, in fact, phenolphthaleinO-glucuronide was readily hydrolyzed by both bovine liver and H. pomatia preparations, especially at pH 5, when examined as positive controls in the studies reported above from these laboratories. (I. Kowalczyk et al., unpublished observations, 1996). Therefore, the optimal conditions for enzymatic hydrolysis vary drastically between different types of glucuronides such as O-ether andN+-quaternary and preferably should be determined for each individual substrate.
Enzymatic hydrolysis of chlorpromazineN+-glucuronide.
Chlorpromazine N+-glucuronide (18 μg/ml) was incubated over 48 hr at 37°C in various buffers with various enzyme preparations (40 U/ml) as follows: bovine liver, pH 5 (□) and pH 7.4 (■); Escherichia coli, pH 5 (▽) and pH 7.4 (▵);Helix pomatia, pH 5 (○) and pH 7.4 (•). CV data not shown: except in the case of E. coli at pH 7.4, these values did not exceed 3%. Only small changes in concentration occurred in the 24–48 hr period. Significant hydrolysis only occurred in the case ofE. coli at pH 7.4 (p < 0.05).
There are indications in the literature that the hydrolysis ofN+-glucuronides does not occur as readily as that of some other types of glucuronides. For example, comparison of the N+-glucuronide and acyl glucuronide of the same aglycone (WY-18, 2511) indicated that under the enzymatic conditions examined, five times more aglycone was produced from the latter than the former conjugate (Janssen et al., 1982). That a relatively long incubation time and high concentration of β-glucuronidase are required to complete the enzymatic hydrolysis of both aliphatic (Beckett and Ali, 1978) and aromatic (Janssen et al., 1982; Sinz and Remmel, 1991b; Rush et al., 1992)N+-glucuronide metabolites has been noted. Also, although the enzymatic conditions utilized in some studies have been reported to lead to complete deconjugation (Breyer-Pfaff et al., 1990; McKillop et al., 1990), in other cases, incomplete deconjugation was noted (Chaudhuri et al., 1976;Dahl-Puustinen and Bertilsson, 1987). Thus hot alkaline treatment of amitriptyline (Dahl-Puustinen and Bertilsson, 1987) and tripelennamine (Chaudhari et al., 1976)N+-glucuronide metabolites led to 2–2.5–fold greater amounts of the respective aglycone than did enzymatic treatment of the same conjugates. Finally, the enzymatic hydrolysis ofN+-glucuronide metabolites is inhibited by the β-glucuronidase inhibitor d-saccharic acid 1,4-lactone (e.g.Kennedy et al., 1977; Hucker et al., 1978a; Rubin et al., 1979; Fischer et al., 1980; Janssen et al., 1982; Sinz and Remmel, 1991b).
Reference Standards.
The unequivocal identification of a metabolite is aided by the availability of an authentic synthetic sample. Unfortunately, there are no known commercial sources of these metabolites. The synthetic approach can be either biochemical or chemical, and both approaches have been used for N+-glucuronide metabolites. The biosynthetic methods include isolation of the metabolite from excreta or the use of enzyme systems. The former is especially useful when a limited quantity of metabolite is needed as a standard for quantitative analysis, as in the cases of amitriptyline (Dahl-Puustinen and Bertilsson, 1987; Breyer-Pfaff et al., 1990) and lamotrigine N+-glucuronides (Remmel and Sinz, 1991). Various approaches to in vitro enzymatic syntheses ofN+-glucuronides can be used, including use of liver homogenates or UGT1 covalently immobilized on an inert matrix, such as agarose beads (Parikh et al., 1976; Lehman et al., 1981; Lehman and Fenselau, 1982; Dulik and Fenselau, 1987). The advantages of the use of such immobilized systems over microsomal systems include the ease of protein removal from the reaction mixture by simple filtration and the potential for greater yield because of increased stability (Parikhet al., 1976). The immobilized enzyme technique has been utilized to synthesize various aliphatic-typeN+-glucuronides of various drugs that include chlorpromazine, cyproheptadine, and tripelennamine (Lehman and Fenselau, 1982; Lehman et al., 1983; Dulik and Fenselau, 1987; Chaudhary et al., 1988). The aromatic-typeN+-glucuronide of lamotrigine has been prepared by use of microsomes, either with or without immobilization into alginate beads (Magdalou et al., 1992).
The organic synthesis of N-glucuronide metabolites generally has posed difficulties, although various secondary-amineN-glucuronides have been obtained by treatment of the primary amine substrate with d-glucuronic acid in a polar solvent (Kaspersen and Van Boeckel, 1987; Babu et al.,1992). The first report of the organic synthesis of aN+-glucuronide metabolite involved the synthesis of pyridine N+-glucuronide by stirring pyridine and methyl (2,3,4-tri-O-acetyl-α-d-glucopyranosyl bromide) uronate at room temperature, followed by removal of the protecting groups of the quaternary intermediate (Aboul-Enein, 1977;Dalgaard, 1983). Other aromatic-typeN+-glucuronide metabolites have been obtained by a similar procedure, including cotinineN+-glucuronide (Caldwell et al.,1992). Use of such a procedure in these laboratories resulted in low yield of quaternary product. It was hypothesized that traces of the quaternary salt, once formed, inhibited further reaction in the reaction mixture. Therefore, an approach was developed by which the use of a two-phase solvent system enabled removal of the quaternary salt as it was formed. This approach has been applied to synthesize a diverse array of aliphatic-type N+-glucuronide metabolites of drugs from various pharmacological classes that include antidepressants, antipsychotics, and H1-antihistamines (Luoet al., 1992b, 1995). The yields were variable, but aN+-glucuronide was obtained with virtually all drugs examined. The approach was applicable when certain other functional groups were present, as with alcohol, alkene, ether, sulfide, sulfone, sulfoxide, and aromatic tertiary amine. Only in the case of nicotine N+-glucuronide has the approach been used to synthesize an aromatic-typeN+-glucuronide metabolite (Seaton et al., 1993).
Isolation and Separation Techniques.
The polarity of N+-glucuronide metabolites makes their isolation and separation from biological samples difficult. Their extraction from samples has most commonly been achieved by solid-phase extraction chromatography. The materials used in columns and cartridges especially have included anion-exchange resin (e.g.DEAE-Sephadex) (Janssen et al., 1982; Lehman and Fenselau, 1982; Lehman et al., 1983; Chaudhary et al.,1988; Breyer-Pfaff et al., 1990; MacRae et al.,1990), non-ionic hydrophobic resin (invariably XAD-2) (Porter et al., 1975; Chaudhuri et al., 1976; Janssen et al., 1982; Chaudhary et al., 1988; McKillop et al., 1990; Luo et al., 1991a, 1991b, 1992b, 1995; Sinz and Remmel, 1991b; Rush et al., 1992), and C18-silica gel (Dahl-Puustinen and Bertilsson, 1987; Dahl-Puustinen et al.,1989; Breyer-Pfaff et al., 1990; Sinz and Remmel, 1991a;Delbressine et al., 1992). Thin-layer chromatography and semi-preparative reversed-phase HPLC1 have commonly been utilized in the subsequent purification and isolation ofN+-glucuronide metabolites. The stationary phases most commonly encountered in these regards are silica gel in thin-layer chromatography (Chaudhuri et al., 1976; Huckeret al., 1978a, 1978b; Janssen et al., 1982;Lehman and Fenselau, 1982; Lehman et al., 1983; Chaudharyet al., 1988) and C8 (Sinz and Remmel, 1991b), C18 (Dahl-Puustinen and Bertilsson, 1987; Dulik and Fenselau, 1987;Dahl-Puustinen et al., 1989; Breyer-Pfaff et al.,1990; Delbressine et al., 1992; Magdalou et al.,1992), or cyano (Luo et al., 1991a, 1991b, 1992b, 1995) material in HPLC. An ion-pairing agent can be used to aid separation of metabolites from endogenous compounds, and sodium dodecylsulfate has been utilized in the analysis of lamotrigineN+-glucuronide (Sinz and Remmel, 1991a).
Spectral Characteristics and Instrumental Techniques.
Both IR and UV spectroscopy have limited use in the identification ofN+-glucuronide metabolites. There is no sharp absorption band in an IR spectrum characteristic of the cationic center. However, the nature of the carboxylic acid group of the glucuronic acid portion of the molecule can be assigned by means of IR spectroscopy. For all N+-glucuronide metabolites isolated from urine and examined by IR spectroscopy (Porter et al., 1975; Chaudhuri et al., 1976; Beider et al., 1983; Takeuchi et al., 1989), bands characteristic of the carboxylate anion were present in the spectrum (i.e.1610-1550 and 1420-1300 cm−1; Williams and Fleming, 1987). Hence the zwitterionic nature of the molecule is indicated. Furthermore, acidification of the examined sample results in a shift of the band at higher frequency (∼1600 cm−1) to a higher wave number (∼1725 cm−1) (Porter et al.,1975; Takeuchi et al., 1989) that is indicative of an unionized aliphatic carboxylic acid group (Williams and Fleming, 1987).
In the case of UV spectroscopy, the absorption characteristics of an aliphatic N+-glucuronide metabolite resembles the aglycone (Breyer-Pfaff et al., 1990), whereas quaternization of an aromatic tertiary amine results in an azomethinium ion with consequent change in the UV chromophore (Beider et al., 1983; McKillop et al., 1990).
The high polarity, thermolability, and lack of volatility ofN+-glucuronide metabolites renders them inappropriate for MS analysis under conventional modes of ionization. Under both electron impact (EI) and chemical ionization (CI), they do not yield a molecular ion (Porter et al., 1975; Chaudhuriet al., 1976; Beider et al., 1983; McKillopet al., 1990; Rush et al., 1992). Furthermore, derivatization to volatile products amenable to CI and EI analysis only occurs if the cationic center of these metabolites is destroyed. However, the development in recent years of soft ionization techniques, especially fast atom bombardment (FAB) and atmospheric pressure ionization (API), including electrospray ionization (ESI), has greatly facilitated identification of N+-glucuronide metabolites. Indeed, the importance of MS to glucuronide metabolite analysis dramatically shifted with the introduction of FAB analysis in the early 1980s from a technique of minor importance to a technique with a key role (Lehman and Fenselau, 1982; Fenselau et al.,1983). With both API and FAB, the general case is that in the positive ion mode, a quasi-molecular ion of the analyte is formed by the addition of one proton. However, in the case ofN+-glucuronide metabolites, the corresponding ion represents the precharged molecular cation (M+) in which the carboxylic acid group of the glucuronic acid moiety is unionized. Such molecular cations have been reported for manyN+-glucuronide metabolites with the use of FAB (tables 1 and 2), whereas with API only a few examples are in the literature (Sinz and Remmel, 1991b; Caldwell et al., 1992;Luo et al., 1994). In these laboratories, we have analyzed many aliphatic-type N+-glucuronide metabolites by both modes of ionization (McKay et al., 1992; G. McKayet al., unpublished observations, 1995). The observed intensities of these ions vary widely and, in fact, in FAB analysis of MPTD (an investigational drug), the molecular cation could not be detected (McKillop et al., 1990).
Along with the molecular cation (M+), other diagnostic ions appear in the API and FAB spectra ofN+-glucuronide metabolites. It is important that these ions be recognized and their origin understood in order to avoid confusion when analyzing extracts from biological samples. The presence of alkali metals in the analytical sample provides diagnostic ions that for the molecular cation appear especially at (M-H + Na)+ and (M-H + K)+. The usual sources of these metal cations are the biological matrix and glassware routinely used in a typical analytical laboratory. It has been observed that when polypropylene-ware is used in place of glassware in preparing solutions of analytes, the intensity of the alkali metal adducts is greatly reduced (McKay et al., 1992). In contrast, alkali metal salts can be deliberately added to the analyte in order to facilitate identification of N+-glucuronide metabolites (Takeuchi et al., 1989). Other types of adducts can arise from interaction of the ions of theN+-glucuronide with components of the analytical sample used for functions such as mobile phase (e.g.methanol) and sample matrix (e.g. glycerol). Also, adduct ions involving more than one metabolite molecule [e.g.(2M + 1)+, (2M + Na)+] have been observed in both API and FAB spectra ofN+-glucuronide metabolites.
The MS fragmentation of N+-glucuronide metabolites under API and FAB modes of ionization has provided information useful in their characterization. However, it should be noted that because of the predominant use of these soft modes of ionization in providing information about the intact molecule or molecular components of complex molecules, and because of the relative infancy of these techniques, there is lack of information on the general fragmentation patterns they induce. Nevertheless, a key aid to the identification of N+-glucuronide metabolites is the ion in the API or FAB spectrum corresponding to the protonated aglycone that is formed from cleavage of the glycosidic bond [i.e. (M-176)+]. Moreover, a less-intense ion attributable to the loss of water from the molecular cation (M-18)+ is generally encountered in the API and FAB spectra of N+-glucuronide metabolites (Caldwell et al., 1992; McKay et al., 1992). Additionally, procedures to induce fragmentation can be utilized. For example, with API, an increase in the sampling cone voltage can give rise to interface-induced fragmentation. Thus at a cone voltage of 50 V but not 15 V, an intense peak at m/z 235 was observed in the ESI spectrum of doxepin N+-glucuronide that coincides in mass/charge ratio with the so-called Cope eliminated product [M-(C6H9O6-NH(CH3)2)]+(G. McKay et al., unpublished observations, 1995). The corresponding peak also appears in the FAB spectrum of doxepinN+-glucuronide (Luo et al., 1991b). Finally, by use of tandem MS, it is possible to delineate the precursor of a fragment (daughter) ion. Thus, by such an approach, it was proven in the case of lamotrigine N+-glucuronide that the ion corresponding to the protonated aglycone mainly arose from the molecular cation (Sinz and Remmel, 1991b).
Definitive identification of a metabolite cannot be made solely by MS analysis. In fact, API or FAB analysis will yield the molecular cation of the N+-glucuronide of the same mass as the pseudomolecular ion of the C- or O-glucuronide of the same aglycone. Certainly by direct comparison of the MS and chromatographic behaviors with that of a synthetic authentic standard, a metabolite can be identified definitively. This contrasts with NMR, with which definitive identification of a metabolite can be made without the availability of a reference standard. The downside is that a far greater amount of analyte is required for NMR than MS analysis.
In fact, 1H NMR has been widely utilized in the identification of N+-glucuronide metabolites isolated from biological samples (tables 1 and 2). In most cases, the use of high-resolution 1H NMR allows definitive assignment of the pertinent uncharacterized structural features of theN+-glucuronide metabolite; namely, the site ofN+-glucuronidation and the anomeric configuration of the glucuronide moiety. The chemical shift ranges for the anomeric proton differ between the two types ofN+-glucuronide, since the azomethine linkage of the aromatic type of N+-glucuronide has a deshielding effect. Thus the chemical shifts for the anomeric protons of the 21 aliphatic type and nine aromatic type ofN+-glucuronide metabolites reported in the literature fall in the distinctly separate ranges of δ 4.3–5.0 and 5.3–5.9, respectively (tables 1 and 2). In virtually all cases, the signal for the anomeric proton was observed as a doublet for which thevicinal coupling constant (J1′,2′) was in the ranges of 6.6–9.6 and 7.8–8.9 Hz for the aliphatic and aromatic typeN+-glucuronides, respectively. This is indicative for both types of N+-glucuronide of atrans diaxially oriented H-1′ and H-2′ of the glucuronic acid moiety. Also, since the coupling constant ranges for the α- and β-anomers of various types of glucuronides have been found to be in the ranges of 2–4 Hz and 7–10 Hz (Kaspersen and Van Boeckel, 1987), respectively, a β-anomer configuration is indicated forN+-glucuronides.
In general, the site of N+-glucuronidation can be established by use of high-resolution 1H NMR because the signals (δ values) for the protons vicinal to the site of quaternization are shifted downfield relative to those of the free base of the aglycone. By such observation, it was established thatN+-glucuronidation of nicotine occurred in the pyridine and not the pyrrolidine ring (Seaton et al., 1993). Another means of delineating the site ofN+-glucuronidation from the 1H NMR spectrum occurs when two identical (usually CH3 or CH2) and similarly substituted alkyl groups are attached to the glucuronidated nitrogen atom. The 1H NMR signal for such alkyl groups respectively appear as one and two singlets for the aglycone and conjugate. This is because for theN+-glucuronide, there are two possible orientations of the alkyl groups about the quaternary nitrogen atom and hence two conformations (Chaudhuri et al., 1976;Breyer-Pfaff et al., 1990; Luo et al., 1992b). For example, the dimethylaminoalkyl group, rather than the pyridine nitrogen of chlorpheniramine, doxylamine, and tripelennamine, was confirmed as the site of N+-glucuronidation by use of such a technique (Luo et al., 1991a, 1992b).
In some cases, the site of conjugation cannot be established definitively by 1H NMR and, therefore, more sophisticated NMR techniques are required. Hence techniques such as 13C NMR, nuclear Overhauser effect (NOE) experiments, and two-dimensional NMR have been used occasionally to establish the site ofN+-glucuronidation (Beider et al.,1983; Takeuchi et al., 1989; MacRae et al., 1990;Sinz and Remmel, 1991b; Caldwell et al., 1992). A case in point is that in heteroaromatic ring systems with two or more aza atoms and no hydrogen substituents, the site of glucuronidation cannot be differentiated by use of 1H NMR. For example, consider the case of lamotrigine, in which glucuronidation occurs in a 3,5,6-trisubstituted 1,2,4-triazine ring. The major site ofN+-glucuronidation was established at 2N by13C NMR in that an upfield shift (δ values) was observed at the C-3 position but not the C-5 and C-6 positions of the triazine ring (Sinz and Remmel, 1991b). It is well established that in13C NMR spectra, a positively charged nitrogen of a heterocyclic system has a shielding effect on the signal of an adjacent carbon atom. Regarding NOE experiments, consider cases where protons are vicinal to the site of glucuronidation. In essence, the NOE experiment involves irradiation of a proton signal such that a change in the intensity of another proton signal indicates that the two protons are close together in the molecule. Thus irradiation of the anomeric proton in cotinine (Caldwell et al., 1992) and tioconazole (MacRae et al., 1990)N+-glucuronides led to enhancements of the signals of the protons of the aromatic ring system bonded to the glucuronic acid. Finally, various two-dimensional techniques, such as two-dimensional J-correlated spectroscopy (COSY) and two-dimensional heteronuclear chemical shift correlation spectroscopy (HETCOR), were utilized to make 1H and 13C assignments of the aromatic N+-glucuronides of cotinine (Caldwellet al., 1992) and croconazole (Takeuchi et al.,1989).
Electrophoresis can be utilized as a means of identification ofN+-glucuronides, namely to demonstrate the zwitterionic character. High-voltage electrophoresis experiments have been conducted with tioconazole N+-glucuronide (MacRae et al., 1990). At pH 2, the metabolite migrated toward the cathode because of the net positive charge (N+, COOH). In contrast, at pH 10, the metabolite did not move because of the lack of net charge (N+, COO−). Other types of glucuronide (e.g. C-linked) of tioconazole would be expected to give a different result; namely, migration toward the cathode at low pH and toward the anode at high pH (MacRae et al., 1990).
N+-Glucuronides as Metabolites in Humans
Identified N+-Glucuronides.
N+-Glucuronide metabolites have been identified as urinary metabolites in human for more than 30 drugs. Of these drugs, those that produce the aliphatic type and the aromatic type ofN+-glucuronides number 23 and 9, respectively. Except for nafimidone (Rush et al., 1990) and oltipraz (Beider et al., 1983), theN+-glucuronide of the parent drug was identified. In the cases of both amitriptyline (Breyer-Pfaff et al., 1990) and nicotine (Byrd et al., 1992; Caldwellet al., 1992; Seaton et al., 1993), theN+-glucuronides of both parent compound and another metabolite(s) were identified. Amitriptyline N-oxide is metabolized to the same quaternary glucuronides as amitriptyline (Becher et al., 1992).
The common appearance of the aliphatic tertiary amine group in drugs of certain pharmacological classes prompted study of whether formation of a N+-glucuronide metabolite was a common feature of these drug classes. Such a metabolite was identified in the urine of patients and/or healthy volunteers administered all four antidepressants (Luo et al., 1995), eight of nine H1 antihistamines (Luo et al., 1991a), and two of ten antipsychotics (Luo et al., 1995) examined. These data, as well as other published reports (table 1), suggest that where an aliphatic tertiary amine is present in their structure,N+-glucuronidation is a general phenomenon of H1 antihistamine and tricyclic antidepressant drugs, but not antipsychotic drugs. Regarding the known aromatic-typeN+-glucuronides, unlike their aliphatic-type counterparts, the aglycones cannot be categorized into a few pharmacological classes. In fact, most of the known aromatic-type conjugates are metabolites of various classes of drugs under development. There are indications that antifungal agents with an imidazole ring commonly form N+-glucuronides in humans. The N+-glucuronides of tioconazole (MacRae et al., 1990) and croconazole (Takeuchi et al., 1989) have been identified in human and rabbit urine, respectively, while the possibility ofN+-glucuronidation of econazole in human has been discussed (Takeuchi et al., 1989; Midgley et al., 1981).
The aliphatic and aromatic tertiary amine groups known to be quaternized are attached to a diverse array of structural units. The quaternized aliphatic tertiary amine can be acyclic or cyclic, as in piperidine, hexahydro-1,4-diazepine, and piperazine ring systems. Also, the aromatic tertiary amine ring systems quaternized by glucuronic acid include imidazole (where one ring nitrogen atom is substituted), pyridine, 1,2,4-triazine, and polycyclic heterocycles.
In those molecules in which N+-glucuronidation could occur at more than one site, the observed site of quaternization can be rationalized in some cases. Where two aliphatic tertiary amines of similar basicity are present in a molecule as part of aminoalkylamine or piperazine structures,N+-glucuronidation has been observed to occur at the nitrogen atom with the less bulky substituent(s) (Luo et al., 1992b). For example, in cyclizine (1-diphenylmethyl-4-methylpiperazine), the observed quaternization occurred at the N-methyl group. However, in many cases, the observed site of quaternization is difficult to rationalize. For example, the N+-glucuronidation of drugs with both aliphatic and aromatic tertiary amine groups has been observed at the former site with some (chlorpheniramine, doxylamine, pheniramine, pyrilamine [Luo et al., 1991a, 1992b], tripelennamine [Chaudhuri et al., 1976; Luo et al., 1991a,1992b]) but at the latter site with others (LY-108380 [Rubin et al., 1979], nicotine [Seaton et al., 1993]). Also, there have been few studies of the N-glucuronidation of molecules that possess a primary or secondary amine group(s) as well as a tertiary amine group. However, recent metabolic studies have been performed with molecules with a five-membered nitrogen heterocyclic ring system with aza atoms as secondary or tertiary amines,i.e. imidazole, 1,2,3-triazole, 1,2,4-triazole, and tetrazole ring systems, with no substituents attached to the ring nitrogen atoms. In fact, only tertiary amine N-glucuronide and not N+-glucuronide metabolite(s) of these substrates were isolated from rhesus monkey urine and various human hepatic preparations (Stearns et al., 1991; Huskey et al., 1993, 1994a, 1994b; Colletti and Krieter, 1994; Perrieret al., 1994; Chiu and Huskey, 1998). Also, in the case of the antipsychotic drug olanzapine administered to healthy volunteers, the major site of N-glucuronidation was the secondary amine group of the tricyclic ring system, rather than an aliphatic tertiary amine group of the exocyclic piperazine ring system (Kassahun et al., 1998). In contrast, only the latter site ofN+-glucuronidation was observed with the structurally related drug clozapine (Luo et al., 1994,1995). In view of the work noted above with olanzapine and relevantin vitro studies (Green and Tephly, 1996), there is need to re-examine the N-glucuronidation of clozapine in humans.
In general, there is need to delineate the factors responsible for selective N-glucuronidation in a molecule with multiple amine groups. Furthermore, few substrates can be cited where the option of N- versus O-glucuronidation has been thoroughly explored. Certainly, in the case of morphine, it is well recognized that in humans, extensive glucuronidation occurs at the alcohol and phenol functional groups but not the aliphatic tertiary amine group. However, that N-glucuronidation can be competitive with O-glucuronidation in vivo is indicated by the observation that the urinary metabolites ofE-10-hydroxyamitriptyline administered to one healthy volunteer included both the O-glucuronide and aliphaticN+-glucuronide metabolites (6% and 2.8% of the dose, respectively) (Breyer-Pfaff et al., 1990).
Pharmacological Importance and Directions for Future Research.
The importance of N+-glucuronidation as a means of eliminating xenobiotics in human varies widely between substrates. For about half of the known substrates, ≥5% of the administered dose is excreted in the urine as the quaternary glucuronide (tables 1 and2). However, only in the cases of doxepin, ketotifen, lamotrigine, and three drugs under development is the reported mean urinary excretion in excess of 20% of the administered dose. The possibility of the fecal elimination of N+-glucuronide metabolites has received little attention. In these laboratories, cyclizineN+-glucuronide (2.6 ± 3.6% of administered dose) was found in the feces of four of five healthy Asian volunteers administered an oral dose of cyclizine (Luo et al., 1992a). This observation raises the possibility of the enterohepatic recycling of xenobiotics viaN+-glucuronide metabolites, but no studies have been reported in these regards. Another possible reason for low observed elimination of a substrate viaN+-glucuronidation is because of competing metabolic pathways. With certain substrates, including phenothiazine antipsychotic agents, there is extensive biotransformation by various routes such that numerous metabolites result. Therefore, it is not surprising that for the seven phenothiazine antipsychotic agents examined, only in the case of chlorpromazine in one patient under chronic treatment with large doses (1600 mg/day) of the drug was theN+-glucuronide detected (Luo et al.,1995; Chaudhary et al., 1988).
The clinical importance of N+-glucuronidation with respect to both pharmacokinetics and pharmacodynamics has not been addressed in many studies. Usually the only available pharmacokinetic data involves urinary excretion and, hence, the data reported in this review. Only rarely has a plasma concentration vs. time curve and associated data of a N+-glucuronide metabolite been reported (Julien-Larose et al., 1983). The enzymology of N+-glucuronidation, including interspecies variation, is discussed in the following article (Green and Tephly, 1998). Active transport processes are involved in the transport of glucuronide metabolites and quaternary ammonium compounds, but only limited data are available forN+-glucuronide metabolites and are suggestive of their active tubular secretion (Remmel and Sinz, 1991) and secretion into the gastrointestinal tract (Breyer-Pfaff et al., 1990). Also data are lacking as to the chemical and enzymatic stability ofN+-glucuronide metabolites in vivo.Finally, as previously noted, the issue of enterohepatic recycling of a xenobiotic via the N+-glucuronide metabolite has not been addressed as yet.
Glucuronidation generally is regarded as a means of detoxication of a substrate. In the case of N+-glucuronide metabolites, there is lack of definitive pharmacodynamic data, but it should be considered that the toxicity of quaternary ammonium compounds as a general class is well recognized, including effects associated with the release of histamine. In the only documented case in which anN+-glucuronide metabolite was taken by a human, not all of the intended dose was administered because of the rapid onset of toxic effects. Administration of an iv infusion of amitriptyline N+-glucuronide to one healthy female volunteer was stopped after 25 min (17.5 mg, 38.5 μmol administered) because of the appearance of flushing and tachycardia (Breyer-Pfaff et al., 1990). Finally, the possibility has been raised that xenobiotic substrates for the enzyme(s) involved inN+-glucuronidation will inhibit the metabolism of certain endogenous substrates of the enzyme(s), especially steroids (Sharp et al., 1992). Therefore, in view of these limited data, it is clear that more studies are required before assessment can be made of the clinical importance of theN+-glucuronidation pathway.
Acknowledgments
The research reported herein of Dr. Hong Luo and Ms. Iwona Kowalczyk and the contributions of fellow members of the Drug Metabolism and Drug Disposition Research Group (Drs. K. K. Midha, J. W. Hubbard, and G. McKay) are gratefully acknowledged.
Footnotes
-
Send reprint requests to: Dr. E. M. Hawes, College of Pharmacy and Nutrition, University of Saskatchewan, 110 Science Place, Saskatoon, SK S7N 5C9 Canada.
-
This work was supported by the Medical Research Council of Canada (Program Grant PG-11472 to Drs. K. K. Midha, E. M. Hawes, J. W. Hubbard, and G. McKay).
- Abbreviations used are::
- UGT
- UDP-glucuronosyltransferase
- HPLC
- high-performance liquid chromatography
- IR
- infrared
- UV
- ultraviolet
- MS
- mass spectrometry
- EI
- electron impact
- CI
- chemical ionization
- FAB
- fast atom bombardment
- API
- atmospheric pressure ionization
- ESI
- electrospray ionization
- NMR
- nuclear magnetic resonance
- NOE
- nuclear Overhauser effect
- The American Society for Pharmacology and Experimental Therapeutics