A Tertiary N-Glucuronide Unique to Humans
1996 ASPET N-Glucuronidation of Xenobiotics Symposium
Abstract
In humans, a major metabolite of the atypical antipsychotic olanzapine in the plasma and in the urine was found to be anN-glucuronide. Unexpectedly, the glucuronic acid moiety was linked through a nitrogen of the benzodiazepine nucleus of olanzapine by way of a secondary amine linkage, rather than through a nitrogen on the piperazine substituent of the nucleus, to give a quaternary ammonium glucuronide. Derivatization with phenylisothiocyanate to yield a thiourea adduct indicated that conjugation occurred via a secondary amine. Subsequently, mass spectrometry and nuclear magnetic resonance studies with the isolated metabolite and later with the synthesized metabolite indicated that the glucuronide was linked at the 10- position of olanzapine. This phase 2 metabolite was only detected in the plasma and urine of human subjects and not in mice, rats, or monkeys; a trace of this metabolite was detected in dog urine. TheN-10 glucuronide was resistant to enzymatic and base hydrolysis but was cleaved under acidic conditions. Formation of anN-glucuronide metabolite directly with the benzodiazepine nucleus has not previously been reported.
Olanzapine (2-methyl-4-(4-methyl-1-piperazinyl)10H-thieno[2,3-b[1,5]benzodiazepine; fig. 1) is a new atypical antipsychotic agent with a thienobenzodiazepinyl structure. Olanzapine is effective in the treatment of both positive and negative symptoms of schizophrenia, with minimal extrapyramidal side-effects (Beasleyet al., 1996a; Beasley et al., 1996b; Baldwin and Montgomery, 1995). After oral administration, olanzapine was well absorbed and extensively metabolized in both humans and experimental animals (Mattiuz et al., 1997; Kassahun et al., 1997). The plasma elimination half-life of olanzapine was ∼3 hr in rodents and monkeys, ∼9 hr in dogs, and much longer in humans (∼27 hr). In humans, olanzapine underwent biotransformation via conjugative and oxidative pathways (Kassahun et al., 1997).
The structures of olanzapine and itsN-glucuronides.
In this article, information is presented on the isolation, structural characterization, and species-dependent formation of anN-glucuronide metabolite of olanzapine, 10-N-glucuronide (fig. 1). The 10-N-glucuronide conjugate of olanzapine was unusual in that it was (1) resistant to hydrolysis by β-glucuronidase, (2) produced only in humans (of five species studied), (3) not amenable to facile in vitroproduction using human liver microsomes, and (4) to the best of our knowledge, without precedence in the literature, although the benzodiazepinyl moiety is a structural feature of many therapeutic agents. Consequently, it was very important to establish its chemical structure fully.
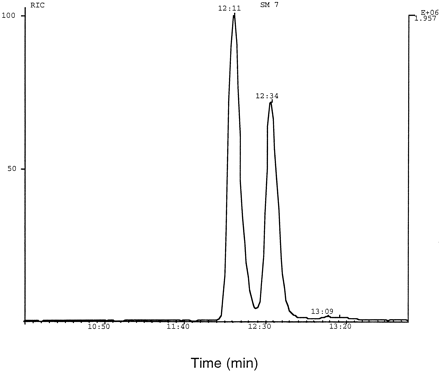
Most of the data on the formation of the 10-N-glucuronide in humans was generated from a clinical study in which healthy volunteers were administered an oral dose of 14C-olanzapine (12.5 mg). An HPLC3 radiochromatogram obtained from an extract (aqueous fraction after extraction of basified urine with ethyl acetate) of a urine sample from one volunteer (4–8 hr after dose) is shown in fig. 2. Analysis by electrospray LC-MS indicated that the major component (peak 2, fig.2) and peak 3 (fig. 2) were N-glucuronides of olanzapine.
HPLC radiochromatogram from the aqueous fraction of an ethyl acetate extract of a urine sample (4–8 hr after dose) from a healthy volunteer administered an oral dose of14C-olanzapine. Peaks 2 and 3 were characterized as 10- and 4′-N-glucuronides of olanzapine, respectively.
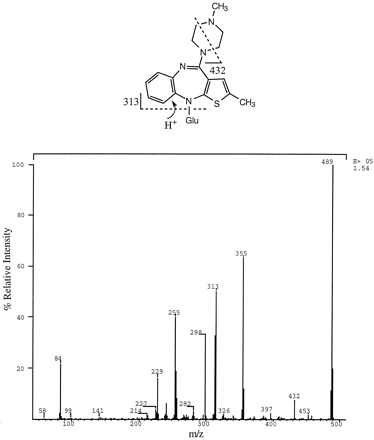
The minor N-glucuronide (peak 3, fig. 2) was identified as the quaternary ammonium–linked 4′-N-glucuronide (fig. 1) by comparing its LC-MS/MS (fig. 3) properties with those obtained from a sample of a synthetic standard.
Product ion spectrum of m/z 489 (M+) of a metabolite isolated from urine (peak 3, fig. 2) and characterized as the 4′-N-glucuronide conjugate of olanzapine. Possible structural assignment for two diagnostic fragment ions is shown above. The ion at m/z 282 is likely due to the combined loss of CH3NH2 and glucuronic acid. Glu, glucuronic acid.
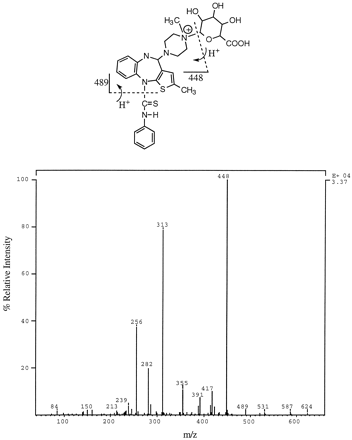
The structure of 10-N-glucuronide (peak 2, fig. 2) was elucidated as follows: The metabolite was isolated by preparative HPLC and analyzed by LC-MS under isocratic HPLC conditions. The isolated 10-N-glucuronide peak was resolved into two closely eluting components, as shown in fig. 4. Both components afforded an MH+ ion at m/z 489 and nearly identical product ion spectra (figs.5 and 6). Both product ion spectra (precursor m/z 489) contained an ion at m/z 313 (MH-176)+, which is likely due to loss of dehydroglucuronic acid. A neutral loss scan of 176 and 57 (loss of CH2CH-NH-CH3 from the methyl piperazine moiety of olanzapine) daltons yielded a peak corresponding tom/z 489. The ratio of m/z 313 to 489 was much higher in the product ion spectrum of 4′-N-glucuronide (fig.3), compared with that obtained for 10-N-glucuronide (figs.5 and 6). This observation is consistent with the ease with which the glycosidic bond is broken in the quaternary 4′-N-glucuronide, in comparison with the glycosidic linkage in the tertiary 10-N-glucuronide.
LC-MS/MS trace generated by obtaining the product ion spectrum (precursor m/z 489) of 10-N-glucuronide. The 10-N-glucuronide metabolite was isolated by preparative HPLC from urine samples of healthy volunteers administered 14C-olanzapine. The two large peaks were identified as “isomers” of 10-N-glucuronide.
Product ion spectrum of m/z 489 (MH+) from the “isomer” of 10-N-glucuronide with a retention time of 12:11 min (fig. 4). Possible structural assignment for some of the fragment ions is shown above.
Product ion spectrum of m/z 489 (MH+) from the “isomer” of 10-N-glucuronide with a longer HPLC retention time (12:34 min, fig. 4). Possible structural assignment for some of the fragment ions is shown above.
Chemical hydrolysis of a sample of 10-N-glucuronide isolated from urine provided additional evidence that the major metabolite in urine was a tertiary N-glucuronide conjugate. Incubation (1 hr, 50°C) of a sample of the conjugate with either 3 or 6 N HCl resulted in the complete disappearance of the HPLC peak due to 10-N-glucuronide and appearance of a new peak at the HPLC retention time of olanzapine. Treatment of a sample of the conjugate with 0.1 N HCl resulted in a hydrolysis rate of 6%, while 1 and 2 N HCl solutions caused 60%–75% hydrolysis of the glucuronide. Hydrolysis of the conjugate was not evident after treatment of a sample with 0.1 or 1.0 N NaOH nor did the glucuronide release the aglycone upon treatment with β-glucuronidase from either Helix pomatia or Escherichia coli.
Further information on the site of glucuronidation was obtained from a derivatization experiment with phenyl isothiocyanate. Olanzapine and 4′-N-glucuronide have secondary amines at position 10, and both formed the corresponding thiourea derivatives as shown in fig.7 for 4′-N-glucuronide. However, as expected, 10-N-glucuronide failed to form such a derivative, indicating that the secondary nitrogen at position 10 of the metabolite was substituted (glucuronidated).
Product ion spectrum of m/z 624 (M+) obtained from the phenyl isothiocyanate derivative of the 4′-N-glucuronide conjugate of olanzapine. Possible structural assignment for two of the fragment ions is shown above.
For further evidence of structure, the 10-N-glucuronide was chemically synthesized and afforded MS and nuclear magnetic resonance data, which were consistent with the proposed structure. Analysis of a sample of the synthetic standard by HPLC resulted in two peaks with retention times identical to the corresponding peaks obtained from a sample of the metabolite(s) isolated from urine. The product ion spectra of the synthetic conjugates were also nearly identical to the corresponding spectra obtained from the isolated metabolite(s). Thus the major metabolite in human urine was identified as olanzapine 10-N-glucuronide.
The finding that the 10-N-glucuronide HPLC peak resolves into two distinct peaks (fig. 4) under optimal HPLC conditions raises the possibility that there are two isomers of the 10-N-glucuronide. Although the NMR spectrum of the conjugate was obtained from a sample that contained the two “isomers,” there was only a single signal for the anomeric proton as well as the aromatic protons of the conjugate. This indicates that the two peaks are not likely to be the α and β-anomers of the conjugate. During storage at −70°C, there was some conversion of one form to another. From the current data, it is not clear what kind of isomeric relationship the conjugates might have, although they do not appear to be regioisomers.
Urinary olanzapine 10- and 4′-N-glucuronides were estimated to account for approximately 13% and 2% of the administered dose, respectively. In extracts of fecal samples from the volunteers, two radioactive components were detected and were identified as olanzapine 10-N-glucuronide and olanzapine. Approximately 8% of the dose was eliminated via the feces as 10-N-glucuronide and another 2% as olanzapine. Thus approximately 21% of a single dose of olanzapine is eliminated via the 10-N-glucuronidation pathway by way of urine and feces.
Analysis of plasma extracts by HPLC with radiochemical detection indicated that 10-N-glucuronide was a major circulating metabolite. The levels of olanzapine, 10-N-glucuronide, and 4′-N-desmethyl olanzapine (a metabolite detected in all species studied) were determined in plasma obtained from patients on steady-state olanzapine (daily doses of 5–20 mg), using HPLC (Catlowet al., 1995). The concentration of theN-glucuronide conjugate was determined as olanzapine-equivalent after acid hydrolysis to olanzapine. A total of 204 plasma samples were analyzed. The average (range) concentrations of olanzapine, 10-N-glucuronide, and 4′-N-desmethyl olanzapine were found to be approximately 24 (3–67), 10 (trace-70), and 7 (trace-19) ng/ml, respectively. This data, together with that of the 14C study, indicate that while the parent compound is the single largest entity in plasma, olanzapine 10-N-glucuronide is likely to be the main circulating metabolite in humans.
The metabolic formation of 10-N-glucuronide was studied in CD-1 mice, Fischer 344 rats, beagle dogs, and rhesus monkeys administered single oral doses of olanzapine (5 to 15 mg/kg). LC-MS/MS analysis of urine (all species), plasma (mice, rats, dogs), and bile (rats) failed to reveal the presence of 10-N-glucuronide in these species, except for a trace amount in dog urine. However, oxidative metabolites of olanzapine were detected and measured in the plasma of animals (Chiu and Franklin, 1995). Olanzapine 4′-N-glucuronide was also formed only in humans, as would be expected for a quaternary N-glucuronide metabolite.
The two N-glucuronides of olanzapine, i.e.the tertiary N-glucuronide 10-N-glucuronide and the quaternary N-glucuronide 4′-N-glucuronide, were similar inasmuch as both were generated as metabolites in humans only. However, they differed from each other in their chemical properties perhaps as a consequence of the nature of the respective glycosidic linkage. The tertiary N-glucuronide was resistant to β-glucuronidase hydrolysis, whereas the quaternaryN-glucuronide was susceptible. Dilute acid (3 N HCl) completely converted 10-N-glucuronide to the aglycone but had no effect on 4′-N-glucuronide.
In summary, an unusual glucuronide, in which glucuronic acid is linked through the nitrogen of the benzodiazepinyl moiety of olanzapine, has been characterized as a major metabolite of olanzapine in humans. Another N-glucuronide of olanzapine, 4′-N-glucuronide, was also identified in humans. The latter conjugate is similar to that described as a metabolite of clozapine in humans (Luo et al., 1994). Of five species studied, olanzapine 10-N-glucuronide was formed only in humans. This is somewhat unprecedented because the metabolic formation of only quaternary N-glucuronides is known to be largely restricted to humans and other higher primates (Chaudhuri et al., 1976;Hucker et al., 1978; Fischer et al., 1980). But, in the case of olazapine, a tertiary N-glucuronide metabolite unique to humans was generated.
Footnotes
-
Send reprint requests to: Kelem Kassahun, Ph.D., Department of Drug Metabolism, Merck Research Laboratories, West Point, PA 19486-0004.
-
↵1 Current address: Department of Drug Metabolism, Merck Research Laboratories, West Point, PA 19486-0004.
-
↵2 Current address: Department of Drug Metabolism and Spectroscopy, Amgen Bio-Pharma, 3200 Walnut Street, Boulder, CO 80303.
- Abbreviations used are::
- HPLC
- high-performance liquid chromatography
- LC-MS
- liquid chromatography-mass spectrometry
- MS
- mass spectrometry
- The American Society for Pharmacology and Experimental Therapeutics