Abstract
An antipeptide antibody has been produced that recognizes CYP3A4 and exhibits greater than 90–95% inhibition on CYP3A4-mediated reactions [Wang RW and Lu AYH (1997) Drug Metab Dispos25:762–767]. The inhibitory epitope of the 21-amino acid peptide, corresponding to residues 253 to 273 of CYP3A4, has been identified to reside in a 7-amino acid sequence (LEDTQKH: residues 261–267 of CYP3A4). This conclusion was based on the reversal of antibody inhibition of testosterone 6β-hydroxylation when peptides with overlapping sequence in this region were preincubated with the antibody. In immunoblotting analysis, this antibody did not recognize CYP3A5 or CYP3A7 in microsomes prepared from baculovirus-infected cells containing these two expressed isoforms. In addition, the antipeptide antibody did not inhibit testosterone 6β-hydroxylation or midazolam 1′- and 4-hydroxylation in microsomes containing expressed CYP3A5 and CYP3A7. Because the corresponding sequence in CYP3A5 (LNDKQKH) and CYP3A7 (LKETQKH) differs from CYP3A4 by only two amino acids, six peptides with either one or two amino acid changes were used to determine which amino acid is essential for antibody-antigen interaction. Our data indicate that Glu, Asp, and Thr in the 7-amino acid sequence of CYP3A4 are critical determinants of selectivity among CYP3A isoforms.
At least three structurally related cytochrome P-450 isoforms in the CYP3A gene subfamily are known to exist in human liver (Wrighton and Stevens, 1992; Guengerich, 1995). CYP3A4, the predominant form, is expressed in virtually all adult human livers, whereas CYP3A5 is polymorphically expressed in about 20 to 30% of adult human livers. CYP3A7, on the other hand, is fetal liver specific. All three enzymes metabolize a variety of therapeutic agents and endogenous substrates, although it is uncertain whether these three isoforms exhibit identical substrate specificities. Because of their structural relatedness, no chemical probes are available to distinguish these enzymes. In addition, antibodies produced against CYP3A4 generally recognize other CYP3A isoforms (Gelboin et al., 1995; Belloc et al., 1996).
To establish the contribution of each one of the CYP3A isoforms in the metabolism of a specific substrate in human liver microsomes, it is desirable to have antibodies inhibiting only one of the three CYP3A isoforms. One way to achieve this goal is to use a peptide with a unique amino acid sequence as antigen, so that antibodies can discriminate minor differences in the amino acid sequence among structurally related isoforms. In a previous paper (Wang and Lu, 1997), we described the production of both inhibitory and noninhibitory anti-CYP3A4 antibodies against a 21-amino acid peptide (residues 253–273 of CYP3A4). Preliminary immunoblotting data indicated that these antibodies recognize only CYP3A4 and not CYP3A5. In the present study, we examined further the specificity of this antipeptide antibody toward CYP3A5 and CYP3A7. The inhibitory epitope on this 21-amino acid peptide has been mapped to reside in a 7-amino acid sequence. Moreover, we established that three amino acids, Glu, Asp, and Thr, in this short sequence play a critical role in recognizing CYP3A4 isoforms by this unique antipeptide antibody.
Materials and Methods
Materials.
Testosterone, 6β-hydroxytestosterone, glucose 6-phosphate, NADP, and glucose 6-phosphate dehydrogenase were purchased from Sigma (St. Louis, MO). Midazolam, 1′-hydroxy midazolam, and 4-hydroxy midazolam were gifts from Hoffmann La Roche (Nutley, NJ). All other reagents and solvents were of high analytical grade supplied by Fisher Scientific (Fair Lawn, NJ). Vectors (pCRII and pBlueBac) and insect cells (sf21) were obtained from Invitrogen Corp. (Carlsbad, CA). The peptides were synthesized on an Applied Biosystems 430A peptide synthesizer using solid-phase chemistry by PeptidoGenic Research & Co, Inc. (Livermore, CA). The purity of synthesized peptides was greater than 90% by high-performance liquid chromatography (HPLC)1 and IonSpray mass spectrometry analysis. Human liver microsomal preparations were kindly provided by Dr. Judy Raucy (Agouron Institute, La Jolla, CA). Microsomes prepared from baculovirus-infected cells containing CYP3A5 were obtained from Gentest Corp. (Woburn, MA). Antibody against purified CYP3A4 protein was kindly provided by Dr. Jerome Lasker (Mount Sinai Medical Center, New York, NY). Inhibitory antipeptide antibody against a 21-amino acid peptide (VKRMKESRLEDTQKHRVDFLQ: residues 253–273 of CYP3A4) was prepared in this laboratory as described (Wang and Lu, 1997).
Cloning and Expression of CYP3A4 and CYP3A7 in Baculovirus System.
The coding sequence for CYP3A7 was amplified from a fetal human liver Quick-Clone cDNA library (Clontech Laboratories, Inc., Palo Alto, CA) using a forward primer, 5′-GATCTAGATGGATCTCATCCCAAACTTGGCCG-3′, and a reverse primer, 5′-GTCTCTAGAGCAAACCAGAAGTCCTTAGG-3′. The PCR fragment was cloned into pCRII, and the plasmid was transformed inEscherichia coli. The entire cDNA fragment was sequenced from both directions, and the sequence was confirmed to be identical with that reported previously (Komori et al. 1989). Plasmids pGem-7/CYP3A4 and pGem-3Z/CYPOR containing the full-length cDNAs for CYP3A4 (Gonzalez et al., 1988) and NADPH-cytochrome P-450 reductase (Yamano et al., 1989), respectively, were provided by Dr. Frank Gonzalez (National Cancer Institute, Bethesda, MD). The entire coding region of each cDNA was excised from the vectors and inserted into baculovirus shuttle vector, pBlueBac (CYP3A4,XbaI/KpnI; NADPH-cytochrome P-450 reductase,EcoRI; and CYP3A7, XbaI). Recombinant virus was produced using the method described by the manufacturer (Invitrogen Corp.). After two runs of plaque purification, the virus was then amplified in high-titer stock for protein expression. Sf21 insect cells were grown at 27°C in complete Sf900 medium (BRL, Gaithersburg, MD) to a density of 2 × 106 cells/ml (400 ml in total) in 1-liter spinner flasks with enlarged blades at 90 rpm. Cells were infected by the virus encoding CYP3A4 or CYP3A7 at approximate multiplicity of infection of 1.0 and by the virus encoding NADPH-cytochrome P-450 reductase at multiplicity of infection of 0.1. Medium containing 1 mg hemin/ml in the form of a hemin-albumin complex was added. After 72 h, cells were harvested by centrifugation and resuspended in 0.1 M potassium phosphate buffer, pH 7.5, containing 20% glycerol. Cells were sonicated and lysed, and microsomes were prepared by differential centrifugation and resuspended in 0.1 M phosphate buffer, pH 7.5, containing 0.25 M sucrose, 1 mM EDTA, 0.5 mM dithiothreital, and 1.15% KCl, and frozen at -80°C until use. Total P-450 content was measured by CO difference spectrum (Omura and Sato, 1964), and protein concentration was determined by bicinchoninic acid method (Smith et al., 1985).
Western Blots.
Liver microsomes from human (15 μg) or microsomes from baculovirus-infected cells containing expressed CYP3A4, CYP3A5, and CYP3A7 (0.5 pmol) were subjected to 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Laemmli, 1970) and transferred to a nitrocellulose membrane as described (Towbin et al., 1979). The nitrocellulose sheets were blocked with nonfat dry milk, incubated with antibody, and then treated with125I-Protein A (Amersham Corp.).
Immunoinhibition.
Immunoinhibition was conducted by preincubating microsomes for 30 min at room temperature with various amounts of rabbit preimmune IgG or antipeptide IgG. After incubation, the mixtures were incubated for 10 min with 100 μM testosterone or 25 μM midazolam in 100 mM potassium phosphate buffer (pH 7.4) with 1 mM EDTA, 6 mM MgCl2, and an NADPH-generating system consisting of 10 mM glucose 6-phosphate, 1 mM NADP, and 0.14 units of glucose 6-phosphate dehydrogenase in a total volume of 0.2 ml. Reactions were quenched by adding 0.2 ml of methanol and centrifuged at 14,000g for 10 min. The supernatants were injected directly for HPLC analysis.
Reversal of Immunoinhibition.
In control experiments, sufficient amounts of antibody were incubated with human liver microsomes to achieve greater than 90% inhibition of testosterone 6β-hydroxylation. To determine whether a peptide could reverse the antibody inhibition, various amounts of peptides, varying in length and sequence from the 21-amino acid peptide, were preincubated with the antibody at room temperature for 2 h. Microsomes were then incubated with the IgG-peptide mixture for 30 min at room temperature, and testosterone 6β-hydroxylase activity was determined. The extent of reversal of immunoinhibition was indicative of the extent of interaction between the peptide and the antibody.
HPLC Analysis.
The HPLC used was a Shimadzu SCL 10A system controller consisting of two LC 10AS pumps, a SIL 10A automatic sample injector, and a SPD10A UV-VIS spectrophotometric detector. Chromatographic analyses were carried out on a Zorbax SB C8 column (4.6 mm × 75 mm, 3.5 μm) at a flow rate of 2 ml/min by a linear gradient elution with mobile phase consisting of buffer A (10 mM ammonium acetate in H2O) and buffer B (10 mM ammonium acetate in 90% acetonitrile and 10% methanol). 6β-Hydroxytestosterone and testosterone were eluted from the column with 25 to 60% of buffer B in 7 min and monitored at 254 nm with retention times of 3.1 and 6.6 min, respectively. Midazolam and its metabolites were eluted with 25 to 65% of buffer B in 7 min and monitored at 254 nm. The retention times for 4-hydroxy midazolam, 1′-hydroxy midazolam, and midazolam were 4.5, 5.0, and 6.5 min, respectively.
Results
Mapping of Inhibitory Epitope.
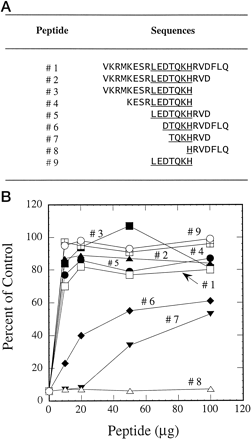
Using a 21-amino acid peptide (corresponding to residues 253–273 of CYP3A4) conjugated to keyhole limpet hemocyanin, we recently have reported the production of both inhibitory and noninhibitory antibodies against CYP3A4 (Wang and Lu, 1997). To map the inhibitory epitope on this 21-amino acid peptide, several peptides were designed by deletions and specific alterations. Reversal of antibody inhibition on testosterone 6β-hydroxylation by peptides was indicative of peptide-antibody interaction, whereas lack of reversed inhibition indicated that the peptide did not possess the epitope to interact with the antibody. The 21-amino acid peptide that served as a positive control fully reversed the inhibition caused by the antibody. A wide range of peptide concentrations was used in these experiments so that both the affinity and extent of peptide-antibody interaction could be evaluated. Figure 1A shows the amino acid sequence of 9 peptides ranging from the 21-amino acid sequence (peptide 1) to a 7-amino acid sequence corresponding to residues 261 to 267 of CYP3A4 (peptide 9). Other peptides contained deleted sequences from either one or both ends. All peptides containing the LEDTQKH sequence (peptides 1–5 and peptide 9) could reverse antibody inhibition, whereas peptides containing part of this sequence (peptides 6–7) were only partially active (Fig. 1B). Peptide 8, which contains only one of the seven amino acids (His at 267), was totally inactive. Because peptide 9 and peptides 1 to 5 were fully active in reversing antibody inhibition of testosterone 6β-hydroxylation, one could conclude that the inhibitory epitope resides in this 7-amino acid sequence.
Reversal of antibody inhibition on testosterone 6β-hydroxylation by peptides.
A, peptides with overlapping sequences. B, IgG (0.2 mg) was preincubated with 10 to 100 μg peptides for 2 h at room temperature. Human liver microsomes (containing 0.04 nmol cytochrome P-450) were then added and incubated for 30 min at room temperature. After addition of the testosterone and NADPH-generating system, reaction mixtures were incubated at 37°C for 10 min. The control activity of testosterone 6β-hydroxylation was 5.85 nmol/min/nmol. In the absence of peptides, greater than 90% of testosterone 6β-hydroxylation was inhibited by antibody.
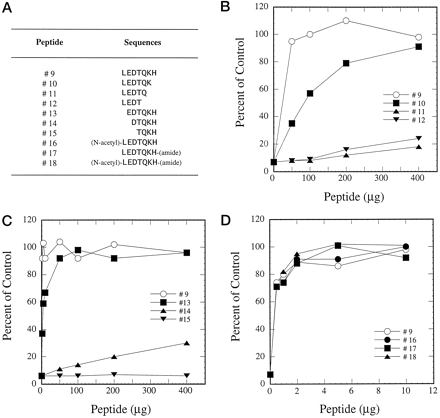
Further experiments were designed to determine whether the 7-amino acid peptide represents the minimum required sequence for peptide-antibody interaction. More peptides were synthesized by deleting additional amino acids from either the N- or C-terminal end (Fig.2A). Removal of His at position 267 (peptide 10) resulted in less affinity between peptide-antibody interaction whereas deletion of His and Lys (peptide 11) or His, Lys, and Gln (peptide 12) caused loss of inhibition reversibility by these peptides (Fig. 2B). Similarly, removal of one amino acid from the N terminus (peptide 13) slightly decreased the affinity for peptide-antibody interaction (Fig. 2C). However, when two or three amino acids were removed from this end (peptides 14 and 15), the capability for these peptides to interact with the antibody was diminished (Fig. 2C). N-Acetylation of the amino group (peptide 16), amidation of the carboxyl group (peptide 17), or both (peptide 18) did not affect the ability of the 7-amino acid peptide to interact with the antibody, indicating that the free carboxyl or amino group of the peptide is not critical in the antigen-antibody interaction (Fig. 2D). All these results indicate that this 7-amino acid sequence is required for optimal interaction between the 21-amino acid peptide and the inhibitory anti-CYP3A4 peptide antibody.
Reversal of antibody inhibition on testosterone 6β-hydroxylation by peptides.
A, sequences of peptides. B–D, peptides 9 to 18 were used in the preincubations as described in Fig. 1.
Antibody Specificity.
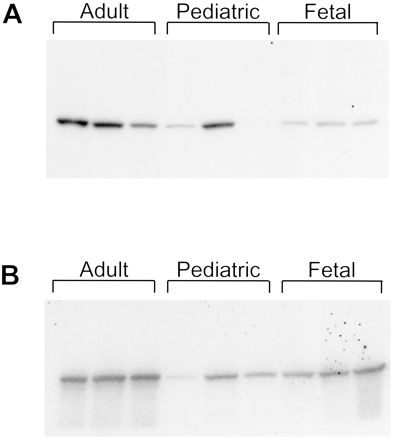
When examined by immunoblotting, antipeptide antibody against CYP3A4 only recognized CYP3A4 but not CYP3A5 and CYP3A7 in microsomes prepared from baculovirus-infected cells expressing CYP3A isoforms (Fig.3A). In contrast, antibodies produced against purified CYP3A4 recognized all three CYP3A isoforms (Fig. 3B). Antipeptide antibody recognized a single protein band in liver microsomes prepared from all three adult and two pediatric subjects (Fig. 4A). Based on the specificity of this antipeptide antibody as shown in Fig. 3A, and the immunoinhibition results to be described, CYP3A4 should be the protein band in the liver microsomes from adult and pediatric subjects, whereas the faint protein band in fetal liver microsomes was either CYP3A4 (as a minor component) or another structurally related CYP3A isoform(s), which probably is not CYP3A7. Antibodies against purified CYP3A4 presumably reacted with all CYP3A isoforms, including CYP3A4, CYP3A5, and CYP3A7 in all human liver microsomes (Fig. 4B).
Western blot detection of CYP3A4, CYP3A5, and CYP3A7.
Microsomes prepared from control insect cells or baculovirus-infected cells containing expressed CYP3A4, CYP3A5, and CYP3A7 were immunoblotted with antipeptide antibody (A) or antibody against purified CYP3A4 (B).
Western blot detection of CYP3A isoforms.
Human liver microsomes from adult, pediatric, and fetal subjects were immunoblotted with antipeptide antibody (A) or antibody against purified CYP3A4 (B).
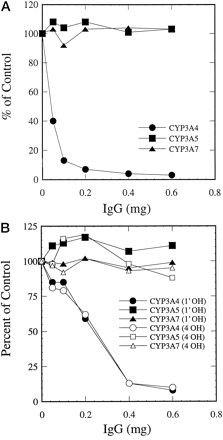
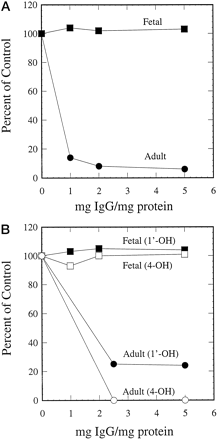
Consistent with the immunoblotting results, antipeptide antibody against CYP3A4 inhibited CYP3A4-mediated testosterone 6β-hydroxylation (Fig. 5A), midazolam 1′-hydroxylation, and 4-hydroxylation (Fig. 5B) in microsomes containing expressed CYP3A4, but had no effect on CYP3A5- or CYP3A7-dependent metabolism of testosterone and midazolam in microsomes containing expressed CYP3A5 or CYP3A7. The remarkable specificity of this antibody was also demonstrated in adult and fetal liver microsomal preparations. Although testosterone 6β-hydroxylation (Fig.6A) and midazolam 1′- and 4-hydroxylation (Fig. 6B) were strongly inhibited by the antipeptide antibody in adult human liver microsomes, metabolism of these two compounds in fetal liver microsomes was not affected at all. These results indicate that CYP3A4 is the major enzyme responsible for testosterone and midazolam metabolism in adult human livers, but played little or no role in the metabolism of these two compounds in fetal human livers.
Effect of antipeptide antibody on CYP3A-mediated reactions.
Microsomes prepared from baculovirus-infected cells containing expressed CYP3A4/P450 reductase, CYP3A5/P450 reductase, and CYP3A7/P450 reductase were preincubated with 0.1 to 0.6 mg of preimmune IgG or antipeptide IgG. Testosterone 6β-hydroxylase (A) and midazolam 1′- and 4-hydroxylase (B) activities were measured. The control activities: testosterone 6β-hydroxylation (24.94, 7.57, and 35.68 nmol/min/nmol P-450 for CYP3A4, CYP3A5, and CYP3A7, respectively); midazolam 1′-hydroxylation (5.45, 9.63, and 29.44 nmol/min/nmol P-450 for CYP3A4, CYP3A5, and CYP3A7, respectively); and midazolam 4-hydroxylation (1.55, 0.94, and 1.42 nmol/min/nmol P-450 for CYP3A4, CYP3A5, and CYP3A7, respectively).
Effect of antipeptide antibody on CYP3A-mediated reactions.
Adult and fetal human liver microsomes containing 0.1 mg proteins were preincubated with 0.1 to 0.5 mg of preimmune IgG or antipeptide IgG. Testosterone 6β-hydroxylase (A) and midazolam 1′- and 4-hydroxylase (B) activities were measured. The control activities: testosterone 6β-hydroxylation (1.62 and 0.59 nmol/min/mg protein for adult and fetal liver microsomes); midazolam 1′-hydroxylation (1.55 and 0.78 nmol/min/mg protein for adult and fetal liver microsomes); and midazolam 4-hydroxylation (0.46 and 0.36 nmol/min/mg protein for adult and fetal liver microsomes).
Critical Amino Acid Residues in CYP-Antibody Interaction.
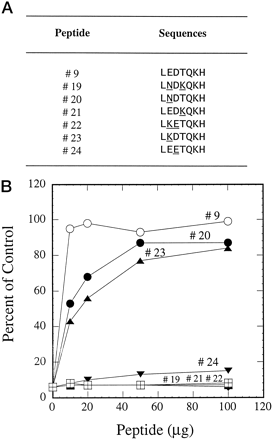
CYP3A5 and CYP3A7 differ from CYP3A4 in only two amino acids in residues 261 to 267. Because this 7-amino acid sequence has been identified to be the inhibitory epitope on the 21-amino acid peptide against this anti-CYP3A4 peptide antibody, experiments were designed to determine the amino acid residues critical for the interaction between the antibody and the three CYP3A isoforms using peptides containing either one or two alterations (Fig. 7A). Again, control experiments showed that peptide 9 fully reversed the inhibition caused by the antibody (Fig. 7B). Converting the 7-amino acid sequence of CYP3A4 to that of CYP3A5 (peptide 19) or CYP3A7 (peptide 22) by changing two amino acids resulted in total loss of inhibition reversibility by these peptides. A single amino acid change either decreased the affinity of peptide-antibody interaction (peptides 20 and 23) or caused the total loss of activity (peptides 21 and 24).
Reversal of antibody inhibition on testosterone 6β-hydroxylation by peptides with modified sequences.
A, sequences of peptides containing the epitope sequence from CYP3A4 or modified sequences from CYP3A5 or CYP3A7 with either a 1- or 2-amino acid alteration. The modified amino acids are underlined. B, peptides 9 and 19 to 24 were used in the preincubations as described in Fig. 1.
Discussion
A previous study has shown that when rabbits were immunized with keyhole limpet hemocyanin-conjugated 21-amino acid peptide (253–273 of CYP3A4), all rabbits produced highly specific antibodies against CYP3A4 by immunoblotting. However, antibodies produced by certain rabbits are inhibitory to CYP3A4-mediated reactions, whereas antibodies produced by some other rabbits are noninhibitory. To understand the production of inhibitory antibodies, the present study was conducted to determine the inhibitory epitope on this 21-amino acid peptide. A series of peptides with overlapping sequences or with specific alterations were synthesized and used to map the inhibitory epitope on the 21-amino acid peptide by their abilities to reverse the inhibition of CYP3A4-mediated reactions caused by the antibody. The peptide containing a 7-amino acid sequence (residues 261–267) was shown to fully reverse antibody inhibition, whereas further deletion from either end of the peptide resulted in partial or complete loss of this ability. These results suggest that the inhibitory antibody is produced against this 7-amino acid epitope. Attempts were also made to map the epitopes against noninhibitory antibodies using an immunoblotting approach. After microsomes containing expressed CYP3A4 were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane, inhibitory or noninhibitory peptide antibodies that were preincubated with various peptides were used for detection of CYP3A4. Peptides 1 to 7 and 9 can block the immunoblotting abilities of the noninhibitory antibodies as well as the inhibitory antibodies to CYP 3A4 to different degrees. Because immunoblotting is much less quantitative than the enzyme activity assay, results were inconclusive regarding the epitope on the 21-amino acid peptide recognized by the inhibitory or noninhibitory antibodies. We could only conclude from the semiquantitative data (not shown) that the inhibitory and noninhibitory epitope on the 21-amino acid peptide may be different. To our surprise, antiserum collected from the rabbits that were immunized with keyhole limpet hemocyanin-conjugated 7-amino acid peptide only recognized CYP3A4 by immunoblotting, but did not inhibit the CYP3A4-mediated testosterone 6β-hydroxylation. Apparently, production of inhibitory antibodies in rabbits is more complex than just presenting the animal with carrier proteins containing the epitope sequence.
Examination of the sequence alignment of the three CYP3A isoforms revealed five amino acid differences between CYP3A4 and CYP3A5 or between CYP3A4 and CYP3A7 in this 21-amino acid region. In terms of the 7-amino acid sequence of the inhibitory epitope, CYP3A5 differs from CYP3A4 only in residues 262 (Asn versus Glu) and 264 (Lys versus Thr) whereas CYP3A7 differs from CYP3A4 only in residues 262 (Lys versus Glu) and 263 (Glu versus Asp). Changing both amino acid residues from the CYP3A4 sequence to the corresponding two amino acid residues in the CYP3A5 or CYP3A7 sequence resulted in complete loss of the ability of these modified peptides to reverse antibody inhibition. Alteration of any one of the residues, namely residues 262, 263, and 264 in the CYP3A4 sequence, would cause the peptides either not to interact with the antibody or to interact with the antibody with reduced affinity and to a lesser extent. Thus, it is clear that Glu, Asp, and Thr in the 7-amino acid sequence of CYP3A4 are critical determinants of selectivity among CYP3A4, CYP3A5, and CYP3A7. This remarkable specificity is also confirmed by the observation that this antibody only inhibited CYP3A4-, but not CYP3A5- and CYP3A7-mediated testosterone 6β-hydroxylation and midazolam 1′- and 4-hydroxylation. Because anti-CYP3A4 antibody inhibited testosterone and midazolam metabolism in adult liver microsomes by more than 90% but failed to inhibit the metabolism of these two compounds in fetal liver microsomes, one can conclude that CYP3A4 is the predominant enzyme responsible for the metabolism of testosterone and midazolam in adult liver, but not in fetal liver. Because CYP3A7 is the most abundantly expressed cytochrome P-450 in fetus, presumably, testosterone and midazolam metabolism are catalyzed by this isoform in fetal liver microsomes.
Highly specific and strong inhibitory antibodies produced against either purified cytochrome P-450 or a unique peptide sequence of a particular cytochrome P-450 are valuable tools in drug metabolism studies (Thomas et al., 1986; Gelboin, 1993; Adams et al., 1997), especially if selective chemical inhibitors are not available. Because most antipeptide antibodies are not inhibitory or only partially inhibitory (Myers et al., 1990; Cribb et al., 1995; Laurenzana et al., 1995; Nakamura et al., 1995a,b), it remains a challenge to find ways to consistently generate inhibitory antibodies against specific peptides.
Acknowledgments
The authors thank Mr. Rick Mumford and Dr. Paul Thomas for their valuable suggestions and helpful discussions; Dr. Judy Raucy for providing human liver microsomes; Dr. Jerome Lasker for antibody against purified CYP3A4; Dr. Fred Guengerich for providing E. coli cell membranes expressing CYP3A isoforms in the initial study; and Ms. Terry Rafferty for preparation of the manuscript.
Footnotes
-
Send reprint requests to: Regina W. Wang, Department of Drug Metabolism, RY80-D100, Merck Research Laboratories, P.O. Box 2000, Rahway, NJ 07065. E-mail: regina_wang{at}merck.com.
-
Part of this work was presented previously at the Experimental Biology 98 in San Francisco, CA.
-
↵1 The abbreviation used is: HPLC, high-pressure liquid chromatography.
- Received June 4, 1998.
- Accepted September 17, 1998.
- The American Society for Pharmacology and Experimental Therapeutics