Abstract
This study in human liver microsomes was undertaken to establish whether paroxetine stereoselectively inhibits the oxidative metabolism of metoprolol in vitro, and whether the in vivo observed magnitude of the paroxetine-metoprolol interaction was predictable from these in vitro data. Two distinct approaches were used: inhibitory effect of paroxetine on 1) the formation of α-hydroxymetoprolol andO-desmethylmetoprolol from the individual metoprolol enantiomers and 2) on the depletion of the enantiomers from the incubation mixture. Nonspecific binding of both metoprolol and paroxetine to human liver microsomes was also investigated. Whereas metoprolol displayed negligible binding, paroxetine was extensively bound to microsomal proteins. This was taken into account in order to obtain unbiased Ki values and unbound concentrations of paroxetine. In the substrate depletion experiments, the intrinsic clearance (CLint) of (R)-metoprolol was larger than that of (S)-metoprolol. Paroxetine caused a concentration-dependent decrease in CLint of both enantiomers and abolished the stereoselectivity. In the metabolite formation experiments paroxetine did not stereoselectively affect α-hydroxylation, but preferentially inhibited theO-demethylation of the (R)-enantiomer versus the (S)-enantiomer. The use of unbound paroxetine concentrations in the two in vitro methods yielded comparable predicted increases in area under the curve (1.7–1.9 and 2.2–2.5 for (S)- and (R)-metoprolol, respectively) but underestimated the in vivo observed changes of about 7- and 10-fold, respectively. In conclusion, this study showed that paroxetine abolishes the stereoselective metabolism of metoprolol due to a stereoselective inhibition of the O-demethylation toward (R)-metoprolol. Furthermore, the extent of the in vivo metoprolol-paroxetine interaction was substantially underestimated by either one of the two in vitro approaches used when a competitive mechanism was assumed.
Metoprolol is a cardioselective β-blocker, clinically used in the treatment of hypertension, angina pectoris, and arrhythmia. The drug is marketed as a racemic mixture, but its pharmacological effect resides in the (S)-enantiomer (Lennard et al., 1986). Metoprolol is metabolized through α-hydroxylation (10% of dose),O-demethylation (65% of dose), andN-dealkylation (10% of dose) (Borg et al., 1975). In vitro data indicate that α-hydroxylation is catalyzed almost entirely, andO-demethylation partially, via CYP2D6 (Otton et al., 1988). Furthermore, it is well established that the pharmacokinetics of metoprolol are stereoselective: after oral administration of the racemate, extensive metabolizers (EMs1) for CYP2D6 clear the (R)-enantiomer more rapidly than the (S)-enantiomer; in poor metabolizers, this stereoselectivity is not observed (Lennard et al., 1983). In vitro data have indicated that the stereoselectivity in EM subjects is the result of the high-affinity component of the O-demethylation, presumably CYP2D6, exhibiting significant enantioselectivity for the (R)-enantiomer (Kim et al., 1992).
We showed recently that multiple-dose paroxetine intake affects the stereoselective pharmacokinetics and pharmacodynamics of metoprolol in healthy volunteers (Hemeryck et al., 2000). Paroxetine caused a substantial increase in the area under the curve of both (R)- and the pharmacologically active (S)-enantiomer, and it abolished the stereoselective aspects of metoprolol clearance. This in vivo interaction was anticipated based on the results of an in vitro study demonstrating that paroxetine is a potent competitive inhibitor of the oxidative metabolism of racemic metoprolol in human liver microsomes (Belpaire et al., 1998).
The aims of the present in vitro study with human liver microsomes were 2-fold. The first aim was to establish whether the microsomal oxidative metabolism of metoprolol was stereoselectively inhibited by paroxetine. Two distinct approaches were used: study of paroxetine's inhibitory effect on the formation of α-hydroxymetoprolol (α-OHM) andO-desmethylmetoprolol (O-DMM) on one hand, and on the depletion of the metoprolol enantiomers from the incubation mixture on the other hand. The second aim of this study was to see whether the in vitro methods could quantitatively predict the interaction between the metoprolol enantiomers and paroxetine observed in healthy volunteers (Hemeryck et al., 2000).
Materials and Methods
Chemicals and Reagents.
(R)- and (S)-metoprolol hydrochloride, (RS)-metoprolol succinate, α-OHM, and O-DMM were kind gifts from Astra Hässle AB (Mölndal, Sweden). Paroxetine hydrochloride (GlaxoSmithKline, Welwyn, UK) was obtained from the manufacturer. Atenolol, nadolol, dibucaine, β-NADP, and d-glucose 6-phosphate were purchased from Sigma Chemical Co (St. Louis, MO). Glucose-6-phosphate dehydrogenase was purchased from Roche Molecular Biochemicals (Mannheim, Germany).
Human Liver Microsomes.
Samples of five histological normal livers (HL 1, 5, 6, 7, and 9) were obtained from brain dead liver transplant donors. The Ethics Committee of the Ghent University Hospital granted ethical approval for these experiments. The five organ donors (aged 12, 19, 28, 62, and 32 years old) were genotyped as EMs with respect to CYP2D6. Liver samples were sliced in portions and placed in vials, frozen in liquid nitrogen, and stored at −80°C until preparation of microsomes by differential ultracentrifugation (Amar-Costesec et al., 1974). The microsomal pellets were suspended in a 0.05 M potassium phosphate buffer (pH 7.4). The protein content of the microsomal preparations was determined by the method of Bradford (1976). Microsomal preparations were aliquoted, frozen, and stored at −80°C until use. From these five microsomal preparations, a pool was prepared for use in the substrate depletion experiments.
Incubation Conditions.
Metabolite formation experiments
(R)- and (S)-metoprolol were incubated with microsomal protein (1 mg/ml; five individual donors) and glucose-6-phosphate dehydrogenase (2 U/ml) in 100 mM potassium phosphate buffer (pH 7.4) to a final incubation volume of 500 μl. After preincubation (5 min at 37°C), the reaction was initiated by addition of 50 μl of NADPH-generating system [NADP (1 mM), glucose 6-phosphate (10 mM), and MgCl2 (5 mM), final concentrations]. Incubations were performed at 37°C in a shaking water bath for 20 min; the reaction was terminated by addition of 25 μl of NaOH 5 N.
Preliminary experiments indicated that the formation of α-OHM andO-DMM from 40 μM (RS)-metoprolol, was linear up to 1 mg/ml microsomal protein and up to 30 min of incubation time.
Substrate depletion experiments.
The incubation conditions were those described for the metabolite formation experiments, except for the following changes: (R)- and (S)-metoprolol were incubated with 2 mg/ml of microsomal protein (pooled human liver microsomes); after 1 min of preincubation, incubations were done up to 60 min of incubation time, and the reaction was stopped by addition of 25 μl of HClO4, 60%.
Preliminary experiments showed that the disappearance of 2 μM racemic metoprolol was log-linear up to 60 min of incubation time for microsomal protein concentrations up to 2 mg/ml.
Measurement of Nonspecific Binding of Metoprolol and Paroxetine to Microsomes.
Nonspecific binding of metoprolol and paroxetine to human liver microsomes was determined by equilibrium dialysis using a Dianorm apparatus (Dianorm, Münich, Germany) with Teflon dialysis cells of 0.2-ml capacity per side and a cellophane dialysis membrane (Visking, Medicell Int. Ltd., London, UK) with a molecular cutoff of 12,000 to 14,000 Da. The cells were rotated in a water bath maintained at 37°C at 10 rpm for 3 h. Preliminary experiments indicated that equilibrium was reached in the 2-h dialysis time.
For metoprolol (2 μM), the binding was investigated at a microsomal protein concentration of 2 mg/ml. For paroxetine, the binding was measured at varying paroxetine concentrations (10–750 μM) in pooled human liver microsomes at a fixed microsomal protein concentration (1 mg/ml) for the determination of the binding parameters. Likewise, at a fixed paroxetine concentration (10 μM), in the linear portion of the binding curve, the unbound paroxetine fraction was measured at microsomal protein concentrations of 0.5, 1, and 2 mg/ml. The mixtures consisting of paroxetine, microsomes, and potassium phosphate buffer (0.05 M, pH 7.4) were subjected to equilibrium dialysis versus an equal volume of buffer.
Based on the monophasic Scatchard plots, a standard saturable binding model with one binding component was fitted to the data of paroxetine (McLure et al., 2000) (WinNonlin, version 1.5, Pharsight Corp., Mountain View, CA):
HPLC Assay of α-Hydroxy- andO-Desmethylmetoprolol.
Analyses of α-OHM and O-DMM were performed with a modified HPLC method according to Otton et al. (1988). After incubation and termination of the reaction by addition of NaOH, 100 to 450 μl of the supernatant was transferred to a glass tube and 50 μl of the internal standard (atenolol, 2 ng/μl in water) and 3.0 ml of a dichloromethane-butanol mixture (85:15, v/v) were added. After vortexing and centrifugation, the organic phase was transferred onto a fresh tube and evaporated under a gentle stream of nitrogen. The residue was dissolved in a 100-μl mobile phase of which 60 μl was injected onto the column. The HPLC system consisted of a Gilson 307 pump and a Gilson 234 automatic injector (Gilson, Middelton, WI) with a 100-μl loop (Rheodyne, Cotati, CA), a model 980 programmable fluorescence detector (Applied Biosystems, Ramsey, NJ), and a HP 3395 integrator (Hewlett Packard, Palo Alto, CA). A Symmetry C18 column (150 mm × 3.0-mm i.d., 5 μm) with a Symmetry C18 Sentry Guard column (20 mm × 3.0-mm i.d., 5 μm) (Waters, Milford, MA) was used to separate α-OHM, O-DMM, and the internal standard at ambient temperature. The mobile phase consisted of a water-acetonitrile mixture (91:9, v/v) containing 1% (v/v) triethylamine adjusted to pH 3 with orthophosphoric acid, and was pumped at a flow rate of 0.6 ml/min. Detection was by fluorescence at an excitation wavelength of 193 nm without emission filter.
Calibration curves were constructed by spiking microsomes not containing the NADPH-generating system with α-OHM (10–200 ng/ml) andO-DMM (50–1000 ng/ml). Peak height ratios were determined using the internal standard method. Linearity of the α-OHM andO-DMM calibration curves was assessed over the concentration range using least-squares regression analysis.
For α-OHM and O-DMM, the within-day coefficient of variation (CV) (n = 6) at high (160 and 800 ng/ml for α-OHM and O-DMM, respectively), medium (100 and 500 ng/ml, respectively), and low (20 and 60 ng/ml, respectively) concentrations were all below 15%, and the mean values were all within ±15% deviation of the nominal value. For between-day variability (n = 16), the CV values at high, medium, and low concentrations were all below 13% and the mean values were all within ±6% deviation of the nominal value. At the lower limit of quantification (10 and 50 ng/ml for α-OHM and O-DMM, respectively), the within-day CV values (n = 6) were less than 5% and the mean values were within ±16% deviation of the nominal value.
Trace impurities of α-OHM and O-DMM in the metoprolol enantiomers presented less than 2% of the α-OHM and O-DMM formation at the highest concentration of the enantiomers tested (3 mM) in the metabolite formation experiments.
HPLC Assay of Metoprolol.
Metoprolol was determined by HPLC with fluorescence detection. After incubation and termination of the reaction by HClO4 60%, 15 μl of the internal standard (nadolol, 10 ng/μl in water) was added, and the pH was adjusted to 2 to 3 by addition of KOH 5 N. Subsequently, 500 μl of hexane was added, followed by brief vortexing to remove impurities. After centrifugation, the organic layer was aspirated and 100 μl of the sample was injected onto the column.
The HPLC system consisted of a Varian 9010 solvent delivery system (Varian, Sunnyvale, CA), an automatic injector with a 100-μl loop (AS 2000 A, Merck, Darmstadt, Germany), a HP 1046 A fluorescence detector, and a HP 3395 integrator (Hewlett Packard). Metoprolol and the internal standard, nadolol, were separated at ambient temperature using a Supelcosil LC-8-DB column (150 mm × 4.0-mm i.d., 5 μm) with a Supelguard LC-8-DB Guard column (20 mm × 4.0-mm i.d., 5 μm) (Supelco Inc., Bellafonte, PA). The mobile phase consisted of 50 mM potassium dihydroxyphosphate/acetonitrile mixture (86:14, v/v), containing 1% triethylamine, adjusted to pH 3 with orthophosphoric acid, and was delivered at a flow rate of 1 ml/min. Detection was done by fluorescence at excitation and emission wavelengths of 220 and 295 nm, respectively.
A metoprolol calibration curve ranging from 0.5 to 2 μM was constructed by spiking microsomes not containing the NADPH-generating system. Peak height ratios were determined using the internal standard method. Linearity of the calibration curve was assessed over the concentration range using least-squares regression analysis.
The within-day CV values (n = 6) at high (1.8 μM), medium (1.5 μM), and low (0.6 μM) metoprolol concentrations were all below 4%, and the mean values were all within ±6% deviation of the nominal value. For between-day variability (n = 6), the CV values at high, medium, and low concentrations were all below 10%, and the mean values were all within ±5% deviation of the nominal value. At the lower limit of quantification (0.5 μM), the within-day CV value (n = 6) was less than 5%, and the mean values were within ±2% deviation of the nominal.
HPLC Assay of Paroxetine.
Paroxetine was determined with an HPLC assay as described by Hemeryck et al. (2000), except for the following changes. For the buffer compartments of the dialysis cells, 10 μl of the internal standard (dibucaine; 1 mg/ml in water) was added to 120 μl of appropriately diluted sample. One hundred microliters was subjected to HPLC analysis. Calibration curves in buffer, constructed over the range of 1 to 10 μM, were linear. For the microsomal compartments of the dialysis cells, 10 μl of HClO4 was added to the appropriately diluted sample. After brief vortexing and centrifugation, 10 μl of the internal standard and 10 μl of KOH 5 N were added. Again, the sample was vortexed and centrifuged, and 100 μl was injected onto the HPLC column. Calibration curves with microsomal protein (1 mg/ml) ranging from 2.5 to 10 μM were linear.
The within-day CV values (n = 6) at high (8 μM for buffer and microsomal samples) and low (0.6 and 3 μM for buffer and microsomal samples, respectively) paroxetine concentrations were all below 6%, and the mean values were all within ±11% deviation of the nominal value. For between-day variability (n = 3), the CV values at high and low concentrations were all below 10%, and the mean values were all within ±13% deviation of the nominal value. At the lower limit of quantification (0.5 and 2.5 μM for buffer and microsomal samples, respectively), the within-day CV value (n = 6) was less than 7% and the mean values were within ±7% deviation of the nominal.
Inhibition Experiments.
Metabolite formation experiments
α-OHM and O-DMM formation rates were measured at (R)- and (S)-metoprolol concentrations in the range of 10 to 3000 μM in the five human liver microsomes (HL 1, 5, 6, 7, and 9). The effects of 0.1, 0.3, 1, 3, 10, and 30 μM paroxetine as inhibitor on the formation of α-OHM and O-DMM were examined at concentrations of 20, 40, and 80 μM (R)- and (S)-metoprolol, respectively, in the same five preparations.
Formation of α-OHM and O-DMM was expressed as picomoles per minute per milligram of protein. For both metabolites and in the absence of inhibitor (control curves), a two-enzyme Michaelis-Menten equation was fitted to the data points consisting of the metabolite formation rate at varying substrate concentrations by nonlinear regression analysis using a proportional weighing factor of 1/Yobs2 (WinNonlin, version 1.5, Pharsight Corp.). The use of the proportional weighing factor was justified based on an improvement of goodness-of-fit as evaluated by the Akaike Information Criterion (AIC) (Akaike, 1976).
Apparent inhibition constants (Ki,app) of paroxetine for the α-hydroxylation and O-demethylation of the metoprolol enantiomers were calculated using a modified two-enzyme Michaelis-Menten model with competitive inhibition for the high-affinity component at three different substrate concentrations (20, 40, and 80 μM) by nonlinear regression analysis:
Initial estimates for Km1,Vmax1, and L(=Vmax2/Km2) were provided by the kinetic parameters obtained from the control curves. The choice of this model was based on the following criteria: 1) lowest AIC, 2) lowest standard errors on the computed parameters, and 3) how well the Km1,Vmax1, and L estimates with inhibitor agreed with those estimated in the absence of inhibitor. The mean Ki,app values were corrected for futile binding to microsomes. When Ki,app≪ KD, theKi,app can be converted to a “true” inhibition constant (Ki), according to the following equation:
Substrate depletion experiments.
(R)- and (S)-metoprolol were incubated with pooled human liver microsomes at a concentration of 2 μM for 0, 10, 20, 40, and 60 min. For these experiments, pooled human liver microsomes were used as a microsomal preparation with “average” metabolic activity as it was found that certain individual preparations displayed insufficient metabolic activity to allow the estimation of the in vitro t1/2. The concentration of the enantiomers (2 μM) was much lower than theKm value estimated for metoprolol α-hydroxylation and O-demethylation, from which can be expected that this substrate concentration does not saturate the metabolism of metoprolol. Paroxetine was tested at three different concentrations (0.5, 1, and 2 μM, added concentrations) for its inhibitory effect on the disappearance rate of (R)- or (S)-metoprolol from the incubation mixture. The amounts of (R)- and (S)-metoprolol remaining at the different time points was expressed as a percentage of the amount att = 0 min. The slope (=−k) of the log remaining substrate versus incubation time, calculated by least-squares regression analysis, was used to calculate the in vitrot1/2 according to the following: in vitrot1/2 = −0.693/k.
The total added paroxetine concentrations were converted to unbound concentrations by multiplication of thefu,mic at 2 mg/ml. This is justified on the basis that these added concentrations were much smaller than the estimated KD for nonspecific binding of paroxetine to microsomes.
Prediction of the in Vivo Metoprolol-Paroxetine Interaction from in Vitro Data.
Based on metabolite formation experiments
In case of both competitive and or noncompetitive inhibition, the ratio of the in vitro CLint in the presence and the absence of the inhibitor can be expressed by the following equation, when [S] ≪ Km (Ito et al., 1998):
Assuming that the protein binding of metoprolol is not affected by paroxetine coadministration, the AUC ratio in the presence and absence of inhibitor can be calculated as follows (Ito et al., 1998):
As paroxetine could be subject to active uptake in the hepatocytes, the calculations were also done by setting [I]uequal to the total paroxetine plasma concentrations, as a highly conservative estimate of the concentration available to inhibit CYP2D6.
Based on substrate depletion experiments.
The in vitro CLint of the metoprolol enantiomers in the absence and in the presence of inhibitor was estimated using the following equation (Obach, 1999):
For orally administered drugs whose clearance is mainly dependent upon hepatic metabolism, the AUC equalsD/fu · CLint (Lin and Lu, 1997). Assuming that the protein binding of metoprolol is not affected by paroxetine coadministration, the AUC ratio in the presence and in the absence of inhibitor can theoretically be predicted by:
Statistical Analysis.
All results are expressed as mean ± S.D. Statistically significant differences in kinetic parameters andKi values between (R)- and (S)-metoprolol were tested by means of a t test for paired samples (Statistica '99 for Windows, StatSoft, Inc., Tulsa, OK).
Results
Nonspecific Binding of the Drugs under Study.
Metoprolol, at a concentration of 2 μM, and at a microsomal protein concentration of 2 mg/ml, displayed negligible binding (<10%) to the microsomes. Consequently, neither the added metoprolol concentrations nor the kinetic parameters determined in this study need to be corrected for futile binding.
Paroxetine, however, was substantially bound to the microsomal proteins. The increase in unbound fraction (fu,mic) occurring with the increase in total drug concentration indicated a saturable binding. The Scatchard plots were linear, indicating a saturable binding with a single binding component (data not shown). At a microsomal protein concentration of 1 mg/ml, the KD andBmax values were 48 ± 20 and 410 ± 64 μM, respectively.
At 10 μM paroxetine, the fu,mic was 0.17 ± 0.01, 0.10 ± 0.02, and 0.08 ± 0.01, at a microsomal protein concentration of 0.5, 1, and 2 mg/ml respectively.
Inhibition Experiments.
Metabolite formation experiments
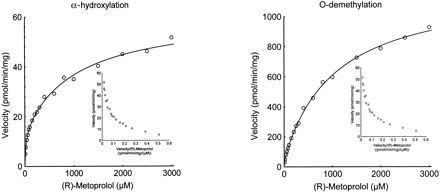
Formation of both α-OHM and O-DMM from (R)- and (S)-metoprolol displayed biphasic kinetics. Figure1 shows representative velocity versus substrate plots of α-OHM and O-DMM from (R)-metoprolol for microsomes from HL 1. The corresponding Eadie-Hofstee plots are shown as an insert. The mean (±S.D.) estimates of the apparent Michaelis-Menten constants and the intrinsic clearances of the α-hydroxylation and O-demethylation of the metoprolol enantiomers in five human liver microsomes are given in Table 1. There were no statistically significant differences between the two enantiomers inVmax and Kmvalues of the high- and low-affinity enzymes catalyzing α-hydroxylation. For the O-demethylation, statistically significant differences between the two enantiomers were found for theVmax values of the high- and low-affinity enzymes but not for the Km values. TheVmax of the high-affinity enzyme was higher for the (R)-enantiomer, whereas the reverse was true for the low-affinity enzyme. The mean (±S.D.) CLintvalues of the high-affinity component catalyzing metoprolol α-hydroxylation were 0.58 (±0.33) and 0.65 (±0.28) μl/min/mg for the (R)- and (S)-enantiomer (nonsignificant), respectively. For theO-demethylation pathway the values were 3.11 (±1.65) and 1.41 (±0.57) μl/min/mg (p < 0.05), respectively.
Representative velocity versus substrate plots of α-OHM (left) and O-DMM (right) formation from (R)-metoprolol by microsomes from HL 1.
The corresponding Eadie-Hofstee plots are shown as an insert. A two-enzyme Michaelis-Menten equation was fitted to the data.
Estimates of the mean (±S.D.) apparent kinetic parameters of α-hydroxymetoprolol and O-desmethylmetoprolol formation from (R)- and (S)-metoprolol in five human liver microsomal preparations (HL 1, HL 5, HL 6, HL 7, and HL 9)
Paroxetine was tested as an inhibitor of α-hydroxylation andO-demethylation of the metoprolol enantiomers in five human livers. Estimates of the apparent inhibition constants (Ki,app) and the average inhibition constants of paroxetine corrected for futile binding (Ki), on both reactions and both enantiomers, are presented in Table 2. Whereas no stereoselective inhibition of paroxetine was seen for the high-affinity enzyme catalyzing α-hydroxylation, a trend (p = 0.054) toward stereoselective inhibition of the high-affinity component involved in metoprololO-demethylation was observed: paroxetine preferentially inhibited (R)-metoprolol O-demethylation in all four livers for which evaluation was possible. In one liver, HL 6, noKi values of paroxetine on theO-demethylation pathways could be determined due to insufficient inhibition by paroxetine (the maximum inhibition was only about 28% at the highest paroxetine concentration tested).
Apparent inhibition constants (Ki,app) and inhibition constants of paroxetine corrected for futile binding (Ki), for inhibition of (R)- and (S)-metoprolol α-hydroxylation and O-demethylation in five human liver microsomal preparations (HL 1, HL 5, HL 6, HL 7, HL 9) (metabolite formation experiments)
Substrate depletion experiments.
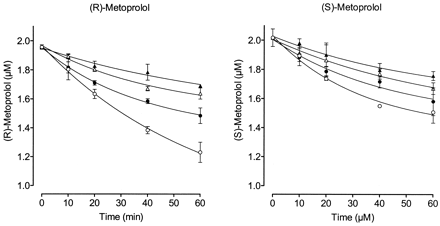
The decline in metoprolol enantiomer concentrations over time during incubation followed a mono-exponential decay. Figure2 depicts the disappearance of the individual metoprolol enantiomers from the incubation mixture as a function of incubation time in the absence and in the presence of 0.5, 1, and 2 μM paroxetine (total added concentrations). With afu,mic = 0.08 at 2 mg/ml microsomal protein concentration, unbound paroxetine concentrations of 0.04, 0.08, and 0.16 μM, respectively, were obtained. The CLintin the absence of inhibitor was larger for (R)-metoprolol than for (S)-metoprolol (Table3). Paroxetine caused a concentration-dependent decrease in CLint of both enantiomers. With increasing paroxetine concentrations, the (R)/(S) CLint ratio decreased and ultimately approached unity.
Decline in metoprolol enantiomer concentrations as a function of incubation time in pooled human liver microsomes.
Mean ± S.D of three independent determinations are presented. A mono-exponential function was fitted to the data in the absence (○) and in the presence of 0.04 (●), 0.08 (▵), and 0.16 (▴) μM unbound paroxetine in the incubation mixture.
Mean (±S.D.) intrinsic clearance of (R)- and (S)-metoprolol in pooled human liver microsomes in the absence and in the presence of 0.5, 1, and 2 μM paroxetine (total added concentrations) (substrate depletion experiments; three independent determinations)
Prediction of the in Vivo Metoprolol-Paroxetine Interaction from in Vitro Data.
Table 4 shows the predicted AUC ratios for the metoprolol enantiomers (as calculated by eq. 6) based on mean inhibition constants corrected for futile binding (Ki), and estimated (according to eq. 5) maximum unbound and total plasma concentrations of paroxetine ([I]) versus the observed AUC ratios from our study in healthy volunteers (Hemeryck et al., 2000). The in vivo observed increase in enantiomer AUC caused by paroxetine intake was substantially underestimated as well in the case of unbound as in the case of total paroxetine concentrations.
Prediction of the in vivo interaction between metoprolol and paroxetine, based on in vitro inhibition constants of paroxetine corrected for futile binding (Ki) and the estimated in vivo hepatocytosolic inhibitor concentrations ([I])
In Table 5 the AUC ratios predicted according to eq. 8 are presented, based on substrate depletion data in the absence and in the presence of 0.08 μM unbound paroxetine. This paroxetine concentration approximates the theoretically expected unbound paroxetine concentration in vivo (eq. 5). Here, too, the predicted AUC ratios substantially underestimated the in vivo observed changes.
Prediction of the in vivo interaction between metoprolol and paroxetine, based on in vitro substrate depletion experiments with (R)- and (S)-metoprolol in the absence and in the presence of 0.08 μM unbound paroxetine in the incubation mixture
Discussion
The purpose of the present study was 2-fold: in the first part we examined whether paroxetine exerted a stereoselective effect on the oxidative metabolism of metoprolol in human liver microsomes. The second part of this study was concerned with evaluating two different in vitro approaches to predict the extent of the in vivo observed changes.
In this study, the nonspecific binding to human liver microsomes of both substrate and inhibitor was taken into account. Several recent papers advocated that futile binding should be taken into account to obtain unbiased Km andKi values (Obach, 1996, 1999; Obach et al., 1997; McLure et al., 2000; Venkatakrishnan et al., 2000), in particular for in vitro-in vivo extrapolation purposes. Metoprolol displayed negligible binding to microsomes, a finding that is compatible with the observations of Madani et al. (1999). Paroxetine, on the other hand, was extensively bound to microsomes, consistent with the results of others who reported that, in general, lipophilic bases display important futile binding to microsomal proteins (Obach et al., 1997;Tucker, 1998; Obach, 1999; McLure et al., 2000). For paroxetine, the added (total) inhibitor concentrations and theKi,app values were therefore corrected for nonspecific binding.
To assess a possible stereoselective inhibition, the effect of paroxetine was studied both on the disappearance of the individual enantiomers and on the formation of α-OHM and O-DMM from the metoprolol enantiomers.
The substrate depletion experiments showed that the (R)-metoprolol disappeared faster from the incubation mixture than the (S)-enantiomer, a result consistent with in vivo data demonstrating that, in extensive metabolizer subjects, the (R)-enantiomer is more rapidly cleared (Lennard et al., 1983).
The apparent kinetic parameters of both oxidative pathways obtained from the metabolite formation data were, in general, in keeping with previously published results, although somewhat lowerVmax values were found (Kim et al., 1992). No distinct enantioselectivity for the high-affinity components involved in α-hydroxylation of the individual enantiomers could be observed, whereas this was not true for the O-demethylation: the intrinsic clearances were significantly greater for the (R)-enantiomer compared with those obtained for the (S)-enantiomer, attributable to differences inVmax. Furthermore, the intrinsic clearances obtained from the substrate depletion experiments were comparable with those from the metabolite formation experiments, calculated as the sum of the intrinsic clearances of the high-affinity α-hydroxylase andO-demethylase enzymes. This suggests that mainly the high-affinity enzymes, presumably CYP2D6, involved in both reactions, account for virtually all catalytic activity in the substrate depletion experiments.
Paroxetine affected the stereoselective metabolism of metoprolol in human liver microsomes. Increasing concentrations of paroxetine reduced, and ultimately abolished, the stereoselectivity of the disappearance of the metoprolol enantiomers from the incubation mixture. Metabolite formation experiments indicated that the stereoselective inhibition by paroxetine of the in vitro metoprolol metabolism is not due to stereoselective inhibition of the α-hydroxylation pathway, but is caused by a preferential inhibition of (R)-metoprolol O-demethylation. These findings are compatible with the in vivo observation that multiple-dose paroxetine abolishes the stereoselective metabolism of metoprolol (Hemeryck et al., 2000). A similar stereoselective effect was seen for verapamil on the metabolism of pseudoracemic metoprolol in human liver microsomes (Kim et al., 1992).
The second part of this study evaluated whether the in vivo observed increase in AUC (Hemeryck et al., 2000) was predictable from in vitro data. For that purpose, two distinct approaches for the prediction of the in vivo paroxetine-metoprolol interaction were evaluated. The first method used the predictive model proposed by Ito et al. (1998) in which in vitro determined Ki values are combined with theoretically estimated in vivo inhibitor concentrations. The second method is based on the in vitro half-life from substrate depletion experiments, from which in vitro intrinsic clearances of the metoprolol enantiomers can be estimated in the absence and in the presence of inhibitor. The in vitro half-life approach has already been applied for the prediction of human drug clearances (Obach et al., 1997; Obach, 1999), but can theoretically be used for estimating the overall intrinsic clearance of a drug in the absence and in the presence of a specific inhibitor concentration. The ratio of the intrinsic clearance in the absence and the presence of a specific inhibitor concentration can then be used as a predictor of the in vivo increase in AUC. This approach is only valid when the drug under study is orally administered and its clearance is mainly dependent on hepatic metabolism (Lin and Lu, 1998).
One of the controversies is whether total or unbound plasma concentrations of inhibitor should be used (Bertz and Granneman, 1997;Ito et al., 1998; Lin and Lu, 1998; Tucker, 1998; von Moltke et al., 1998). Ito et al. (1998) proposed the use of the maximum theoretically unbound inhibitor concentration in the plasma for the estimation of the unbound inhibitor concentration available for inhibition of enzyme activity in vivo. This is in accordance with the basic tenet of pharmacokinetics that only unbound drug diffuses across the hepatic membranes. As paroxetine might be actively transported into the hepatocytes, we also considered the use of total paroxetine plasma concentrations for the setting of [I]u in the predictive model. However, this only marginally improved the predictions made.
For the substrate depletion experiments, an unbound paroxetine concentration of 0.08 μM was considered, since this concentration approaches the maximum attainable unbound paroxetine concentration in the plasma (calculated according to eq. 5).
The extent of the in vivo interaction between the metoprolol enantiomers and paroxetine was substantially underestimated with both approaches. Even when total paroxetine plasma concentrations were used in the predictive model, the magnitude of the interaction was markedly underestimated.
Several in vitro and in vivo issues could confound the predictions made (Bertz and Granneman, 1997; Ito et al., 1998; Lin and Lu, 1998; Tucker, 1998; von Moltke et al., 1998). One of the in vitro problems involves futile binding of the inhibitor to the human liver microsomes. In our study, this was taken into account.
In vivo, the presence of inhibitory metabolites of paroxetine could have contributed to the extent of inhibition observed. The M2 metabolite in particular has been shown to be a strong CYP2D6 inhibitor in vitro (Crewe et al., 1992). However, this metabolite is unlikely to contribute to the overall inhibition by paroxetine, since in vivo it is rapidly conjugated and excreted in the urine (Sindrup et al., 1992;Hiemke and Härtter, 2000). It could also be that paroxetine's inhibitory effect is not restricted to the liver. Indeed, for drugs with a substantial first pass metabolism, the systemic bioavailability is the product of the fractions of the dose absorbed and surviving intestinal and hepatic metabolism. In extensive metabolizers for CYP2D6, metoprolol is a medium- to high-clearance drug, exhibiting a substantial first pass effect (Lennard et al., 1986). However, it is unlikely that there is an important contribution to the overall inhibition by paroxetine at the level of the gut since CYP2D6 expression in human small intestine is very low compared with that in human liver (Madani et al., 1999), suggesting that paroxetine's inhibitory effect is restricted to the hepatic extraction of metoprolol.
Other cytochromes, aside from CYP2D6, involved in metoprolol metabolism in vivo could possibly also be inhibited by paroxetine. This is not very likely because paroxetine has only minimal inhibitory effects on the other major cytochrome P450 isoforms (Greenblatt et al., 1999).
Another reason for the discrepancy between the predicted and the observed increase in the AUC of metoprolol after paroxetine treatment could be that the mechanism of inhibition is not purely competitive, as is generally accepted. Indeed, competitive inhibition does not preclude mechanism-based inhibition, but this has not been tested for paroxetine. In a preliminary experiment, we evaluated this possibility using human liver microsomes from one donor. Paroxetine, in the presence of a NADPH-generating system, produced a preincubation time-dependent loss of α-hydroxylation and, to a lesser extent,O-demethylation activity of metoprolol, suggesting mechanism-based inhibition (data not shown). Additional experiments, also with other CYP2D6 substrates, are required to confirm and further characterize this mechanism of inhibition. In the meantime, mechanism-based inhibition is a possible explanation for the underestimation of the extent of interaction in vivo.
A possible limitation for the prediction of the in vivo observed extent of AUC increase after multiple paroxetine intake is related to interindividual variability. The mean (±S.D.) baseline log of the metoprolol metabolic ratios of our volunteers was −0.88 ± 0.31, indicating that the subjects in our study (Hemeryck et al., 2000) can be classified as rather rapid extensive metabolizers in the Caucasian population (McGourty et al., 1985). The fact that our subjects had high CYP2D6 activity could explain why the inhibition is so substantial. It has indeed often been reported that the effect of a metabolic inhibitor in the individuals with the highest clearance results in the largest effects (Bertz and Granneman, 1997).
Only one paper reported on the in vitro-in vivo extrapolation of interaction data between paroxetine and other drugs, i.e., desipramine (von Moltke et al., 1995). In this report, the hepatocytosolic inhibitor concentration was estimated based on total paroxetine plasma concentrations and in vitro liver:water partition ratios. This resulted in a reasonably accurate prediction of the interaction. However, there are major concerns regarding this approach, and since its theoretical basis is unclear, the satisfactory results of the prediction could be fortuitous (Tucker, 1998).
In conclusion, our study showed that the in vivo observed stereoselective inhibitory effect of paroxetine on the clearance of the metoprolol enantiomers is caused by stereoselective inhibition of theO-demethylation toward (R)-metoprolol. The work presented here also shows that the extent of the in vivo metoprolol-paroxetine interaction, based on the assumption of a competitive inhibition mechanism, was substantially underpredicted by either one of the two in vitro approaches used in this study.
Acknowledgments
We thank Prof. Dr. B. Hemptinne and M. Van der Vennet of the Ghent University Hospital Liver Transplantation Team. Critical review of the manuscript by Prof. Dr. M. G. Bogaert is acknowledged.
Footnotes
-
Send reprint requests to: F. M. Belpaire, Heymans Institute of Pharmacology, Ghent University Medical School, 9000 Ghent, Belgium. E-mail: Frans.Belpaire{at}rug.ac.be
-
This study was supported by Grant G.0011.97 from the National Fund for Scientific Research, Belgium and by Grant 0104197 from the Ghent University Research Foundation.
- Abbreviations used are::
- EM
- extensive metabolizer
- α-OHM
- α-hydroxymetoprolol
- O-DMM
- O-desmethylmetoprolol
- HPLC
- high-performance liquid chromatography
- CV
- coefficient of variation
- AIC
- Akaike Information Criterion
- AUC
- area under the curve
- fu,mic
- unbound fraction of paroxetine in the microsomal compartment
- Ki,app
- apparent inhibition constant
- Received August 14, 2000.
- Accepted January 12, 2001.
- The American Society for Pharmacology and Experimental Therapeutics