Abstract
The objective of the present study was to examine the accuracy of using unbound brain concentration determined by a brain homogenate method (Cub), cerebral spinal fluid concentration (CCSF), and unbound plasma concentration (Cup) as a surrogate for brain interstitial fluid concentration determined by brain microdialysis (Cm). Nine compounds—carbamazepine, citalopram, ganciclovir, metoclopramide, N-desmethylclozapine, quinidine, risperidone, 9-hydroxyrisperidone, and thiopental—were selected, and each was administered as an intravenous bolus (up to 5 mg/kg) followed by a constant intravenous infusion (1–9 mg/kg/h) for 6 h in rats. For eight of the nine compounds, the Cubs were within 3-fold of their Cm; thiopental had a Cm 4-fold of its Cub. The CCSFs of eight of the nine compounds were within 3-fold of their corresponding Cm; 9-hydroxyrisperidone showed a CCSF 5-fold of its Cm. The Cups of five of the nine compounds were within 3-fold of their Cm; four compounds (ganciclovir, metoclopramide, quinidine, and 9-hydroxyrisperidone) had Cups 6- to 14-fold of their Cm. In conclusion, the Cub and CCSF were within 3-fold of the Cm for the majority of the compounds tested. The Cups were within 3-fold of Cm for lipophilic non–P-glycoprotein (–P-gp) substrates and greater than 3-fold of Cm for hydrophilic or P-gp substrates. The present study indicates that the brain homogenate and cerebral spinal fluid methods may be used as surrogate methods to predict brain interstitial fluid concentrations within 3-fold of error in drug discovery and development settings.
For drugs with an intended action in the central nervous system it is assumed that unbound drug in brain interstitial fluid is in direct contact or in equilibrium with the site of action (de Lange and Danhof, 2002). Therefore, in preclinical and clinical pharmacokinetic/pharmacodynamic studies, it is critical to determine the concentration in the interstitial fluid for brain-targeted compounds. Unbound plasma concentration (Cup) has been used to represent the unbound concentration in tissue (Wilkinson, 2001). Because the brain is separated from the plasma by the blood-brain barrier (BBB) and the blood cerebrospinal fluid barrier (BCSFB), Cup may not represent the interstitial fluid concentration (Davson and Segal, 1995; Hammarlund-Udenaes et al., 2008; Liu et al., 2008).
Microdialysis has been considered as a standard approach to measure interstitial fluid concentration (Cm) (Joukhadar and Müller, 2005; Chaurasia et al., 2007). Although this technique has been developed for more than 2 decades, it is primarily used for determination of neurotransmitters and not drug concentrations in the brain. The main limitations of this technique include high resource requirements, low throughput, and special surgical skills to set up the experiment. In addition, many compounds in the discovery stage are often very lipophilic, and it is difficult to apply microdialysis technique to study these compounds because of high nonspecific binding. Importantly for ethical reasons, this method cannot be used routinely to measure the interstitial fluid concentration in clinical trials, although it has been used to monitor glucose metabolism such as lactate and pyruvate ratio as a marker for ischemia in brain trauma patients in a few life-threatening situations (Benjamin et al., 2004; Hillered et al., 2006; Chaurasia et al., 2007; Helmy et al., 2007). Because of these limitations, several alternative methods, such as brain homogenate, brain slice, and cerebral spinal fluid (CSF), have been proposed and used to estimate brain unbound drug concentrations (Liu et al., 2008).
A brain homogenate method has been proposed as a surrogate approach to estimate brain unbound concentration (Cub) (Kalvass and Maurer, 2002). In this approach the unbound fraction in brain tissue is estimated by the unbound fraction in brain homogenate, which is determined using equilibrium dialysis or ultracentrifugation. Several indirect validation studies have been published, such as comparing the projected brain to plasma concentration ratios with the observed ratios, or comparing the unbound brain fraction determined by brain homogenate method with that determined by brain slice method, or by CSF method (Kalvass and Maurer, 2002; Maurer et al., 2005; Becker and Liu, 2006; Liu et al., 2006; Summerfield et al., 2006, 2008). Recently, Fridén et al. (2007) showed that Cub predicted Cm within 3-fold of error for 10 of 15 compounds. In that study, the microdialysis for 14 of the 15 compounds was compiled from the literature, and all the data were generated using different methods or in different species.
Another commonly used method in both preclinical and clinical studies is the CSF concentration (CCSF). Although CSF is in direct contact with brain tissue and there are discontinuous gap junctions found in most areas of the ventricular ependyma, CCSF may not always represent the interstitial fluid concentration because of the convective bulk flow of brain interstitial fluid from brain tissue into CSF, and different transporters expressed at the BBB and BCSFB (de Lange and Danhof, 2002; Shen et al., 2004). However, a pragmatic question is how large is the difference between CSF concentration and the Cm.
Studies have not been reported to examine and compare the Cub, CCSF, Cup, and Cm for multiple compounds under identical experimental conditions. The objective of the present study was to compare the accuracy of using Cub, CCSF, and Cup as a surrogate to predict Cm. We selected nine model compounds representing different physicochemical properties and various P-glycoprotein (P-gp) transport activities. The results from this study will help us to understand whether the Cub, CCSF, and Cup can be used as surrogates to predict the interstitial fluid concentration in drug discovery and development setting and to identify potential caveats of these methods.
Materials and Methods
Chemicals. Carbamazepine, metoclopramide, N-desmethylclozapine, quinidine, and thiopental were obtained from Sigma-Aldrich (St. Louis, MO). Risperidone and 9-hydroxyrisperidone were obtained from SynFine Research (Richmond Hill, ON, Canada). Citalopram and ganciclovir were synthesized at Roche Palo Alto, LLC (Palo Alto, CA) with purity greater than 98%. All the other chemicals used in the experiments were of the highest available grade.
Brain Microdialysis. Jugular and femoral-cannulated male Sprague-Dawley rats (250–350 g), with a surgically implanted microdialysis guide cannula and a dummy probe (CMA/12; CMA Microdialysis, Solna, Sweden), were purchased from Charles River Laboratories, Inc. (Wilmington, MA). The guide cannula was implanted in the prefrontal cortex of rat brain using a stereotaxic instrument at 3.2 mm anteroposterior, 1.0 mm mediolateral, and 0.5 mm dorsoventral to the bregma point and secured to the skull with screws and dental cement. The animals were acclimatized to the laboratory environment for 3 to 5 days before the study. At approximately 16 h before dosing, the rats were placed into individual BASi RATURN systems (Bioanalytical Systems, Inc., West Lafayette, IN) for freely moving animals with food and water ad libitum. The dummy probes were replaced with CMA 12/2-mm probes (CMA Microdialysis) and perfused with an artificial CSF solution (147 mM NaCl, 2.7 mM KCl, and 1.2 mM CaCl2) at 1 μl/min overnight using microdialysis pumps (CMA/102; CMA Microdialysis). On the day of the study, the outlets were connected to the BASi Refrigerated Honeycom Fraction Collector (Bioanalytical Systems, Inc.) at 4°C and perfused at 1 μl/min. Rats (n = 5–6) received an intravenous bolus dose followed by intravenous infusion via the femoral cannula for carbamazepine (1.5 mg/kg and 1 mg/kg/h), citalopram (10 mg/kg and 3 mg/kg/h), ganciclovir (0 mg/kg and 3 mg/kg/h), metoclopramide (0.5 mg/kg and 1 mg/kg/h), N-desmethylclozapine (1 mg/kg and 1.33 mg/kg/h), quinidine (5 mg/kg and 9 mg/kg/h), risperidone (0.5 mg/kg and 1 mg/kg/h), and thiopental (0.35 mg/kg and 1 mg/kg/h). Compound 9-hydroxyrisperidone was monitored after dosing its parent drug risperidone (0.5 mg/kg and 1 mg/kg/h). Carbamazepine was prepared in 2-hydroxypropyl-β-cyclodextrin, and N-desmethylclozapine was prepared in 0.5% dextrose at pH 8. All the other compounds were prepared in saline. Blood samples were collected via jugular vein cannula at 0.25, 1, 2, 4, 5, and 6 h after start of infusion for ganciclovir, at 2, 4, 5, and 6 h for citalopram, and at 1, 2, 4, 5, and 6 h for the remaining compounds. Blood was then centrifuged to obtain plasma. The perfusate samples were serially collected from each animal at 0.5-h intervals from 1 h predose to 6 h postdose. At 6 h, animals were euthanized by carbon dioxide asphyxiation. Approximately 100-μl CSF samples were collected via cisterna magna puncture from each rat. Brain samples were also collected. All the samples were stored at –20°C before analysis.
In Vitro Recovery of the Microdialysis Probe. The in vivo recovery of the microdialysis probe for each compound was estimated using in vitro microdialysis by a gain method. A CMA 12/2-mm probe was immersed in the artificial CSF solution containing 100 ng/ml of testing compound in a 1.5-ml tube at 37°C and perfused with the artificial CSF solution at 1 μl/min for 4 h. The dialysate was collected every 0.5-h interval and stored at –20°C before analysis. The ratio of the concentration in the dialysate versus that in the solution was calculated as the in vitro recovery.
Protein Binding. The in vitro unbound fraction in brain homogenate and plasma for each compound was determined using a 48-well Rapid Equilibrium Dialysis device (Linden Bioscience, Woburn, MA). Brain tissue was homogenized in 2 volumes (w/v) of 0.9% saline. Brain homogenate or plasma was spiked with a compound for a concentration of 1000 ng/ml. Two-hundred microliters of the matrix was added to the donor side of a dialysis chamber. The receiver side contained 350 μl of Sorenson's buffer. The dialysis apparatus was maintained on a shaking device at 37°C for 4 h. The drug concentrations were determined as described below.
Sample Analysis. The brain tissues were homogenized in 2 volumes (w/v) of 0.9% saline. Fifty microliters of brain homogenate or plasma and 200 μl of internal standard in acetonitrile were mixed in 96-well polypropylene plates. For the protein binding samples, 25 μl of diluted brain homogenate or plasma samples was mixed with 25 μl of control buffer, and 25 μl of buffer samples was mixed with 25 μl of control brain homogenate or plasma to yield identical matrix between donor and receiver side of samples. The samples were then mixed with 150 μl of acetonitrile containing internal standard. The acetonitrile mixtures were vortexed and then centrifuged at 1800g for 10 to 15 min. Aliquots of the supernatant were transferred to a 96-well plate and diluted with equal volume of water before analysis by high-performance liquid chromatography combined with tandem mass spectrometry (HPLC/MS/MS). Aliquots of the CSF (30 μl) or perfusate samples were mixed with 20 μl of internal standard solution in acetonitrile in 96-well glass tubes and analyzed by HPLC/MS/MS.
The standard curves were prepared by spiking a known amount of compound into blank matrix and then processing according to procedure described previously for each matrix. The HPLC/MS/MS system consisted of either a Shimadzu (Kyoto, Japan) ternary pump (Shimadzu LC-10A) or an Agilent Technologies (Santa Clara, CA) quaternary pump HPLC system, an HTS-PAL autosampler (LEAP Technologies, Carrboro, NC), and a PE Sciex API 4000 (PerkinElmerSciex Instruments, Waltham, MA) mass spectrometer with a turbo ion spray interface (PerkinElmerSciex Instruments). A 10-μl aliquot of each sample was injected onto a reverse-phase column. The HPLC/MS/MS conditions for the nine compounds can be found in Table 1. The concentration of all the samples was within the linear range of quantitation for all the assays. The low limit of quantitation for the nine compounds was 0.5 to 2 ng/ml for plasma, 1 to 7.5 ng/g for brain, and 0.05 to 0.25 ng/ml for CSF and the dialysate. The assay accuracy was between 80 and 120%.
HPLC/MS/MS conditions for the nine compounds
Data Analysis. The unbound fractions determined from diluted brain tissue homogenates were corrected to yield an estimate of unbound fraction in the intact brain tissue using eq. 1 (Kalvass and Maurer, 2002).  where fu,brain and fu,homogenate represent the unbound fraction in brain tissue and unbound fraction in brain homogenate. Dilution is the dilution factor for the brain homogenate.
where fu,brain and fu,homogenate represent the unbound fraction in brain tissue and unbound fraction in brain homogenate. Dilution is the dilution factor for the brain homogenate.
Results
The compounds selected in the present study include acidic, basic, and neutral compounds with diverse structures and physicochemical properties (Table 2). The recovery of the microdialysis probes was determined in vitro by measuring the gain in the dialysis solution for each compound and ranged from 18.3 ± 4.1 to 55.2 ± 5.7% (mean ± S.D.) with an average recovery of 34.6 ± 11.0% (Table 3). The unbound fractions of each compound in rat plasma and brain were determined using equilibrium dialysis. The rat plasma unbound fractions ranged from 0.0625 ± 0.0047 to 0.9720 ± 0.319. The rat brain tissue unbound fraction ranged from 0.00569 ± 0.00030 to 0.855 ± 0.546 (Table 3). The brain unbound fractions were equal to or lower than the unbound fraction of plasma for this set of compounds.
Physicochemical and P-gp transport properties of the nine compounds
In vitro microdialysis probe recovery, unbound plasma and brain fraction, plasma, brain, and CSF concentration, and Cm of the nine compounds in rats (mean ± S.D., n = 3-6)
All of the concentrations were from the samples collected at 6 h after start of infusion.
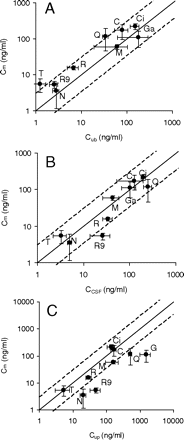
To study the relationship of drug concentrations in different compartments, the studies were designed to reach steady state for both the plasma and brain concentrations. As shown in Fig. 1, the concentration in plasma and brain compartments reached steady state at 1 to 4 h after the start of infusion. All the analyses of the plasma, brain, CSF, and interstitial fluid concentration were based on the concentrations at 6 h after the start of infusion (Table 3; Fig. 2).
The -folds of difference of the Cub, CCSF, and Cup over Cm at 6 h after the start of infusion are shown in Table 4, and the relationships between Cub, CCSF, or Cup and Cm are presented in Fig. 2. For eight of the nine compounds, their Cubs were within 3-fold of their Cm. The Cm of thiopental was 4-fold of its Cub (Fig. 2A; Table 4). For eight of the nine compounds, their CCSFs were within 3-fold of their Cm. The CCSF of 9-hydroxyrisperidone was 5-fold of its Cm (Fig. 2B; Table 4). The Cup of the nine compounds was equal to or greater than their Cm (Fig. 2C; Table 4). For five of the nine compounds, their Cups were within 3-fold of their Cm. The Cup of ganciclovir was 14-fold of its Cm.
The -fold difference of use of the unbound brain concentration measured by brain homogenate method (Cub), CSF concentration (CCSF), and unbound plasma concentration (Cup) to predict the unbound brain interstitial concentration measured by brain microdialysis (Cm) of the nine compounds
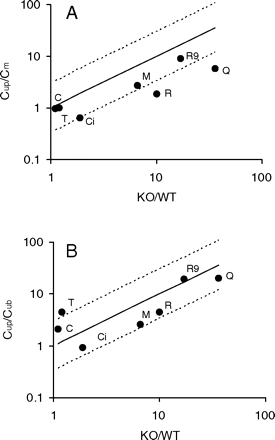
In the present study, compounds whose ratio of brain/plasma concentration in mdr1a/1b knockout mice over the brain/plasma concentration in wild-type mice (KO/WT) greater than 2 are defined as P-gp substrates. Among the nine compounds, five compounds are P-gp substrates, and the rest are non–P-gp substrates or their P-gp transport activities are not known (Table 2). For those P-gp substrates, the range of the KO/WT ratios is 6.6 to 36. For non–P-gp substrates, the range of KO/WT ratios is 1.1 to 1.9. The KO/WT ratios correlated Cup/Cm ratios for the seven compounds (r = 0.89; Fig. 3A). The KO/WT was within 3-fold of Cup/Cm for five of the seven compounds. The KO/WT ratios for the other two compounds, quinidine and risperidone, were 5- and 6-fold greater than their Cup/Cm ratios, respectively. The KO/WT ratios also correlated Cup/Cub ratios for the seven compounds (r = 0.78; Fig. 3B). The KO/WT was within 3-fold of Cup/Cub for six of the seven compounds. The Cup/Cub for thiopental was 4-fold of its KO/WT.
Discussion
The major findings in the present study are 1) the Cub predicted the Cm within 3-fold of error for eight of the nine compounds and 4-fold for one of the nine compounds; 2) the CCSF predicted the Cm within 3-fold of error for eight of the nine compounds and 5-fold for one of the nine compounds; and 3) the Cup predicted the Cm within 3-fold of error for five of the nine compounds and overpredicted the Cm for the other four compounds (6–14-fold). These results support the use of brain homogenate or CSF as a surrogate for the interstitial fluid concentration in drug discovery and development settings.
The brain homogenate predicted steady-state brain unbound concentration for eight of nine compounds within 3-fold of error for the Cm. Only thiopental showed a 4-fold difference. This correlation is better than the results reported recently by Fridén et al. (2007), who showed that Cub predicted Cm within 3-fold for 10 of the 15 compounds, and the other five compounds showed approximately a 5-fold error. In that study, the microdialysis data for all the compounds except for CP-122721 were collected from the literature and generated under very different experimental conditions, including recovery methods (in vitro and in vivo), microdialysis probe locations, animal species (rats or rabbits), and dosing routes. For CP-122721, the in vivo microdialysis and in vitro brain homogenate studies were carried out in the same laboratory, and its Cub and Cm were very similar.
Rat unbound plasma concentration (solid circles) and brain unbound interstitial drug concentration measured by brain microdialysis (open circles) versus time profiles (mean ± S.D., n = 3–6) of nine compounds after an intravenous bolus dose and followed by an constant intravenous infusion for 6 h in rats.
The relationship of brain unbound interstitial drug concentration measured by brain microdialysis (Cm) and brain unbound drug concentration measured by brain homogenate method (Cub, A), CSF concentration (CCSF, B), and unbound plasma concentration (Cup, C) at steady state in rats (mean ± S.D., n = 3–6). The Cubs were calculated from the total brain concentration and brain unbound fraction. All of the concentrations represent the values at 6 h after start of an intravenous bolus and followed with a constant intravenous infusion. The solid and dashed lines represent unity and 3-fold boundaries, respectively. Symbols for compounds are defined in Table 1.
Because the brain homogenate method can be much more easily implemented in the drug discovery setting, the present study only focused on the evaluation of the brain homogenate method. The concern for the brain homogenate method is that brain homogenization may change the drug binding properties by destroying cell structure and unmasking binding sites that are not accessible to a drug in vivo. In addition, the unbound fraction in brain tissue may not be extrapolated from the unbound fraction in the diluted brain tissue homogenate if the drug only presents in the interstitial space. In our previous work, we showed that the brain homogenate method was similar to brain-slice method in prediction of Cm (Becker and Liu, 2006). This conclusion is also supported by the data reported by Fridén et al. (2007). The unbound fraction, a reciprocal of the unbound volume being reported in the article, determined by the brain homogenate method and by the brain slices method was within 3-fold for 15 compounds except for one compound, gabapentin. This compound showed 4-fold difference (Fridén et al., 2007).
The relationship of the ratio of Cup/Cm and the ratio of KO/WT (A) and the ratio of Cup/Cub and the ratio of KO/WT (B). The solid and dashed lines represent unity and 3-fold boundaries, respectively. Symbols for compounds are defined in Table 1.
CSF concentration has been considered closely related to the interstitial fluid concentration and used as a surrogate for the interstitial fluid concentration in preclinical and clinical studies. CSF is separated from blood by BCSFB and is in direct contact with brain tissue. The ependymal lining of the ventricles allows diffusional and convectional exchange with the brain interstitium (Abbott, 2005). Because the drug transporters at the BBB and BCSFB are different, the CCSF is not necessarily identical to the interstitial fluid concentration, but it is not clear whether much difference exists between CCSF and the interstitial fluid concentration and the potential errors of using CCSF as surrogate for the interstitial fluid concentration. In the present study, we observed that CCSF was within 3-fold of error for eight of the nine compounds and 5-fold for the other compound. These results support the theory that CCSF may be used as a surrogate in drug discovery and development to predict the interstitial fluid concentration in the brain.
CSF as a surrogate for the interstitial fluid concentration is supported by the data presented by Shen et al. (2004), who compiled CCSF and Cm for 20 compounds. The CCSF was within 3-fold of error for 17 of the 20 compounds and 4- to 5-fold for 2 of the 17 compounds (Liu et al., 2006). Only morphine-6-glucuronide showed large discrepancy between CCSF and Cm. The CCSF of morphine-6-glucuronide was 19-fold lower than its Cm. This discrepancy may be because of the experimental conditions where CCSF and the interstitial fluid concentration did not reach equilibrium as the brain half-life is longer than its plasma half-life (Bouw et al., 2001). Experimental variability might also contribute to the discrepancy because the CCSF and Cm data were obtained from separate studies (Stain-Texier et al., 1999). The ratio of total brain concentration of morphine-6-glucuronide versus the Cm was 0.11 as reported by Stain-Texier et al. (1999) but was 0.20 as reported by Bouw et al. (2001). Further studies are needed to assess the difference between CCSF and Cm for morphine-6-glucuronide.
Plasma represents the easiest accessible matrix as compared with brain tissue and CSF; therefore, the unbound plasma concentration has been used as the primary surrogate for the interstitial fluid concentration, particularly in clinical studies. Because of the existence of the BBB, Cup is often equal to or higher than the interstitial fluid concentration (Liu et al., 2008). The results from the present study are consistent with this view. For the three lipophilic non–P-gp substrates, their Cup was same as their Cm, but for the four P-gp substrates and the two hydrophilic unknown transporter substrates, their Cup values were 2- to 14-fold of their Cm. The results from the present study also confirm our hypothesis that CCSF more accurately predicts the interstitial fluid concentration of the brain than Cup does (Liu et al., 2006).
Under the assumption that the only difference between the mdr1a/1b gene KO and the WT mice is P-gp at the BBB, the unbound plasma and brain concentration ratio in the WT mice can be estimated from the KO/WT ratio (Liu et al., 2008). The results from the present study are consistent with this projection. The efflux ratios observed in mdr1a/1b mice correlated the ratios of Cup/Cm and Cup/Cub in the rat. The KO/WT ratios were within 3-fold of the Cup/Cm ratios for five of the seven compounds and within 3-fold of the Cup/Cub for six of the seven compounds. Therefore, one may use KO/WT ratio to semiquantitatively estimate the unbound plasma and brain concentration ratio, assuming no species difference in P-gp activity at the BBB (Liu et al., 2008).
Risperidone is a P-gp substrate with a KO/WT ratio of 10 in P-gp KO mice (Doran et al., 2005), but interestingly its Cup was only 2- and 4-fold greater than its Cm and Cub in rats, respectively. This finding is consistent with the literature. Summerfield et al. (2006) observed the predicted brain/plasma ratio in rats was 0.76 based on the unbound fraction in plasma and brain tissue. This projected value is within 3-fold of the observed in vivo brain/plasma ratio 0.3, suggesting low or no significant efflux activity at the BBB.
One of the limitations of the present study is that in vitro recovery was used to calibrate the microdialysis probes. In vitro recovery methods may be less accurate compared with the in vivo recovery methods, such as no-net-flux and retrodialysis methods (Lönnroth et al., 1987; Olson and Justice, 1993; Wang et al., 1993). However, there is a tradeoff between theoretical requirements for the recovery and practical possibilities when performing microdialysis (Chaurasia et al., 2007). The in vivo recovery methods are resource-intensive and thus were not suitable in the drug discovery setting. Because the main goal of this work was to assess which concentration is a more appropriate surrogate of Cm, the use of in vitro recovery should not affect our conclusions.
In summary, the present study supports that the Cub and CCSF can be used as a surrogate for the interstitial fluid concentration in drug discovery and development setting. The errors of these methods for most compounds will probably be less than 3-fold. The Cup may predict the interstitial fluid concentration for lipophilic and nonefflux substrates but overpredict the interstitial fluid concentration for polar or efflux substrates. The future research needs to further assess the utilities and limitations of brain homogenate and CSF methods, and to refine the current surrogate methods to improve their accuracy.
Acknowledgments
We thank Alan Yau, Cuiping Chen, Yuen Tam, Marina Fridlib, Mey Lee, David Huynh, Phil McVey, and Pam McLawhon for their contribution to this project.
Footnotes
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.108.024125.
-
ABBREVIATIONS: Cup, unbound plasma concentration; BBB, blood-brain barrier; BCSFB, blood-cerebrospinal fluid barrier; Cm, unbound brain interstitial fluid concentration measured by brain microdialysis; CSF, cerebral spinal fluid; Cub, unbound brain concentration measured by brain homogenate method; CCSF, CSF concentration; P-gp, P-glycoprotein; HPLC/MS/MS, high-performance liquid chromatography combined with tandem mass spectrometry; KO, knockout; WT, wild type; CP-122721, (+)-(2S,3S)-3-(2-methoxy-5-trifluoromethoxybenzyl)amino-2-phenylpiperidine.
- Received August 22, 2008.
- Accepted December 29, 2008.
- The American Society for Pharmacology and Experimental Therapeutics