Abstract
Quinoline was used to probe the steric and electronic contributions to rates of aromatic oxidation of nitrogen-containing, multiring substrates by cytochrome P450 (P450) enzymes. The regioselectivity of the P450 oxidation of quinoline was determined experimentally by identifying and measuring the ratios of metabolites. The laboratory results were compared with those obtained computationally by modeling the electronic effects for aromatic hydroxylation of the substrate. Calculated values predict 8-hydroxyquinoline to have the lowest relative activation energy, whereas 3-hydroxyquinoline was calculated to have the highest relative activation energy. In contrast, 3-hydroxyquinoline was produced to a much greater extent relative to 8-hydroxyquinoline. The sharp contrast observed between the computationally obtained energies and the ratios of products identified experimentally indicates that steric factors play a role in determining the regioselectivity of P450 enzymes with quinoline. To further probe steric contributions to product formation, isoquinoline was used as a substrate and the results were compared with those obtained with quinoline. Isoquinoline N-oxide was determined to be the major metabolite of isoquinoline with all of the P450 enzymes used. These results provide further evidence for the steric influence on the regioselectivity of P450 enzymes with quinoline.
The cytochrome P450 enzymes are a superfamily of monooxygenases that function to metabolize both endogenous and exogenous compounds (Ortiz de Montellano, 1995). These enzymes are large contributors to both the prevention and induction of chemical toxicities and carcinogenicity. Although many of these processes result in detoxification, occasionally a more toxic compound is formed (Jones et al., 2002). Since P4501 enzymes play a central role in bioactivation and detoxification, the ability to predict the products of P450-mediated reactions is a valuable tool. This tool can assess potential risks associated with environmental exposures as well as assist in drug design (Jones et al., 2002).
The cytochrome P450 enzymes accommodate a vast array of xenobiotics by metabolizing compounds of a broad chemical diversity. An individual isoform can metabolize multiple substrates and a single substrate at multiple positions. The P450 family catalyzes a wide variety of reactions, including aromatic oxidation, nitrogen and sulfur heteroatom oxidations, and aliphatic hydroxylation (Ortiz de Montellano, 1995). These processes are accomplished through the unique characteristics of the cytochrome P450 family, in that the transition states of reactions are not tightly bound and the enzyme catalyzes the activation of molecular oxygen (Jones and Korzekwa, 1996). Thus, for many reactions, ratios of metabolites produced reflect the intrinsic ease of oxidation at a given position in a molecule, whereas for other reactions oxidation is directed to a specific position by the binding orientation of the substrate.
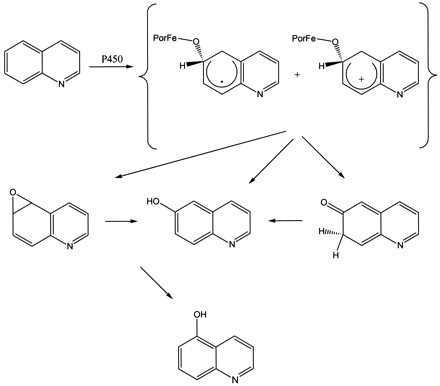
Many substructures of drugs metabolized by cytochromes P450 consist of multiple aromatic rings containing a nitrogen atom. Predictive models for aromatic hydroxylation have been created (Jones et al., 2002), but the effects of multiple rings and the presence of a heteroatom on product ratios have not been assessed. Several factors can influence the regioselectivity of P450 enzymes with quinoline (Fig. 1). The intrinsic ease of a given position on the molecule to undergo oxidation may govern which products are ultimately formed. However, these enzymes can also mediate the epoxidation of aromatic substrates. The epoxides formed can then be nonenzymatically converted into ketones and phenols (Fig. 2). Therefore, the phenols produced can either be the result of initial attack at the hydroxylated position or initial attack at the adjacent position. The regioselectivity of P450 enzymes may also be influenced by steric factors. Steric effects on the binding affinity or orientation of quinoline with P450s may lead to metabolites that do not reflect the most energetically favored position for initial attack.
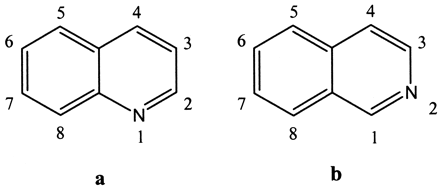
Quinoline (a) and isoquinoline (b) with nomenclature shown.
Positions 1, 4, 5, and 8 are sterically hindered.
Mechanism of aromatic hydroxylation with quinoline as a substrate.
Formation of 5-hydroxyquinoline can occur through initial attack at position 6 followed by formation of quinoline-5,6-epoxide and subsequent ring opening to form the 5-phenol.
The experiments described herein help access the electronic and steric interactions that influence regioselectivity of P450 enzymes with quinoline. This information may lead to a better understanding of the metabolism of heterocyclic and multiple aromatic rings by P450 enzymes.
Materials and Methods
Chemicals and Reagents. Quinoline, quinoline N-oxide, 2-hydroxyquinoline, 4-hydroxyquinoline, 5-hydroxyquinoline, 6-hydroxyquinoline, 8-hydroxyquinoline, 3-aminoquinoline, isoquinoline, isoquinoline N-oxide, 5-hydroxyisoquinoline, and 3-hydroxyisoquinoline were purchased from Aldrich Chemical Co. (Milwaukee, WI). Catalase and NADPH were purchased from Sigma-Aldrich (St. Louis, MO). Dilauroylphosphatidylcholine was purchased from Avanti Polar Lipids (Alabaster, AL). All solvents were purchased from J. T. Baker (Phillipsburg, NJ). All other chemicals were of the highest purity commercially available.
Synthesis of 3-Hydroxyquinoline. 3-Hydroxyquinoline was synthesized from 3-amino quinoline as described by Bergeron et al. (1999). 3-Aminoquinoline and sodium sulfite were heated to reflux in water for 3 days. The reaction mixture was basified to pH 8 with 30% NaOH and brought to reflux. The product was purified on a gravity silica column.
Enzyme Preparation. CYP2E1, 1A2, and 3A4 were purchased from PanVera Corp. (Madison, WI) and used according to the recommended protocol for the RECO system. Purified CYP2A6 was a gift from the laboratory of Dr. Alan Rettie at the University of Washington. Cytochrome P450 reductase was expressed and purified as described previously (Rock et al., 2001). P450cam was expressed in Escherichia coli and purified with Pharmacia Hi-Trap nickel columns (Pharmacia, Peapack, NJ) along with putadiredoxin, putadiredoxin reductase (French et al., 2001), and cytochrome b5 (Locuson et al., 2003).
Expression and Purification of CYP2B4. The truncated 2B4 (glutathione S-transferase) P450 expression plasmid was kindly provided by Dr. M. J. Coon at the University of Michigan Medical School. The truncated CYP2B4 was fused to glutathione S-transferase at the N terminus for purification as previously described (Vaz et al., 1996).
Enzyme Incubations. Incubations with CYP2E1, 3A4, and 1A2 were carried out with 0.5 mM quinoline according to PanVera's protocol with the addition of 0.04 mg/ml catalase. A concentration of 0.5 mM substrate was incubated with 0.5 μM P450cam, 1.0 μM putiaredoxin reductase, 5 μM putiaredoxin, 1 mM NADH, and 0.04 mg/ml catalase in a reaction buffer of 0.1 M potassium phosphate, pH 7.4. Purified CYP2A6 and CYP2B4 were incubated at a concentration of 100 nM with 0.5 mM substrate, 200 nM P450 reductase, 100 nM cytochrome b5, 20 μg of dilauroylphosphatidylcholine, and 50 mM NADPH in 0.1 M potassium phosphate buffer, bringing the reaction volume to 1 ml. All incubations were run in a Forma Scientific shaker bath at 30°C for the bacterial enzymes and 37°C for the mammalian enzymes (Thermo Forma, Marietta, OH). Each incubation was terminated after 30 min with 100 μl of methanol. Each sample was filtered with a 4 mm × 0.25 mm nylon Supelco ISO-Disc filter (Supelco, Bellefonte, PA).
Analytical Methods. Liquid chromatography/mass spectroscopy was used to identify and quantify quinoline metabolites. 4-Hydroxyquinoline was used as an internal standard. Chromatograms were obtained using positive electrospray ionization and monitoring a mass-to-charge ratio of 146. Liquid chromatography/mass spectrometry analysis was performed using a Finnigan AQA liquid chromatography/mass spectrometer (ThermoQuest; ThermoFinnigan, San Jose, CA) coupled to a ThermoQuest Surveyor high-performance liquid chromatography (HPLC) system equipped with an Agilent Hypersil BDS-C18 column (2.0 × 125 mm; Agilent Technologies, Palo Alto, CA). The column method began with 100% 10 mM ammonium acetate buffer adjusted to a pH of 5.1 with acetic acid at a flow rate of 100 μl/min. After 1 min, the solvent composition was ramped to 25% methanol over a period of 4 min. The solvent composition was then slowly ramped to 30% methanol over a period of 25 min. This ratio was held for 5 min before ramping to 70% methanol over 5 min. The ionizing voltage was 4 kV, the orifice-skimmer voltage was 20 V, and the probe temperature was 225°C. Products were also quantified by monitoring their maximum UV absorption with a ThermoQuest Finnigan Surveyor photodiode array detector. Quinoline-5,6-epoxide was identified by its UV absorption spectrum. 8-Hydroxyquinoline was quantified using gas chromatography/mass spectroscopy (GC/MS). Each quinoline incubation was extracted with ethyl acetate and the quinoline phenols were derivatized for GC/MS with N-methyl-N-(t-butyldimethylsilyl)-trifluoroacetamide, which was purchased from Regis Technologies, Inc. (Morton Grove, IL). A ThermoQuest Finnigan Voyager GC/MS equipped with a cross-linked methyl siloxane capillary column (25 m × 0.2 mm × 0.5 μm; Agilent Technologies) was used for GC/MS analysis. The column method began at a temperature of 100°C, which was maintained for 1 min. The temperature was then ramped to 190°C at a rate of 30°C/min. The temperature was then slowly ramped to 230°C at a rate of 3°C/min. Electron impact ionization was used to monitor ion 202. The incubations with isoquinoline were analyzed using HPLC with photodiode array detection.
Computational Methods. Gas-phase transition state calculations were performed with Gaussian 98 (Gaussian, Inc., Carnegie, PA) at the MP2 level using the 6-31G* basis set. A methoxy radical was used as a surrogate for the heme active oxygen species in the calculations and was added to each carbon of quinoline to obtain relative heats of formation of the tetrahedral intermediate. This smaller model compound has been confirmed to give very similar results to the t-butoxy radical, which in turn has been shown to reproduce the P450 active oxygen species with respect to kinetic isotope effects for hydrogen atom abstraction (Manchester et al., 1997). The methoxy radical has also been shown to be a well suited model compound for aromatic hydroxylation of single ring-containing compounds (Jones et al., 2002). The transition states were found by following the most negative eigenvalue after the methoxy radical oxygen-aromatic carbon bond was lengthened to 1.95 Å. Transition states were confirmed with frequency calculations that had only one negative eigenvalue. Relative activation energies of the transition states were then obtained. A methoxide cation was added to each carbon of quinoline to obtain the relative heats of formation for each epoxide opening to the given position.
Results
The regioselectivity results for quinoline are shown in Table 1. Several mammalian P450s were used in these experiments along with one bacterial enzyme, P450cam. Quinoline N-oxide was found to be the major metabolite of quinoline with CYP3A4 and 2A6. In contrast, 3-hydroxyquinoline was found to be the major metabolite with CYP2B4, 2E1, and 1A2, whereas quinoline N-oxide was not detected with CYP1A2 and found in minute quantities with CYP2E1 and CYP2B4.
Metabolism of quinoline by P450 enzymes Values are mean rates ± the standard deviation of triplicate incubations. Rates are expressed in pmol product/nmol P450/min.
A significant amount of metabolism occurred at positions 5 and 6 of quinoline. The major metabolite with P450cam, 5-hydroxyquinoline, was produced in significant amounts with all isozymes used. Quinoline-5,6-epoxide was tentatively identified by its UV absorption spectrum and HPLC column retention time (Saeki et al., 1993). This epoxide was produced in the incubations with CYP2A6 and CYP2B4, and to a lesser extent in the CYP2E1 incubation (data not shown). CYP3A4, 2E1, 2A6, and 2B4 all produced 6-hydroxyquinoline as a minor quinoline metabolite.
All of the P450 isozymes used produced 8-hydroxyquinoline as a minor metabolite, with the exception of P450cam, which produced no 8-hydroxyquinoline. The largest amount was produced with CYP2E1, which produced 8-hydroxyquinoline at a rate of 221 pmol/nmol P450/min. The other P450s produced this metabolite in minute quantities.
The results of the computational calculations of the relative free energies of the transition state for aromatic hydroxylation at each position of quinoline are displayed in Table 2. These values represent the electronically favored metabolites of quinoline. The results reveal 8-hydroxyquinoline to require the lowest energy of activation. Formation of 3-hydroxyquinoline is predicted to be the most electronically disfavored oxidation, requiring 3.04 kcal/mol more energy than the formation of 8-hydroxyquinoline. Other electronically disfavored metabolites include 6- and 7-hydroxyquinoline, whereas 4- and 5-hydroxyquinoline are predicted to be electronically favored.
Computationally obtained electronic effects of aromatic hydroxylation of quinoline The tetrahedral intermediate for aromatic hydroxylation of each position of quinoline was computationally modeled to obtain relative heats of formation of the tetrahedral intermediate.
To further investigate the regioselectivity associated with the ring opening of epoxides, we computationally modeled the ring opening of each epoxide to predict which phenols would be produced. Relative heats of formation of the cation product of each quinoline epoxide were obtained, and the results are shown in Table 3.
Computationally obtained electronic effects of quinoline epoxide ring opening to form phenols at the given positions
The calculated values predict an epoxide at the 5,6-position opening to generate the 5-phenol to have the most stable heat of formation of the cation product. This result is in agreement with experimental data demonstrating the isomerization of quinoline-5,6-epoxide to produce 86% 5-hydroxyquinoline and 14% 6-hydroxyquinoline (Agarwal et al., 1986). According to Table 3, epoxides at positions 2,3 and 3,4 would both be expected to open to generate 3-hydroxyquinoline. The cation product produced if quinoline-2,3-epoxide opens to position 3 would be 8.23 kcal/mol more stable than if it were to open to position 2. The cation product produced if the 3,4-epoxide opens to generate the 3-phenol would be 7.85 kcal/mol more stable than if it were to open to generate the 4-phenol. These results also favor the quinoline-7,8-epoxide to open to form 8-hydroxyquinoline by 1.58 kcal/mol.
The results of the experiments performed with isoquinoline (Fig. 1) as a substrate reveal isoquinoline N-oxide to be the major metabolite with the P450 enzymes used. Table 4 compares the quinoline N-oxide produced to the isoquinoline N-oxide produced with each P450 isoform used. CYP2B4 and CYP2E1 demonstrated the greatest increase in N-oxygenation when isoquinoline replaced quinoline as the substrate. CYP2B4 produced an almost 20-fold increase in N-oxygenation when isoquinoline was used as a substrate. CYP2E1 produced a trace amount of quinoline N-oxide but produced 35 times that amount of isoquinoline N-oxide. P450cam produced about twice as much N-oxide from isoquinoline as it did from quinoline. A slight decrease in N-oxygenation was observed upon incubating with isoquinoline when compared with the use of quinoline as a substrate for CYP3A4. However, other minor metabolites of isoquinoline were not detected, whereas quinoline N-oxide made up only 80% of the total metabolism of quinoline by CYP3A4.
Metabolism of quinoline and isoquinoline by P450 enzymes Values are mean rates ± the standard deviation of triplicate incubations. Rates are expressed in pmol product/nmol P450/min.
Discussion
The purpose of this study was to probe the steric and electronic contributions to rates of aromatic oxidation for different cytochrome P450 enzymes. Quinoline was used in these experiments to help assess the regioselectivity with multiring aromatic substrates containing a nitrogen atom. Electronic contributions were analyzed by computationally modeling the transition states of each possible aromatic hydroxylation to obtain relative activation energies. The results of the P450 incubations with quinoline represented a sharp contrast to the computational results. The most likely explanation for this contrast is that steric influence of the enzyme plays a role in determining which products are formed. Although 8-hydroxyquinoline is the most electronically favored aromatic hydroxylation product, it was not produced to a significant extent with any of the enzymes used. Steric interactions with the enzyme could prevent the formation of the tetrahedral intermediate at the 8-position of quinoline. Laboratory results also reveal 3-hydroxyquinoline to be a major metabolite with many of the enzymes used, even though attack at this position is electronically disfavored. Therefore, steric interference with the enzyme must be less for formation of 3-hydroxyquinoline. By this argument, attack at the 5-position of quinoline should lead to the same steric interference as at the 8-position. The significant amount of 5-hydroxyquinoline formed in the P450 incubations therefore requires further discussion.
The regioselectivity of aromatic hydroxylation may be partially disguised through the formation of epoxides, followed by the nonenzymatic conversion of these epoxides into phenols. Based on evidence obtained through kinetic isotope effects, the mechanism of P450-mediated aromatic hydroxylation (Fig. 2) has been suggested to involve initial formation of a tetrahedral intermediate formed by the oxygen atom of enzyme and the carbon atom being attacked. The tetrahedral intermediate then reacts to form either an epoxide or a ketone, which is then converted into the oxidized product (Korzekwa et al., 1989). When the route through the epoxide in Fig. 2 is followed, the final hydroxylated product can be the result of initial attack either at that position or at the adjacent carbon atom. A direct epoxide formation mechanism was ruled out by the observation that synthetically prepared epoxides of chlorobenzenes could not open to generate meta-phenols, although meta-phenols were produced from P450 incubations with chlorobenzene (Selander et al., 1975a,b). Evidence adding further support to the tetrahedral intermediate formation mechanism has recently been obtained theoretically through hybrid density functional calculations performed by de Visser and Shaik (2003). The authors discovered a porphyrin proton-shuttle mechanism in which benzene is enzymatically converted to phenol and ketone, in addition to the nonenzymatic production of these species by conversion of an epoxide to phenol and ketone. Although other mechanisms are possible, such as direct insertion to the Π bond, we only consider this single mechanism for simplicity. Obviously, if another mechanism is involved, this could be a source of error in our calculations.
The production of 5-hydroxyquinoline can then be explained by attack at the 6-position followed by ring closure to the 5,6-epoxide and subsequent nonenzymatic ring opening to a phenol at the 5-position (Fig. 2). Since quinoline-5,6-epoxide has been shown to isomerize to 86% 5-hydroxyquinoline and 14% 6-hydroxyquinoline with heat (Agarwal et al., 1986), it is likely that the 5-hydroxyquinoline metabolite results from initial attack at the 6-position. The relative energies obtained from modeling the ring opening of the epoxides also reveal quinoline-5,6-epoxide opening to the 5-position to produce a cation product that is 7.31 kcal/mol more stable than if it were to open to generate the 6-phenol. The results in Table 3 also indicate that a large amount of energy is required for the quinoline epoxides to open to positions 2 and 4 relative to the other positions of quinoline. This explains why 2-hydroxyquinoline and 4-hydroxyquinoline were not obtained with any of the enzymes used, even though initial attack at these positions is energetically favored. Since attack at position 4 may also be sterically hindered, in line with the argument for positions 8 and 5, attack may occur at the more sterically and energetically favorable 2-position. This route would then lead to formation of quinoline-2,3-epoxide followed by collapse to produce 3-hydroxyquinoline. The small amount of 8-hydroxyquinoline formed may result from attack at the 7-position leading to quinoline-7,8-epoxide followed by ring opening to the 8-phenol. This route seems likely, since an epoxide opening to the 8-position is 1.58 kcal/mol more stable than the 7-position, which also explains why 7-hydroxyquinoline was not detected in any of the P450 incubations.
The regioselectivity of aromatic hydroxylation was assessed using quinoline as a substrate. The results suggest that steric interactions contribute to the regioselectivity of P450 enzymes with quinoline. Significantly less steric interactions are associated with metabolism at positions 2, 3, 6, and 7, relative to positions 1, 4, 5, and 8 (Fig. 1). The major metabolite of quinoline with P450s 2E1, 2B4, and 1A2 is 3-hydroxyquinoline, whereas quinoline N-oxide is the major metabolite with CYP3A4 and CYP2A6, and is produced to a significant extent by P450cam. Therefore, CYP2E1, 2B4, and 1A2 would be expected to have larger steric components contributing to product formation relative to CYP3A4, 2A6, and P450cam, assuming N- oxidation is energetically favored over aromatic hydroxylation.
To test this hypothesis, isoquinoline was used as a substrate. The nitrogen atom in isoquinoline is in position 2, which should be more sterically accessible relative to position 1, as in quinoline (Fig. 1). Isoquinoline was expected to produce isoquinoline N-oxide as the major metabolite, provided that N-oxidation is electronically favored over aromatic hydroxylation. Specifically, incubating isoquinoline with CYP2E1 and CYP2B4 was expected to shift metabolism from the 3-position to the 2-position of the molecule.
The results of the incubations with isoquinoline are consistent with our observations that CYP2B4 and CYP2E1 have greater steric components associated with their regioselectivity, relative to CYP3A4 and P450cam. Incubating quinoline with CYP2B4 and CYP2E1 produced relatively low levels of the electronically favored, but sterically hindered N-oxide. Incubating isoquinoline with CYP2B4 and CYP2E1 shifted metabolism to the sterically and electronically favored N- oxygenation pathway. Incubating isoquinoline with P450cam produced twice as much N-oxide as produced from quinoline, and 5-hydroxyisoquinoline was produced at less than half the rate as 5-hydroxyquinoline. Although a slight decrease in the production of N-oxide was observed with CYP3A4 on using isoquinoline as a substrate, the total metabolism of the substrate was shifted to the production of N-oxide. These results provide further evidence for large steric contributions to regioselectivity with CYP2B4 and CYP2E1 and the relatively smaller steric contributions to regioselectivity with CYP3A4 and P450cam.
Potential steric interactions contributing to the regioselectivity of quinoline were found to be more prominent with CYP2E1, 2B4, and 1A2 compared with those of 3A4, 2A6, and P450cam. These findings are in agreement with previous studies demonstrating CYP3A4 to be free of excessive steric interactions (Korzekwa et al., 1990). However, CYP1A2 and CYP2E1 have been shown to be free of excessive steric interactions with substrates 2-methyl anisole, 4-methyl anisole, and α-chloro-p-xylene (Higgins et al., 2001). CYP2B4 has also been shown to be free of steric interactions (White et al., 1984). Therefore, the experiments performed with quinoline have helped to identify possible steric interactions associated with the oxidation of multiring aromatic substrates by CYP2B4, 2E1, and 1A2.
This is the first time this computational model has been extended to aromatic compounds containing multiple or heterocyclic rings. Although the energetics of single ring-containing aromatic compounds have been successfully predicted with this model (Jones et al., 2002), it is possible that a different model is required for multiple ring-containing or heterocyclic compounds. For simplicity, we have also assumed a mechanism for aromatic oxidation involving initial formation of the tetrahedral intermediate followed by collapse to products. If another mechanism is involved, this could be a source of error in our calculations. Therefore, the contrast observed between the experimental regioselectivity of the P450-mediated reactions and the computational predictions may be attributed to deficiencies in the electronic model.
In conclusion, it is likely that binding and orientation effects play a role in the regioselectivity of the oxidation of quinoline by P450 enzymes and that CYP3A4, 2A6, and P450cam are less sterically demanding than CYP2E1, 2B4, and 1A2. However, this conclusion is based on results obtained computationally by modeling the electronic effects on product formation and on the production of N-oxide, which is assumed to be electronically favored over aromatic hydroxylation. Therefore, deficiencies in the computational method may also contribute to the contrast observed between the experimental regioselectivity and the computational predictions.
Footnotes
-
↵1 Abbreviations used are: P450, cytochrome P450; HPLC, high-performance liquid chromatography; GC/MS, gas chromatography/mass spectroscopy.
-
This work was supported by National Institute of Environmental Health Sci-ences Grant 09122.
- Received September 9, 2003.
- Accepted December 2, 2003.
- The American Society for Pharmacology and Experimental Therapeutics