Abstract
The enantioselective sulfoxidation of the prochiral anthelmintic compounds albendazole (ABZ) and fenbendazole (FBZ) was investigated in liver, lung and small intestinal microsomes obtained from healthy sheep and cattle. The microsomal fractions were incubated with a 40 μM concentration of either ABZ or FBZ. Inhibition of the flavin-containing monooxygenase (FMO) system was carried out by preincubation with 100 μM methimazole (MTZ) either with or without heat pretreatment (2 min at 50°C). ABZ and FBZ were metabolized to the (+) and (-) enantiomers of their sulfoxide metabolites, named albendazole sulfoxide (ABZSO) and oxfendazole (OFZ), respectively. ABZ sulfoxidation rates were higher (p < 0.001) than those observed for FBZ. The FMO-mediated liver sulfoxidation of ABZ was enantioselective (100%) toward the (+) ABZSO production in both species. Liver sulfoxidation of FBZ by FMO was also enantioselective toward (+) OFZ (sheep = 65%; cattle = 79%). Cytochrome P450 was found to be mainly involved in the production of (-) ABZSO in the liver. MTZ did not affect the sulfoxidation of ABZ by lung microsomes, which may indicate that FMO is not involved in the production of ABZSO in this tissue. A significant (p < 0.05) inhibition of (-) ABZSO production by liver microsomes was observed after ABZ incubation in the presence of erythromycin (cattle = 21%) and ketoconazole (sheep = 36%). Both CYP3A substrates induced a reduction in the production of (-) ABZSO (sheep = 67–78%, cattle = 50–78%) by lung microsomes. Overall, the results reported here contribute to the identification of the metabolic pathways involved in the biotransformation of benzimidazole anthelmintics extensively used for parasite control in ruminants.
Livestock animals are exposed to a variety of xenobiotic agents (i.e., veterinary drugs, feed-additives, pesticides, pollutants, etc.) during their production cycles. These compounds are likely to be metabolized by different enzymatic systems from both hepatic and extra-hepatic tissues. The metabolic activity of the flavin-containing monooxygenase (FMO) and cytochrome P450 (P450) systems plays a major role in determining the persistence of therapeutically used drugs in target species, which may additionally impose a risk to the consumer as a consequence of the permanence of drug residue levels in edible tissues. Metabolic interactions with either the FMO or P450 enzymatic systems may drastically affect the disposition kinetics of different drugs used in animal production, which will have a relevant impact on the pattern of drug/metabolite residues in edible tissues, a major concern for public health and consumer safety.
Benzimidazole (BZD1) and pro-BZD anthelmintics are extensively metabolized in domestic animals and humans. Their metabolic pattern and the resultant pharmacokinetic behavior are relevant in the attainment of high and sustained concentrations of pharmacologically active drug/metabolites at the target parasite (Lanusse and Prichard, 1993). Albendazole (ABZ; methyl-[(5-propylthio)-1H-benzimidazol-2-yl] carbamate) and fenbendazole (FBZ; methyl-[(5-phenylthio)-1H-benzimidazol-2-yl] carbamate) are BZD derivatives that have been used in our laboratory as model compounds to characterize different factors affecting the pharmacokinetic and biotransformation processes in ruminant species. The BZD-sulfoxide derivatives, albendazole sulfoxide (ABZSO) and oxfendazole (OFZ), are the main anthelmintically active metabolic products found systemically after ABZ (Hennessy et al., 1989) and FBZ (Short et al., 1987; Lanusse et al., 1995) administration to sheep and cattle. The absence of ABZ parent drug in plasma has been attributed to a first-pass oxidation in the liver. In fact, the oxidation of ABZ to ABZSO has been shown to be catalyzed by the liver microsomal mixed function oxidases in rats (Moroni et al., 1995), pigs (Souhaili-el Amri et al., 1987), lambs (Galtier et al., 1986), calves (Lanusse et al., 1993), and humans (Rawden et al., 2000). On the other hand, the parent drug FBZ and its sulfoxide metabolite are found in the bloodstream after the administration of both FBZ and OFZ to sheep (Lanusse et al., 1995). The hepatic sulfoxidation of FBZ to form OFZ has been shown in rats (Murray et al., 1992), horses (Montesissa et al., 1989; McKellar et al., 2002), pigs, sheep, and cattle (Montesissa et al., 1989). Furthermore, both anthelmintically active sulfoxide derivatives undergo a second, slower and irreversible oxidative step which forms the inactive sulfone (ABZSO2 and FBZSO2) metabolites, which are also found in the bloodstream after administration of their respective parent sulfides.
Albendazole sulfoxide and OFZ have an asymmetric center in the sulfur atom of their side chain. This nucleophilic sulfur atom is attached to four different functional groups, which results in an asymmetric molecule nonsuperimposable with its mirror image. Thus, two ABZSO and OFZ enantiomers have been identified (by chiral separation) in the plasma of sheep after administration of ABZ, FBZ (prochiral molecules) (Delatour et al., 1990), and OFZ (Sánchez et al., 2002). It has been shown that (+) ABZSO is the main enantiomeric form recovered in plasma (Delatour et al., 1991) and in tissues of parasite location (Alvarez et al., 2000) following ABZ treatment to sheep and ABZSO administration to cattle (Cristofol et al., 2001). Similarly, (+) OFZ prevails in the systemic circulation after both FBZ (Delatour et al., 1990) and OFZ (Sánchez et al., 2002) administration to sheep. Such differences between the plasma and tissue availabilities of the (+) and (-) enantiomeric forms of ABZSO and OFZ were attributed to the relative contribution of the FMO- and P450-dependent oxygenases to ABZ and FBZ hepatic sulfoxidation.
Biotransformation takes place predominantly in the liver, although metabolic activity is apparent in extrahepatic tissues such as the lungs and gastrointestinal (GI) tract. The catalytic activity of several mixed function oxidases have been characterized in lung tissue (Philpot and Smith, 1984) and GI mucosa (Thummel et al., 1997). For instance, CYP2F3 cloned from goat lung tissue has been implicated in the oxidation of 3-methylindole (a metabolic product of the amino acid l-tryptophan) into reactive pneumotoxic intermediates (Wang et al., 1998). On the other hand, it has been shown that CYP3A4 is the major isoenzyme involved in the biotransformation of many xenobiotics in human small intestinal mucosa (Kolars et al., 1994; Thummel et al., 1997). CYP3A4-mediated biotransformation in the small intestine largely contributes to the first pass metabolism of midazolam in humans (Paine et al., 1996; Thummel et al., 1997). Although the intestinal sulfoxidation of ABZ has been studied in the rat (Redondo et al., 1999), there is no information available on the oxidative biotransformation of BZD anthelmintics in extrahepatic tissues in ruminant species. It has been shown that the pattern of metabolism affects the systemic availability of anthelmintically active BZD molecules and that interference with their liver biotransformation accounts for enhanced drug activity (Lanusse and Prichard, 1993). Despite the knowledge available on the relationship among the metabolism pattern, disposition kinetics, and availability of BZD molecules in target parasites collected from treated animals (Alvarez et al., 2000), the identification of the metabolic pathways involved in the biotransformation of BZD anthelmintics in ruminant species requires further work. The objective of the current work was to characterize the comparative enantioselective sulfoxidation of ABZ and FBZ by hepatic and extrahepatic (lung and small intestinal mucosa) microsomes from sheep and cattle.
Materials and Methods
Chemicals. Reference standards (99% pure) of ABZ, ABZSO, and ABZSO2 were provided by Schering Plough (Kenilworth, NJ). Reference standards (99.5% pure) of FBZ, OFZ, and FBZSO2 were provided by Rhône Mérieux (Lyon, France). Stock solutions (1000 μg · ml-1) of each molecule were prepared in methanol (J. T. Baker, Phillipsburg, NJ). The reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) was purchased from Sigma-Aldrich (St. Louis, MO). Methimazole (MTZ) (Sigma-Aldrich) stock solution (1000 μg · ml-1) was prepared in deionized water. Ketoconazole (KTZ) and erythromycin (ETM) stock solutions (25 mg · ml-1) were prepared in methanol. The solvents used for the chemical extraction and chromatographic analysis were HPLC grade (J. T. Baker). Buffer salts (NaHCO3, Na2HPO4, and CH3COONH4) were purchased from J. T. Baker.
Animals. Three healthy Holstein steers (120–140 kg) and four Corriedale weaned lambs (20–25 kg), fed with high quality lucerne hay and water ad libitum, were used in this experimental work. Liver and lung microsomes were prepared from both sheep and cattle, whereas the small intestinal mucosa microsomal fraction was obtained from cattle. Animals were stunned and exsanguinated immediately according to internationally accepted animal welfare guidelines (AVMA, 2001).
Preparation of Microsomes. After sacrifice, the abdomen was opened and the liver (sheep and cattle) and small intestine (cattle) were removed. Then, the thoracic cavity was opened to obtain the lungs. Samples (approximately 2 cm × 2 cm × 2 cm) of liver and lung (diaphragmatic lobule) tissues were rinsed with ice-cold phosphate buffer (0.01 M, pH 7.3). The gut content was discarded and the organ was opened through a longitudinal incision. Then, the mucosa was washed with the same phosphate buffer and samples of the mucosal tissue were obtained by scraping. All samples were transported to the laboratory in phosphate buffer (0.01 M, pH 7.3) at 4°C. All subsequent operations were performed between 0 and 4°C. For each experimental animal, samples of liver and lung tissues (10–15 g) were cut into small pieces with scissors and washed several times with the phosphate buffer (to remove hemoglobin). Then, all tissue samples were homogenized in phosphate buffer (0.01 M, pH 7.3) with an Ultra-Turrax homogenizer (IKA Works Inc., Wilmington, NC), centrifuged at 10,000g for 20 min, and the resulting supernatant was centrifuged at 100,000g for 60 min. The pellet (microsomal preparation) was suspended in phosphate buffer and stored at -70°C until used for incubation assays. An aliquot of the microsomal preparation was used to determine protein content using bovine serum albumin as a control standard (Smith et al., 1985).
Enzyme Assays. The metabolic activity was assessed by the amount of either (total), (+), and (-) ABZSO (ABZ incubation) or OFZ (FBZ incubation) formed in the presence of NADPH. A typical reaction mixture contained 250 μl of NADPH solution prepared in phosphate buffer (0.01 M, pH 7.3), 100 μl of tissue preparation (1 mg of microsomal protein), and 40 μM ABZ or FBZ (incubated substrates) dissolved in 10 μl of methanol. The incubation mixture was adjusted to 1 ml with phosphate buffer (0.01 M, pH 7.3). Thawed microsomal samples were diluted with the same phosphate buffer. The incubation mixture was allowed to equilibrate (5 min at 37°C) and the reaction was started with the addition of drug substrates. Incubations (60 min at 37°C) were carried out in glass vials in an oscillating water bath under aerobic conditions. The drug substrates were also incubated, under the same conditions, either without microsomes or without NADPH. These incubations were used as controls for possible nonenzymatic drug conversion. All reactions were stopped by the addition of 1 ml of acetonitrile and stored at -20°C until analysis. For the microsomal preparations of the different tissues studied, the sulfoxidation of ABZ was linear up to 60 min, whereas the biotransformation of FBZ to OFZ was linear up to 90 min. The maximal sulfoxidation rates of ABZ and FBZ were obtained using 1 μmol of NADPH in cattle microsomes and 0.5 μmol of NADPH in sheep microsomes.
Competitive inhibition of the FMO activity was obtained by preincubation (5 min at 37°C) of diluted microsomes with the FMO substrate MTZ (100 μM) in the presence of NADPH. Additionally, inactivation of FMO was performed by heating the diluted microsomal preparation (2 min at 50°C) without NADPH, which was immediately chilled in ice (Dixit and Roche, 1984) and followed by a preincubation (5 min at 37°C) with MTZ (100 μM) in the presence of NADPH. The reaction started with the addition of the incubated substrates ABZ or FBZ.
The sulfoxidation of ABZ by liver and lung microsomes was also studied in the presence of the CYP3A substrates ETM and KTZ (Newton et al., 1995; Zweers-Zeilmaker et al., 1999). Three different concentrations of ETM (40, 250, and 500 μM) and KTZ (40, 100, and 200 μM) were assayed in the incubation trials performed with liver microsomes. The concentrations of ETM and KTZ used in the incubations with lung microsomes were 500 and 200 μM, respectively. Incubation mixtures containing ETM were preincubated during 30 min at 37°C in the presence of NADPH, following the methodology reported by Zweers-Zeilmaker et al. (1999). The reaction started with the addition of ABZ. Microsomal preparations containing KTZ were preincubated (5 min at 37°C) without NADPH followed by the addition of ABZ. The reaction was initiated by the addition of NADPH, as previously shown by Newton et al. (1995). Both ETM and KTZ were dissolved in 10 μl of methanol, and parallel control tubes contained the same volume of the solvent. ETM, as well as KTZ, was also incubated in the absence of ABZ under the same conditions to ensure that the presence of these inhibitors in the incubation mixture did not interfere with the chromatographic determination of ABZ metabolites.
Drug/Metabolites Extraction. The internal standard oxibendazole (0.5 μg dissolved in 5 μl of methanol) was added to an aliquot (1 ml) of the inactivated incubation mixture containing acetonitrile/microsomal preparation (1:1). Spiked samples (500 μl), fortified with ABZ, FBZ, and its metabolites, were mixed with 500 μl of acetonitrile followed by the addition of the internal standard. Experimental and fortified samples were mixed using a vortex for 15 s and centrifuged at 10,000g for 5 min. The supernatant fractions were mixed with 5 ml of deionized water and injected into C18 cartridges (Lichrolut; Merck, Darmstadt, Germany) preconditioned with methanol and deionized water. After washing with deionized water (1 ml) and elution with methanol (2 ml), samples were evaporated to dryness under a stream of N2. The dry residue was redissolved in 300 μl of the HPLC mobile phase.
Chromatographic Analysis. Samples were analyzed for ABZ, ABZSO, ABZ sulfone (ABZSO2), FBZ, OFZ, and FBZ sulfone (FBZSO2) by HPLC. Fifty microliters of each extracted sample were injected through an autosampler (Shimadzu SIL 10 A Automatic Sample Injector; Shimadzu Corporation, Kyoto, Japan) into a Shimadzu 10 A HPLC system fitted with a Selectosil C18 (5 μm, 250 mm × 4.60 mm) reverse-phase column (Phenomenex, Torrance, CA) and UV detector (Shimadzu SPD-10A UV detector) reading at 292 nm. The mobile phase was an acetonitrile/ammonium acetate (0.025 M, pH 6.6) elution gradient. The chromatographic conditions were as previously reported (Virkel et al., 2002). The analytes were identified with the retention times of pure reference standards. Chromatographic peak areas of the analytes were measured using the integrator software (Class LC 10, Shimadzu Corporation) of the HPLC system.
During the reverse phase HPLC analysis, the ABZSO and OFZ chromatographic peak fractions were collected into a glass tube. The collected fractions were evaporated to dryness under a N2 stream and redissolved with 150 μl of chiral mobile phase (1% 2-propanol in 0.008 M Na2HPO4 buffer, pH 6.9). Fifty microliters of each sample were injected into the same HPLC system fitted with a chiral stationary phase column (5 μm, 100 mm × 4.0 mm) (Chiral-AGP column; ChromTech, Hägersten, Sweden). This chiral chromatographic method was adapted from a methodology described previously (Delatour et al., 1990). ABZSO and OFZ enantiomers were identified after the chromatographic analysis of a pure racemic standard of each molecule. The relative proportions (percentages) of each antipode were obtained using the integrator software (Class LC 10, Shimadzu Corporation) of the HPLC system.
Drug/Metabolite Quantification. Validation of the analytical procedures for extraction and quantification of ABZ, FBZ, and their metabolites was performed before starting the analysis of the experimental samples from the incubation trials. Known amounts of each analyte (0.25–10 μg · ml-1) were added to aliquots of boiled (inactivated) microsomal preparations, extracted, and analyzed by HPLC (triplicate determinations) to obtain calibration curves and percentages of recovery. Calibration curves were prepared using least-squares linear regression analysis (Instat 3.00; GraphPad Software Inc., San Diego, CA) of HPLC peak area ratios of analytes/internal standard and nominal concentrations of spiked samples. Correlation coefficients (r) were 0.999 (ABZ), 0.997 (ABZSO), 0.998 (ABZSO2), 0.997 (FBZ), 0.999 (OFZ), and 0.998 (FBZSO2). A lack-of-fit test was also carried out to confirm the linearity of the regression line of each analyte. The concentrations in the experimental samples were determined following interpolation using the standard curves. Absolute recoveries were established by comparison of the detector responses (peak areas) obtained for spiked microsomal samples (0.5, 1, 2, and 5 μg · ml-1) and those of direct standards prepared in mobile phase. Drug/metabolite absolute recoveries were 84 to 85% (ABZ), 81 to 89% (ABZSO), 83 to 87% (ABZSO2), 67 to 83% (FBZ), 82 to 99% (OFZ), and 79 to 96% (FBZSO2). Interassay precision coefficients of variation (CVs) were ≤15% and the limits of quantification were 0.18 (ABZ), 0.09 (ABZSO and ABZSO2), 0.13 (FBZ), 0.09 (OFZ), and 0.08 μg · ml-1 (FBZSO2).
Data and Statistical Analysis. The reported data are expressed as mean ± S.D. Metabolic rates are expressed in nmol of metabolic products formed per min · mg-1 of microsomal protein. The ratios (±) represent the average value of the ratio between the rates of production of the (+) and (-) enantiomeric forms obtained in each individual assay. Statistical comparisons were carried out using Instat 3.00 software (GraphPad Software Inc.). Metabolic rates and enantiomeric ratios (±) were statistically compared using the Kruskal-Wallis test (nonparametric analysis of variance). Where significant overall differences (p < 0.05) were observed, further analysis among individual incubation sets was performed using Dunn's test. Statistical comparisons between the sulfoxidation rates of ABZ and FBZ, obtained in the microsomal fraction of the same tissue, were performed using the Mann-Whitney U test. The same nonparametric test was used for statistical comparisons between species. A value of p < 0.05 was considered statistically significant.
Results
ABZ and FBZ were metabolized to their pharmacologically active sulfoxide metabolites by liver and lung microsomes from sheep and cattle, as well as by cattle intestinal microsomes. Only trace amounts of ABZSO2 and FBZSO2 were recovered in some cattle liver microsomal incubations. Conversely, the mean rates of ABZSO2 and FBZSO2 production by sheep liver microsomes were 0.011 ± 0.007 and 0.007 ± 0.002 nmol · min-1 · mg-1, respectively. There was no ABZ or FBZ conversion to their respective sulfone metabolites by lung and intestinal microsomes. No measurable ABZ or FBZ sulfoxidation did occur in blank incubations either without microsomes or in the absence of NADPH.
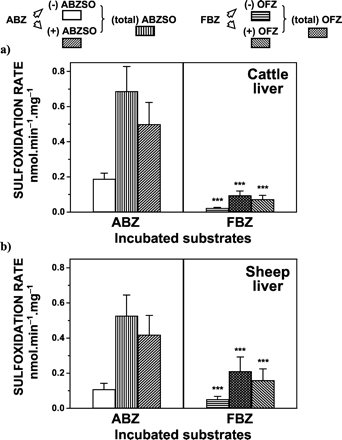
The maximal rate of ABZ sulfoxidation by cattle liver microsomes was 0.69 ± 0.14 nmol of (total) ABZSO formed per min · mg-1 of microsomal protein, which was 30% higher (p < 0.001) than that observed in sheep liver microsomes (0.53 ± 0.12 nmol · min-1 · mg-1). Conversely, the maximal rate of FBZ sulfoxidation to form (total) OFZ by sheep liver microsomes (0.21 ± 0.08 nmol · min-1 · mg-1) was 2.3-fold higher (p < 0.001) than that observed in cattle liver microsomes (0.09 ± 0.03 nmol · min-1 · mg-1). The rates of ABZ sulfoxidation in the liver were 7.7-fold (cattle) and 2.5-fold (sheep) higher (p < 0.001) than those observed for FBZ (Fig. 1). After ABZ and FBZ incubations with control liver microsomes, the rates of production of the (+) enantiomeric forms of ABZSO and OFZ were higher (p < 0.001) than those observed for their respective (-) antipodes (Fig. 1).
In vitro sulfoxidation of ABZ and FBZ by liver microsomes from cattle (a) and sheep (b).
The initial substrate concentration was 40 μM. Data (nmol · min-1 · mg-1 of microsomal protein) are the mean (±S.D.) of 12 determinations. The mean (±S.D.) enantiomeric ratios (±) were: 2.69 ± 0.64 (cattle) and 4.12 ± 1.43 (sheep) for ABZSO; and 3.31 ± 0.55 (cattle) and 3.03 ± 0.53 (sheep) for OFZ. Values are significantly different (⋆⋆⋆, p < 0.001) from those obtained after ABZ incubation.
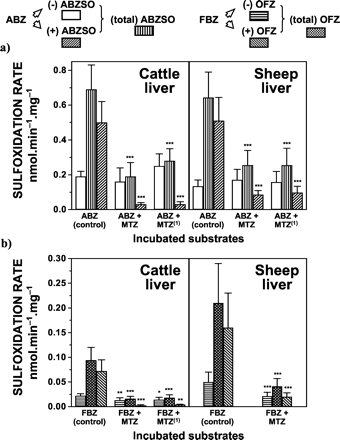
Competitive inhibition of FMO as well as the inactivation of this enzyme system reduced both (total) and (+) ABZSO production following ABZ incubation with cattle and sheep liver microsomes, whereas the formation of (-) ABZSO was not affected (Fig. 2a). Preincubation with MTZ inhibited the liver microsomal sulfoxidation of ABZ to both (total) ABZSO (sheep = 60%, cattle = 72%) and (+) ABZSO (sheep = 83%, cattle = 94%). ABZSO enantiomeric ratios (±) found after ABZ incubation in the presence of MTZ were 0.53 ± 0.08 (sheep) and 0.23 ± 0.06 (cattle). Inhibition of both (total) ABZSO (sheep = 60%, cattle = 59%) and (+) ABZSO production (sheep = 81%, cattle = 94%) was also obtained after thermal inactivation of the FMO followed by preincubation with MTZ (Fig. 2a). ABZSO enantiomeric ratios observed under these experimental conditions were 0.62 ± 0.09 (sheep) and 0.11 ± 0.06 (cattle). Similarly, competitive inhibition of the FMO system by MTZ decreased the production of (total) OFZ (sheep = 80%, cattle = 83%), (+) OFZ (sheep = 87%, cattle = 94%), and (-) OFZ (sheep = 59%, cattle = 36%) after FBZ incubation with liver microsomal fractions from both species (Fig. 2b). OFZ enantiomeric ratios (±) observed after either inhibition or inactivation of the FMO system were: 0.97 ± 0.09 (sheep) and 0.26 ± 0.11 (cattle). Based on the assumption that inhibition or inactivation of FMO leaves the P450 system able to metabolize ABZ and FBZ, the relative involvement of both enzymatic systems in the liver was estimated (Table 1).
Effect of FMO inhibition or inactivation on the in vitro sulfoxidation of ABZ (a) and FBZ (b) by cattle and sheep liver microsomes.
(1) Inactivation of the FMO system was carried out by heat pretreatment (2 min at 50°C) of the microsomal preparation followed by preincubation with MTZ (see Materials and Methods). The initial ABZ or FBZ concentration was 40 μM and the concentration of MTZ used was 100 μM. Data (nmol · min-1 · mg-1 of microsomal protein) are the mean (±S.D.) of 12 determinations. Values are significantly different from control incubations at ⋆, p < 0.05; ⋆⋆, p < 0.01; and ⋆⋆⋆, p < 0.001
In vitro sulfoxidation of ABZ and FBZ by cattle and sheep liver microsomes The involvement of the FMO and P450 systems was estimated by the difference between the sulfoxidation rates in control incubations and those observed after either inhibition or inactivation of FMO. Values are expressed in nmol · min-1 · mg-1 of microsomal protein. Data are the mean of 12 determinations.
The effect of the CYP3A substrates, ETM and KTZ, on the hepatic microsomal sulfoxidation of ABZ is shown in Table 2. Both ETM (cattle) and KTZ (sheep) reduced the conversion of ABZ into (-) ABZSO. Under these experimental conditions, the sulfoxidation of ABZ to (+) ABZSO was not affected. A significant (p < 0.05) reduction (16–21%) in the rate of (-) ABZSO production by cattle liver microsomes was observed after ABZ incubation in the presence of ETM. Also, KTZ inhibited (27–36%) the formation of (-) ABZSO following ABZ incubation with sheep liver microsomes.
Effect of ETM and KTZ on the sulfoxidation of ABZ by liver microsomes from cattle and sheep Data are the mean (±S.D.) of 12 determinations.
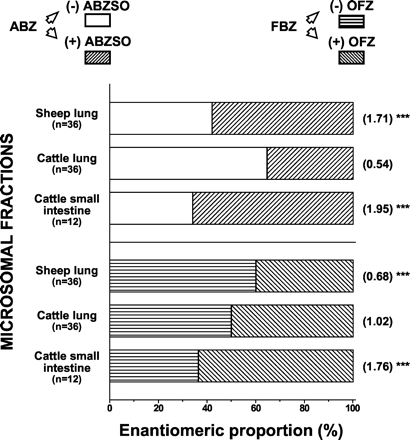
The maximal rate of ABZ sulfoxidation to (total) ABZSO by lung microsomes was 2.5-fold higher (p < 0.001) in sheep (0.20 ± 0.08 nmol · min-1 · mg-1) compared with cattle (0.08 ± 0.03 nmol · min-1 · mg-1). The production of (total) OFZ after FBZ incubation with lung microsomes was also 2-fold higher (p < 0.01) in sheep (0.012 ± 0.004 nmol · min-1 · mg-1) compared with cattle (0.006 ± 0.003 nmol · min-1 · mg-1). Moreover, the rates of ABZ sulfoxidation in lung microsomes from both species were higher (p < 0.001) than those observed for FBZ. The maximal rate of ABZ sulfoxidation by intestinal microsomes was 0.063 ± 0.040 nmol of (total) ABZSO formed per min · mg-1 of microsomal protein, higher (p < 0.001) than that observed for FBZ sulfoxidation to (total) OFZ (0.006 ± 0.003 nmol · min-1 · mg-1) by the same microsomal fraction. The formation of both (total) ABZSO and (total) OFZ in lung and small intestinal microsomes from cattle were similar. Figure 3 shows the enantiomeric proportions and ratios (±) found in the microsomal fractions obtained from extrahepatic tissues.
Extrahepatic microsomal sulfoxidation of ABZ and FBZ.
The initial substrate concentration was 40 μM. The mean enantiomeric proportions are expressed as percentages. The mean enantiomeric ratios (shown in parentheses) are the average value of the ratio between the rates of production (nmol · min-1 · mg-1 of microsomal protein) of the (+) and (-) enantiomers obtained in each individual assay. ⋆⋆⋆, values are significantly different from the enantiomeric ratio observed in cattle lung microsomes at p < 0.001.
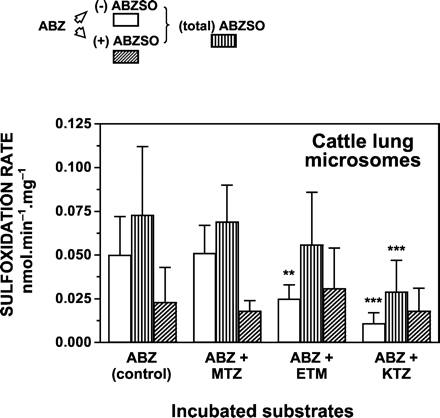
MTZ did not affect the sulfoxidation of ABZ by lung microsomes from both species (Fig. 4; Table 3). On the other hand, the production of (total) ABZSO by lung microsomes was inhibited in the presence of ETM (33%) and KTZ (sheep = 48%, cattle = 60%) (Table 3). Both ETM and KTZ decreased ABZ sulfoxidation to form (-) ABZSO (sheep = 67–78%, cattle = 50–78%) by lung microsomes, whereas the production of the (+) ABZSO enantiomeric form was not affected.
Effect of MTZ, ETM, and KTZ on the in vitro sulfoxidation of ABZ by cattle lung microsomes.
The initial ABZ concentration was 40 μM and the concentrations of MTZ, ETM, and KTZ used were 100, 500, and 200 μM, respectively. Data (nmol · min-1 · mg-1 of microsomal protein) are the mean (±S.D.) of 16 determinations. The mean (±S.D.) enantiomeric ratios (±) were: 0.42 ± 0.21 (ABZ control), 0.37 ± 0.09 (ABZ + MTZ), 1.08 ± 0.53*** (ABZ + ETM), and 1.57 ± 0.49*** (ABZ + KTZ). Values are statistically different from control incubations at ⋆⋆, p < 0.01 and ⋆⋆⋆, p < 0.001.
Effect of MTZ, ETM, and KTZ on the sulfoxidation of ABZ by sheep lung microsomes Data are the mean (±S.D.) of 12 determinations.
Discussion
Aromatic BZD derivatives such as FBZ and OFZ require more extensive hepatic oxidative metabolism than aliphatic derivatives (ABZ and ABZSO) to achieve sufficient polarity for excretion (Hennessy et al., 1993). As a consequence, FBZ is recovered in plasma following its oral/intraruminal administration to sheep and cattle (Short et al., 1987; Lanusse et al., 1995; Sánchez et al., 2002), whereas ABZ is not detected in the bloodstream after its administration as parent drug in both species (Hennessy et al., 1989; Sánchez et al., 2000). Moreover, longer plasma mean residence times and elimination half-lives for FBZ and its metabolites, compared with those of ABZ metabolites, were observed in sheep (Lanusse et al., 1995). The results obtained herein are consistent with those previous pharmaco-kinetic findings and confirm the suggested lower rate of FBZ metabolism. The rates of ABZ sulfoxidation, either in liver, lung, or intestinal microsomes, were higher than those observed for FBZ. Indeed, pharmacokinetic differences between ABZ and FBZ are highly based on their different oxidative metabolism. Although the liver is the main site of ABZ and FBZ biotransformation, sulfoxidation in the intestinal mucosa and lung tissue may contribute to the presystemic metabolism of both anthelmintic drugs. Further studies are required to establish the quantitative importance of both extrahepatic tissues to the overall metabolic clearance of these BZD anthelmintics in ruminant species.
The involvement of the P450 and FMO systems in the liver sulfoxidation of ABZ has been demonstrated in sheep (Galtier et al., 1986), pigs (Souhaili-el Amri et al., 1987), cattle (Lanusse et al., 1993), rats (Moroni et al., 1995), and humans (Rawden et al., 2000). Approximately 30% of ABZ sulfoxidation in human liver is mediated by FMO, whereas the P450 system is the major contributor (∼70%) (Rawden et al., 2000). Similarly, P450 is primarily involved (∼60%) in ABZ hepatic sulfoxidation in rats, although the FMO enzymatic system is also implicated (Moroni et al., 1995). Similar in vitro studies showed the relative involvement of liver FMO (∼32%) and P450 (∼68%) on the liver sulfoxidation of FBZ in rats (Murray et al., 1992). Inhibition of P450-mediated sulfoxidation by piperonyl butoxide demonstrated the participation of this enzymatic system in the hepatic metabolism of FBZ in horses (McKellar et al., 2002). Conversely, it has been demonstrated that FMO is primarily involved in ABZ hepatic sulfoxidation in sheep (Galtier et al., 1986; Lanusse et al., 1993) and cattle (Lanusse et al., 1993), but there is no information on the participation of both metabolic pathways on FBZ metabolism in ruminants. In the current work, the competitive inhibition of the FMO system was carried out by preincubation of microsomes with MTZ. It has also been shown that MTZ may inhibit some P450 isoenzymes (Kedderis and Rickert, 1985). However, P450-mediated metabolism of MTZ was not observed after 60 min of incubation of this antithyroid compound (incubated at 100 μM) with human liver microsomes (Grothusen et al., 1996). This observation may indicate that at the concentration and incubation time used in the current work, MTZ behaves as a competitive FMO inhibitor. Additionally, FMO was inactivated by heat pretreatment of the microsomal preparation followed by MTZ preincubation (Dixit and Roche, 1984). Heat treatment inactivates the FMO system without affecting P450-mediated metabolism (McManus et al., 1987), although some loss of activity of CYP2A6 and CYP2C9 isoenzymes has been reported (Grothusen et al., 1996). Heat treatment partially inhibited P450-mediated sulfoxidation in rat liver microsomes, leading to an overestimation of the involvement of FMO on ABZ metabolism (Moroni et al., 1995). Conversely, equivalent Vmax values for ABZ sulfoxidation were observed in heat-treated compared with MTZ-treated human liver microsomes (Rawden et al., 2000). The findings reported here for ruminant species are in agreement with the latest observations, since there were no statistical differences between ABZ and FBZ sulfoxidation rates despite the methodology used to inhibit or inactivate the liver microsomal FMO system (see Fig. 2). In conclusion, MTZ preincubation as well as heat treatment followed by MTZ preincubation seems to be appropriate for the assessment of the relative contribution of FMO and P450 to ABZ and FBZ hepatic sulfoxidation. Thus, the relative involvement of both enzymatic systems was estimated following the assumption that either inhibition or inactivation of FMO leaves the P450 system able to metabolize ABZ or FBZ (see Table 1). In both sheep and cattle, the FMO-mediated sulfoxidation accounted for 60% of (total) ABZSO production, whereas P450 contributed with 40% of the ABZSO formation. Similarly, FMO was estimated to be the main enzymatic system involved in 80% of total FBZ liver sulfoxidation in both species.
Enantioselectivity of metabolic products occurs when chiral metabolites are generated differentially (in qualitative or quantitative terms) from a single achiral substrate (Testa and Mayer, 1988). Two different Km values for the production of each ABZSO enantiomer have been reported after ABZ (prochiral molecule) incubation with liver microsomes obtained from rats (Moroni et al., 1995) and calves (Virkel et al., 2000). These observations are consistent with the involvement of two different enantioselective enzymatic pathways on the liver sulfoxidation of ABZ. Indeed, FMO and P450 are known to be oppositely enantioselective (Cashman, 1998). In rat liver, the FMO system produces ∼63 to 69% of (+) ABZSO, whereas the P450 isoenzymes 2C6 and 2A1 are mainly involved in the production of (-) ABZSO (Moroni et al., 1995). In the current work, FMO activity accounted for 94% (cattle) and 81% (sheep) of (+) ABZSO production in liver microsomes (see Table 1). In both species, the enantioselectivity of the hepatic FMO system toward (+) ABZSO production was equal to 100%. On the other hand, FMO-mediated sulfoxidation of FBZ generated both (+) OFZ (cattle = 94%, sheep = 88%) and (-) OFZ (cattle = 36%, sheep = 59%) enantiomers (see Table 1), inasmuch as the percentages of enantioselectivity toward (+) OFZ production were 65% (sheep) and 79% (cattle). Besides, both enantiomeric forms of ABZSO and OFZ were produced by the liver microsomal P450 in both animal species (see Table 1). The enantioselectivity of the P450-dependent sulfoxidation in cattle liver microsomes toward the formation of (-) ABZSO (78%) and (-) OFZ (56%) seems to be higher compared with that observed in sheep liver microsomes. In fact, the lower enantiomeric excess estimated for (-) ABZSO (24%) and (-) OFZ (2%) in sheep liver suggests a low enantioselectivity of the P450-mediated sulfoxidation of ABZ and FBZ, respectively, in the ovine species.
Among the P450 superfamily, CYP3A4 appears to be the most important isoenzyme involved in the hepatic sulfoxidation of ABZ in humans (Rawden et al., 2000) and FBZ in rats (Murray et al., 1992). A CYP3A isoenzyme with low enantioselectivity has also been shown to be implicated in ABZ metabolism in rat liver (Moroni et al., 1995). The involvement of CYP1A in the hepatic sulfonation of ABZSO has been suggested in rats (Souhaili-el Amri et al., 1988) and sheep (Galtier et al., 1991; Benoit et al., 1992). However, the role of P450 isoenzymes on ABZ hepatic sulfoxidation in ruminants is unknown. CYP3A substrates such as ETM and KTZ are useful tools to evaluate whether this isoenzyme is involved in the in vitro biotransformation of a given xenobiotic (Newton et al., 1995; Zweers-Zeilmaker et al., 1999). The slight inhibition of (-) ABZSO production caused by ETM (cattle = 16–21%) suggests that CYP3A may not be the primary P450 subfamily involved in the hepatic metabolism of ABZ in this ruminant species. On the other hand, the concentrations of KTZ used to inhibit ABZ metabolism in sheep liver microsomes were higher than its Ki value for CYP3A4 inhibition. Moreover, it has been shown that higher concentrations of KTZ (>10 μM) inhibit CYP1A2, 2D6, and 2C9 activities in human liver microsomes (Newton et al., 1995), whereas its N-deacetyl ketoconazole metabolite, formed in a CYP3A-mediated reaction, undergoes FMO1 and FMO3-dependent N-oxidation (Rodriguez et al., 1999). Certainly, we cannot exclude the competitive inhibition of the FMO-mediated (+) ABZSO production by the N-deacetyl ketoconazole metabolite in sheep liver microsomes. However, inhibition of the production of this enantiomer was not observed after incubation of ABZ in the presence of KTZ. These observations indicate that KTZ may not be considered as a useful inhibitor of CYP3A activity under the experimental conditions described here. Nevertheless, the metabolic interference between ABZ and KTZ should be carefully considered since the concurrent use of both drugs may occur in veterinary therapeutics. Other technical approaches (i.e., use of specific antibodies against the isoenzyme) may be useful to complement the assessment of the participation of CYP3A on ABZ sulfoxidation in sheep and cattle.
Large quantities of ABZ were recovered from tissues of parasite location such as lung parenchyma and small intestinal mucosa in both sheep (Alvarez et al., 1999) and cattle (Sánchez et al., 2000; Cristofol et al., 2001). FBZ has been recovered in both target tissues following oral administration of OFZ to cattle (G. Virkel, L. Mottier, and C. Lanusse, unpublished observations). These findings support the need to study the microsomal biotransformation of each anthelmintic drug in extrahepatic tissues. The relative involvement of FMO (∼49–60%) and P450 (∼40–51%) systems in the sulfoxidation of ABZ by gut epithelium was studied in rats (Redondo et al., 1999). The sulfoxidation of ABZ and FBZ by small intestinal microsomes obtained from cattle was enantioselective (see Fig. 3). The mean ratios (±) between ABZSO and OFZ enantiomers in the gut were similar to those observed in liver microsomes. Further studies should be conducted to determine the relative involvement of FMO and P450 systems on the intestinal sulfoxidation of ABZ and FBZ in ruminants. Although the sulfoxidation of ABZ and FBZ was higher in the liver microsomal fraction, the oxidative metabolism of both anthelmintic drugs in lung tissue and gut epithelium should not be underestimated. Benzimidazole anthelmintics exert their antiparasite effects by binding to parasite tubulin, which produces the subsequent disruption of the tubulin-microtubule dynamic equilibrium (Lacey, 1990). It is well established that both ABZ and FBZ have greater affinity for parasite tubulin than their respective sulfoxides (Lubega and Prichard, 1991). Thus, the data reported here on the oxidative metabolism in extrahepatic tissues may help to understand the relationship between the pharmacokinetic behavior and clinical efficacy for these anthelmintics in ruminants.
The (+) ABZSO enantiomer predominates in plasma (Delatour et al., 1991; Cristofol et al., 2001) and in tissues of parasite location such as lung and GI mucosa (Cristofol et al., 2001), as well as in a target parasite such as the liver fluke Fasciola hepatica (Alvarez et al., 2000). For example, the (+) ABZSO enantiomer represents 91% of the total ABZSO plasma area under the concentration versus time curve (AUC) in cattle and 86% in sheep (Delatour et al., 1991). Such enantioselective kinetic disposition of ABZSO enantiomers was attributed to the relative contribution of the FMO- and P450-dependent oxygenases to ABZ sulfoxidation, which was confirmed in the current work. Moreover, it has been shown that (-) ABZSO, rather than its (+) antipode, would be the main substrate for the P450-mediated formation of the inactive ABZSO2 metabolite (Delatour et al., 1991; Benoit et al., 1992), whereas (+) ABZSO was shown to be the main substrate for a bacteria-mediated sulforeduction to ABZ in the rumen (Virkel et al., 2002). Unfortunately, the relative pharmacological potency of each ABZSO enantiomer is still unknown, but altogether, these observations may indicate a minor contribution of the (-) ABZSO enantiomer (compared with its antipode) to the overall clinical efficacy of ABZ. This consideration could also be relevant for OFZ enantiomers.
The sulfoxidation of ABZ in lung microsomes from both sheep and cattle was enantioselective. The lack of inhibition of ABZ metabolism by MTZ suggests that the FMO system is not involved in the production of ABZSO enantiomers in the lung tissue of both species (see Table 3 and Fig. 4). The FMO system is a family of xenobiotic-metabolizing enzymes found in most tissues of all mammalian species. Five distinct FMO isoenzymes (FMO1 through FMO5) have been identified according to their amino acid sequence identities (Lawton et al., 1994). The expression of these isoenzymes was shown to be tissue-, species-, and temporal-specific. For example, FMO1 and 3 isoforms are expressed in the liver, whereas FMO2 is detected in lung parenchyma (Lawton et al., 1994; Shehin-Johnson et al., 1995). FMO1 predominates in adult male rat and female mouse livers (Cherrington et al., 1998) and during the prenatal trimester in human liver (Hines and McCarver 2002). However, this isoenzyme does not exist in the liver of adult humans (Cherrington et al., 1998; Hines and McCarver, 2002). Liver FMO3 is the dominant FMO isoenzyme in adult female mouse, whereas it is gender independent in adult rat and humans (Cherrington et al., 1998). Only limited information is available about FMO expression in ruminant species. For example, it has been shown that FMO3 is the predominant flavin-containing isoenzyme in sheep female liver (Longin-Sauvageon et al., 1998); however, these authors suggested that the expression profile of FMO in sheep liver microsomes is similar to that observed in mice or adult human liver. Although these isoenzymes share the same catalytic mechanism, the overall substrate size has been shown to be a key factor to determine substrate specificities (Guo et al., 1992). This evidence suggests that FMO3 may be the primarily flavin-containing isoenzyme involved in the hepatic sulfoxidation of ABZ and FBZ. In contrast, ABZ may not be the substrate of the FMO2 isoform located in lung tissue, as is MTZ (Guo et al., 1992). Consequently, only the P450 enzymatic system would be responsible for ABZ metabolism in lung microsomes. Moreover, the inhibition of (-) ABZSO production induced by ETM may suggest that a CYP3A isoenzyme is involved in the metabolism of ABZ in lung tissue (see Table 3 and Fig. 4).
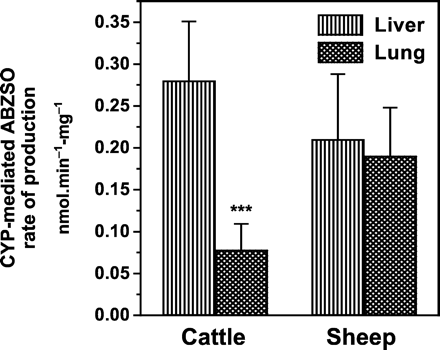
The cytochrome P450-mediated liver sulfoxidation of ABZ was estimated after inhibition of the FMO activity, as explained above. ABZ metabolism in lung tissue is mediated by the P450 enzymatic system (no inhibitory effect of MTZ, a well known FMO substrate). Based on this assumption, the microsomal P450-mediated sulfoxidation of ABZ in liver and lung is compared in Fig. 5.
Comparative P450-mediated ABZ sulfoxidation by liver and lung microsomal fractions obtained from cattle and sheep.
Data (nmol · min-1 · mg-1 of microsomal protein) are the mean (±S.D.) of 12 (liver microsomes) and 36 (lung microsomes) determinations. The ABZ sulfoxidation rate in cattle lung microsomes was lower (⋆⋆⋆, p < 0.001) than that observed in cattle liver microsomes.
In conclusion, the results of the metabolic characterization obtained in the current work confirm the lower rate of FBZ metabolism, compared with ABZ, and the existence of extrahepatic sulfoxidation of both molecules, which may help to explain their in vivo kinetic behavior. The sulfoxidative activity in the small intestinal mucosa and lung parenchyma may contribute to the described presystemic metabolism of these anthelmintic compounds. In the liver, the FMO enzymatic system is mainly involved in the production of the (+) enantiomers of ABZSO and OFZ, whereas P450 produces the (-) antipodes of both sulfoxides. Conversely, the lack of MTZ effect on ABZ sulfoxidation by lung microsomes suggests that the FMO system is not primarily involved in the production of ABZSO in this tissue. Thus, the sulfoxidation of ABZ in the lung parenchyma is mediated by the P450 system. Inhibition of (-) ABZSO production in liver and lung microsomes by ETM may suggest the involvement of a CYP3A isoenzyme in the production of this enantiomer. Altogether, the findings reported in this article are a further contribution to understand the hepatic and extrahepatic biotransformation pathways for widely used anthelmintic drugs in ruminant species.
Footnotes
-
↵1 Abbreviations used are: BZD, benzimidazole; P450, cytochrome P450; ABZ, albendazole (methyl-[(5-propylthio)-1H-benzimidazol-2-yl]carbamate); ABZSO, albendazole sulfoxide; ABZSO2, albendazole sulfone; FBZ, fenbendazole (methyl-[(5-phenylthio)-1H-benzimidazol-2-yl]carbamate); OFZ, oxfendazole; FBZSO2, fenbendazole sulfone; MTZ, methimazole; ETM, erythromycin; KTZ, ketoconazole; DAK, N-deacetyl ketoconazole; HPLC, high-performance liquid chromatography; FMO, flavin-containing monooxygenase; GI, gastrointestinal.
-
Research at the Laboratorio de Farmacología is supported by Consejo Nacional de Investigaciones Cientificas y Técnicas (Argentina), Universidad Nacional del Centro and Agencia Nacional de Promoción Científica y Tecnológica (PICT 08-07277) (all from Argentina).
- Received October 14, 2003.
- Accepted January 29, 2004.
- The American Society for Pharmacology and Experimental Therapeutics