Abstract
Although the promising immunosuppressant, mycophenolic acid (MPA), has many desirable properties and is widely prescribed for organ transplant recipients, its high oral dosage requirement is not understood. Whereas previous Northern blot analysis by Basu and colleagues (2004) located the mRNAs encoding MPA primary metabolizers, UDP-glucuronosyltransferases (UGTs) 1A7, 1A8, 1A9, and 1A10, in human gastrointestinal (GI) tissues, in situ hybridization analysis of mRNAs found that the isozymes were restricted to the mucosal layer of various GI organs. Concomitantly, MPA was glucuronidated by microsomes isolated from normal adjoining specimens. Microsomal studies showed the highest relative rates of metabolism in esophagus, ileum, duodenum, colon, and stomach at pH 6.4; only esophagus showed high pH 7.6 activity. Properties of the recombinant UGTs indicate that MPA is metabolized with pH 6.4 or 7.6 optimum. Activity for 1A7 and 1A9 increased with increasing concentrations up to 2.4 mM, with parallel production of both ether- and acylglucuronides; similarly, 1A8 and 1A10 reached plateaus at 1.6 mM, producing both glucuronides. Km values were 250 to 550 μM. Between 400 and 1600 μM MPA, isozymes generated between 15 and 42% of the acylglucuronides. In effect, high Km values (MPA) are associated with high concentrations to achieve saturation kinetics. Finally, transient inhibition of UGTs in human LS180 colon cells and mouse duodenum by the dietary agent, curcumin, has implications for in vivo pretreatment to reduce MPA glucuronidation to increase the therapeutic index.
The premature clearance of many orally administered therapeutic drugs is a long-standing problem recognized to be associated with metabolism by UDP-glucuronosyltransferases (UGTs). Because lipid solubility of a substrate is a key determinant of its membrane permeability that largely governs absorption, distribution, and excretion, UGT attaches glucuronic acid to an acceptor substrate, converting the lipophile to a soluble innocuous glucuronide to be readily excreted by the hepatobiliary system or the kidney (Dutton, 1980). The isozymes participate in the broad and critical function of detoxifying numerous endogenous metabolites and a vast number of lipid-soluble phenols derived from the diet and environment, including many therapeutic drugs. The realization that the glucuronidation process is a primary contributor to drug inefficiency has been an important impetus over the decades to characterize the UGTs and to develop inhibitors of the system. The system, present in vertebrates from fish to mammals, includes some 35 glucuronidating isozymes encoded by three gene families, including 15 in humans (www.unisa.edu.au/pharm_medsci/Gluc_trans/table1.html).
Mycophenolic acid (MPA) is an example of a highly effective immunosuppressant drug, which undergoes extensive glucuronidation that is not well understood. Despite its high dose requirement with attendant side effects, it is widely prescribed for renal transplant recipients (Jacqz-Aigrain et al., 2000; MacPhee et al., 2000) because of its many desirable properties (Allison and Eugui, 2000). This fungal-derived agent has become a desirable and important drug that is administered alone or in combination to immunosuppress cytotoxic T-lymphocyte proliferation and chronic allograft dysfunction following solid-organ transplantation. Potent MPA inhibition of the rate-limiting enzyme, inosine monophosphate dehydrogenase (IMPDH) (Sintchak et al., 1996), allows high selectivity of type II IMPDH isozyme over type I because of its specific role in de novo synthesis of guanosine in activated mononuclear cell proliferation. Type II is expressed almost exclusively in activated mononuclear cells, whereas type I enzyme is expressed in most other cell types (Allison and Eugui, 2000). Selective inhibition of type II IMPDH prevents proliferation of activated T- and B-lymphocytes, which allows MPA to exert a potent immunosuppressant effect.
Because MPA is generally orally administered, we studied its primary metabolizers, which are distributed in the gastrointestinal (GI) tract. To that end, we found the enzyme cluster, UGT1A7, 1A8, 1A9, and 1A10, among the human UGT1-encoded isozymes (Gong et al., 2001) to be most responsible for converting the drug. Furthermore, the GI-distributed isozymes were shown to be differentially, but strategically located in the mucosal layers throughout the GI tract. Isozymes used a sharp pH 6.4, pH 7.6, or a broad pH 6.4 to 7.6 optimum. In an in vitro assay, each isozyme activity was stimulated by increasing MPA concentration, reaching a maximum at either 1.6 or 2.4 mM, with parallel production of ether-linked and acyl-linked glucuronides and Km values between 250 and 550 μM. Furthermore, we demonstrate that curcumin down-regulated MPA glucuronidation in LS180 colon cells and in mouse duodenum.
Materials and Methods
Cell Lines and Reagents. Serum was obtained from Serologicals Corp. (Norcross, GA). The MTT kit and curcumin were obtained from Sigma-Aldrich (St. Louis, MO). Curcumin was solubilized in fresh DMSO for addition to COS-1 and LS180 (American Type Culture Collection, Manassas, VA) cell cultures or diluted in corn oil for administration by gavage to mice. COS-1 and LS180 cells were grown in Dulbecco's modified Eagle's medium containing 4% and 10% fetal calf serum, respectively. Following treatment, cell viability was monitored by the MTT assay.
In Situ Hybridization. In situ hybridization was carried out with sense and antisense riboprobes from the most unique 5′ regions (1–320 bases) of each mRNA to enhance the specificity of annealing for UGT1-encoded mRNAs (Gong et al., 2001). Each fragment was cloned into the pZErO-1 vector, labeled with [α-35S]CTP (1500 Ci/mM), subjected to alkaline hydrolysis to give, on average, 100- to 200-nt long riboprobes, hybridized overnight with 2 × 106 cpm/μl (Fox and Cottler-Fox, 1993), and washed under high stringency at 60°C. Normal human tissues obtained via the National Disease Research Interchange (Philadelphia, PA) were used. The National Disease Research Interchange collected normal tissue adjoining surgical specimens and from accident victims according to its Institutional Review Board.
Microsomes Isolated from Various Human and Mouse GI Tissues. Microsomes were isolated (Bock et al., 2002) from specimens of human GI tissues collected concurrently with those for in situ studies. Also, C57BL/6 mice (25 g) were treated orally with curcumin according to Animal Study Protocol # 00-019, National Institutes of Health; curcumin (2.5 mg/25 μl DMSO) or DMSO was combined with corn oil and administered by gavage. After 1 h, animals were sacrificed to prepare microsomes. Duodenal tissues were collected and stored at -80°C until microsomes were prepared.
Expression of UGT1A7, 1A8, 1A9, and 1A10 cDNAs in COS-1 Cells. pSVL-based expression units containing a specific UGT-cDNA (Basu et al., 2004) were transfected into COS-1 cells using DEAE-dextran (Ritter et al., 1991) and allowed to incubate 72 h. After expression of UGT1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, 1A10, 2B7, or 2B15, cellular extracts were analyzed biochemically and by Western blot. For Western blots, extracts were solubilized, resolved in a 10% SDS-polyacrylamide gel system, and transblotted onto membranes. Western blots were processed (Ciotti et al., 1999), and specific protein was quantitated with Adobe Photoshop software (Adobe Systems, Mountain View, CA).
Assay for Glucuronidation of Mycophenolic Acid. Homogenates of UGT-transfected COS-1 and LS180 cells were assayed in vitro for MPA glucuronidation (Ciotti et al., 1995). Briefly, common donor substrate, UDP-[14C]glucuronic acid (1.41 mM, 1.4 μCi/μmol), was combined with 200 μM unlabeled MPA, including studies for optimal pH determinations. All incubations were carried out with 100, 150, or 300 μg of cellular protein at pH 6.4, pH 7.0, or pH 7.6 for 1 to 2 h at 37°C, with 0.5 mg of 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid /mg protein to abolish UGT latency. Reactions were linear up to 3 h. To protect AcMPAG against alkaline hydrolysis, pH was adjusted, if needed, to 6.8 to 7.0, and ethanol was added to 66%. MPA-glucuronides were processed, separated by TLC elution, and quantified (Ciotti et al., 1999). Protein was estimated with a BCA kit (Pierce Chemical, Rockford, IL).
Determination of Acylmycophenolic Acid-Glucuronide. To determine the amount of AcMPAG formed at different MPA concentrations, six reactions at each concentration were incubated for 2 h at 37°C, and three reactions were stopped and exposed to 0.2 M NaOH for 2 h at 37°C to hydrolyze AcMPAG (Shipkova et al., 1999). Alkalinized samples were then adjusted to pH 7.0, and all samples were resolved by TLC and quantified (Ciotti et al., 1995). The difference between untreated (total) and NaOH-treated samples represents AcMPAG. Total MPA-glucuronides, MPAG, and AcMPAG are measured as picomoles of MPA-β-glucuronide per unit time.
Results and Discussion
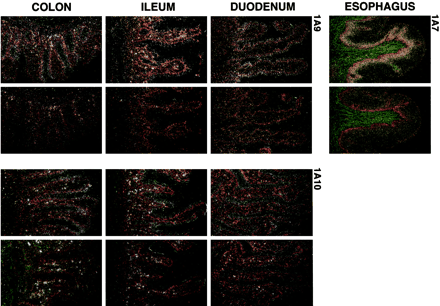
GI Distribution of UGT Isozymes that Metabolize MPA. Among 10 isozymes tested, UGT1A1, 1A3, 1A4, 1A6, 1A7 through 1A10, UGT2B7, and 2B15, we found that 1A7, 1A8, 1A9, and 1A10 were the major metabolizers of MPA. Furthermore, we confirmed by Northern blot analysis (Basu et al., 2004) that the four isozymes are distributed in GI tissues (Strassburg et al., 1998; Basu et al., 2004). In situ hybridization studies show that each mRNA is strategically located in mucosal epithelia of GI tissues (Fig. 1). UGT1A7 mRNA was present at very high levels in esophageal mucosa (Fig. 1, top panel) and at very low levels or not detectable from stomach to colon. Because Northern blot analysis shows that, among the enzymes studied, UGT1A8 is at high levels in stomach and erratically present in other GI tissues, and has the lowest activity toward MPA, we do not display its in situ data. UGT1A9 and 1A10 mRNAs were essentially coequally abundant in duodenal and ileal mucosa and in surface/mucus-secreting goblet cells of colon (Fig. 1); both mRNAs were at low levels in esophagus (not shown).
In situ hybridization of mRNAs coding for UGT1A7, 1A9, and 1A10 in human GI tissues.
For each tissue pair: antisense probe (left) and sense (right) hybridized to mRNA coding for UGT1A7, 1A9, and 1A10 as described under Materials and Methods. Images were magnified 200×. Analysis was done on three separate sets of normal tissues from either surgical specimens or accident victims as described in the Fig. 3 legend.
Since the UGT isozymes exhibiting the highest activity toward MPA are located in the mucosal tissue layer in the respective GI tissues, the isozymes seem strategically located to convert the chemical at the site of absorption, which most likely represents an efficient process that negatively impacts therapeutic index. The location of the most avid metabolizer, UGT1A7, at high levels in esophageal mucosa and low levels in other GI tissues, necessarily reduces its impact. UGT1A10, 1A9, and 1A8, at higher concentrations in other GI tissues, are potentially in more advantageous locations to convert MPA.
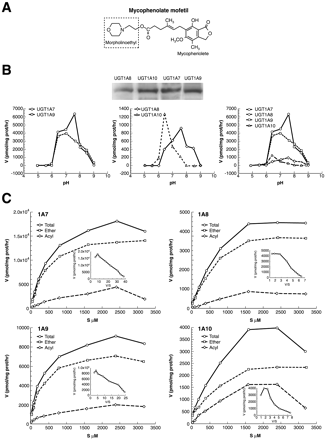
Kinetic Properties of UGT Isozymes that Form Both Ether and Acyl-Linked Glucuronides of MPA. Because there are wide variations in the intraluminal environment bordering the GI mucosal layers harboring UGTs, we determined optimal in vitro pH conditions for MPA conjugation by recombinant isozymes. Immediately upon administration of prodrug mycophenolate mofetil, it is cleaved by luminal and blood esterases, making available a carboxyl group, as well as the 7-hydroxyl for glucuronidation. Hence, we used MPA for substrate (Fig. 2A). Isozymes showed differences in pH optimum. UGT1A7 and 1A8 metabolized MPA with a sharp pH 7.6 optimum, with significant shoulders at pH 6.4 and pH 8.0 (Fig. 2B), respectively. On the contrary, UGT1A9 and 1A10 metabolized best at pH 7.0 and 6.4 (Fig. 2B), respectively. Collectively (Fig. 2B, right), the isozymes exhibited a broad pH range for MPA glucuronidation.
Glucuronidation of mycophenolic acid by recombinant UGTs.
A, structure of mycophenolic acid contained in mycophenolate mofetil. The box defines endogenous esterase cleavage site to release active MPA. B, Western blot and pH optima of UGT1A7, 1A8, 1A9, and 1A10. Recombinant UGTs (150 μg) expressed in COS-1 cells were assayed with 200 μM MPA in 2.0-h incubations as described under Materials and Methods (Ciotti et al., 1995; Basu et al., 2003). C, activity of recombinant UGTs versus MPA concentration. UGT levels were quantitated by Western blot analysis (Ciotti et al., 1999), and six 2-h incubations with equal levels of UGTs were carried out at each concentration. Triplicate reactions were then exposed to 0.2 M NaOH for 2 h at 37°C to hydrolyze AcMPAG (Shipkova et al., 1999). Alkalinized samples were adjusted to pH 7.0, and all samples were resolved by TLC and quantified (Ciotti et al., 1995). The difference between untreated (total) and treated samples represents AcMPAG. Total MPA glucuronides represent MPAG and AcMPAG combined, and are expressed in pmol of MPA-β-glucuronide per unit time. pH optima were 7.6 for UGT1A7 and 1A8, 7.0 for 1A9, and 6.4 for 1A10. Experiments, repeated three times, have a S.E. of ±1 to 5%. For each isozyme, data representing total glucuronide are presented as an Eadie-Hofstee plot in the inset.
Under optimal conditions and equivalent isozyme normalized according to Fig. 2B (Ciotti et al., 1995), we studied the effect of MPA concentration on production of the ether-(MPAG) and acylglucuronide (AcMPAG). Each isozyme showed typical increases in activity with increases in concentrations of MPA that reached saturation-kinetics at 1.6 mM for 1A8 and 1A10, whereas 1A7 and 1A9 required 2.4 mM before achieving maximum activity (Fig. 2C). Also, both ether- and acyl-glucuronides rose in parallel with increasing concentrations and reached peak rates of production at similar concentrations. Whereas 1A7, 1A8, and 1A9 produced 15 to 20% acylglucuronide between 0.4 and 1.6 mM MPA, 1A10 formed 34 to 42% (Table 1) and exhibited a precipitous decline at 3.2 mM. As shown in Table 1, Vmax (18.23 × 103 pmol/mg protein/h) for 1A7 was 2-fold higher than that for 1A9, which was, in turn, 2-fold higher than that for 1A8 and 1A10. Among the four isozymes studied, 1A7 and 1A9 have the lowest Km values of 375 and 250 μM, respectively, whereas 1A8 and 1A10 have slightly higher values at 500 and 550 μM, respectively. Hence, relative Km/Vmax values shown in Table 1 indicate that 1A7, 1A9, 1A8, and 1A10 show the highest relative capacities to glucuronidate MPA. Although 1A10 appears to have the lowest capacity to glucuronidate MPA, it has twice the capacity, 34 to 42%, of the other isozymes to generate the toxic acyl-glucuronide (Table 1; Fig. 2C) (Shütz et al., 1999).
Kinetic parameters for MPA glucuronidation by UGTs
The fact that the four isozymes exhibited nearly linear increases in activity over an expansive concentration range of MPA (Fig. 2C) suggested that the chemical exerts special properties on glucuronidation. Because this atypical increase in activity spanned from 1 to 1600/2400 μM, it was of interest to replot the data using the Eadie-Hofstee equation. Such plots for 1A7, 1A8, and 1A9 are nearly linear (Fig. 2C, insets). Although 1A10 deviated significantly from linearity, there is no evidence that MPA exhibited cooperative effects on its activity. Hence, the capacity of MPA to show such an increase by four isozymes no doubt contributes to its high level of glucuronide production in vivo (Ding et al., 1993; Jacqz-Aigrain et al., 2000). Although MPA is highly prescribed because of its highly selective inhibition of IMPDH II and other beneficial effects (Transplantation Study Group, 1996), its extensive metabolism to glucuronides (Ding et al., 1993; Shütz et al., 1999; Jacqz-Aigrain et al., 2000), no doubt, contributes to high glucuronide accumulation in patient blood, which evidently compromises the efficacy of immunosuppression by MPA. Both MPAG and AcMPAG (M2) were observed at high concentrations in the plasma of transplant recipients receiving mycophenolate mofetil (Shipkova et al., 1999; Wieland et al., 2000), which indicates that our results are consistent with reports that found AcMPAG in humans.
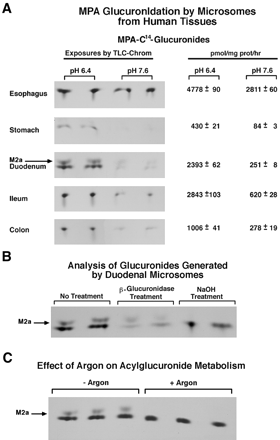
Glucuronidation by Microsomes Isolated from Human GI Tissues. Based on the pH optimizations shown in Fig. 2B, we carried out microsomal glucuronidation studies with MPA. Microsomes prepared from tissue specimen adjoining that used for in situ hybridization showed the relative levels of MPA conjugation as follows: esophagus, ileum, duodenum, colon, and stomach (Fig. 3A). Based on this pH profile and tissue distribution of mRNAs (Fig. 1), 1A10 and 1A9 could account for MPA glucuronidation in duodenum to colon (Fig. 3A). Only esophagus had significant pH 7.6 activity, and each tissue, except stomach, had relatively high pH 6.4 activity. Although we observed the highest pH 6.4 activity and approximately half that at pH 7.6 in esophageal microsomes, this result is not understood, since 1A7 is uniquely most abundant in this tissue (Fig. 1) and has from 2- to 4-fold greater pH 7.6 activity than have 1A8 and 1A9. This unexplained MPA glucuronidation at pH 6.4 in esophageal microsomes could be related to altered phosphorylation of 1A7 as described for 1A1 mutants (Basu et al., 2003). Also, we have evidence that 1A7 shifts its pH optimum for MPA from 7.6 to 6.4 following mutations at serine-432 (N. K. Basu, A. Garza, M. Kovarova, T. Saha, J. Rivera, and I. S. Owens, manuscript in preparation), similar to 1A1 for S435G mutants (Basu et al., 2003). It is possible that the 2:1 activity at pH 6.4 versus pH 7.6 with esophageal microsomes represents a phosphorylation effect in 1A7.
Glucuronidation of MPA by human microsomes from GI tissues.
A, UGT activity in microsomes isolated from tissue adjacent to in situ specimens of Fig. 1. Tissues were collected and stored at -80°C for microsome preparation and, for microsomal activity, were incubated 2 h as described under Materials and Methods. The autoradiogram (left) depicts the TLC plate that resolved glucuronides. Both ether-linked (MPAG) and acyl-linked (AcMPAG) glucuronides comigrate in this system and are the primary band in A, B, and C. M2a is designated as a derivative of AcMPAG, which was identified by Mojarrabi and Mackenzie (1997) and Shütz et al. (1999). Product (right) is mean ± S.E. A total of two esophagi, three stomachs, three duodena, two ilea, and four colons were analyzed three times in triplicates. B, analysis of glucuronide(s) generated by duodenal microsomes. Three sets of duplicate 2-h incubations were carried out with duodenal microsomes at pH 6.4. To one set adjusted to pH 6.8, β-glucuronidase (80 units) was added; incubations continued for 1 h at 37°C. One set was adjusted to 0.2 M NaOH to incubate 2 h at 37°C. M2a is identified under A. C, argon inhibition of the rapidly migrating band (M2a) generated by duodenal microsomes. Three of six duodenal microsomal reactions were flushed with argon, sealed, and incubated 2 h at 4°C before adding UDP-[14C]glucuronic acid; after the addition, tubes were again flushed, sealed, and incubated 2 h at 37°C; triplicate untreated tubes were control.
Characteristics of Glucuronide Products formed with Duodenal Microsomes. Because duodenal microsomes generated two bands during TLC resolution of glucuronides (Fig. 3B), we further characterized the products. As stated, we demonstrated that 1A7, 1A8, 1A9, and 1A10 formed AcMPAG (Fig. 2C); both MPAG and AcMPAG comigrate in the TLC system (Ciotti et al., 1995). The differentially migrating products generated by duodenal microsomes were sensitive to β-glucuronidase, confirming that each is a glucuronide (Fig. 3B). The rapidly migrating product, which we designated M2a, was sensitive to NaOH and consistent with an AcMPAG derivative (Shipkova et al., 1999) that accounted for 21 ± 2% of product at 200 μM MPA. Displacement of oxygen with argon before initiating the glucuronidation reaction eliminated the band (Fig. 3C), indicating that M2a requires oxygen for production. Argon inhibition suggests that duodenal microsomes carry out enzymatic oxidation that creates M2a.
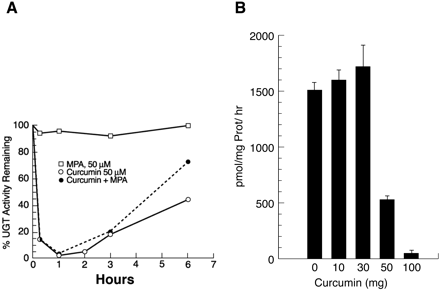
In Vivo Treatment with Curcumin Causes Inhibition of MPA Glucuronidation in LS180 and Mouse Duodenal Microsomes. Because the four GI distributed UGTs show robust conversion of MPA to glucuronides, and because we have observed that UGT1A1 requires phosphorylation for activity, which was inhibited by curcumin (Basu et al., 2003), we analyzed MPA glucuronidation for curcumin inhibition and reversibility in LS180 cells, as well as concentration-dependent inhibition of MPA glucuronidation in mouse duodenum. The time course study showed inhibition by 50 μM curcumin reached 80 to 98% between 0.25 and 1 h, with reversal evident by 3 h in the cell line and 50% recovered by 6 h (Fig. 4A). The combination of MPA and curcumin revealed a similar profile, whereas MPA alone had no effect on its glucuronidation. Additionally, we found curcumin inhibition of MPA glucuronidation in in vivo studies. Preliminary studies indicated that duodenal microsomes isolated from orally treated mice after a 30-min delay caused 100% inhibition of MPA glucuronidation; the delay allowed transit time to reach the tissue. Further studies showed that doses of 10, 30, 50, and 100 mg/kg b.wt. also led to 100% inhibition of duodenal microsomal activity and that 50 and 100 mg/kg maintained 61 to 64 and 94 to 96% inhibition of MPA glucuronidation up to 2 h (Fig. 4B), whereas activity for 10 and 30 mg/kg returned to or slightly above normal. Inhibition of MPA glucuronidation by curcumin, a UGT substrate, followed by recovery is consistent with its transient disruption of kinase activity (Chen and Huang, 1998; Tourkina et al., 2004), an effect that led to the observation that phosphorylation of 1A1 is required for bilirubin activity (Basu et al., 2003). Importantly, the MTT assay showed no cellular toxicity in the LS180 cells. Hence, curcumin inhibition indicates that this agent has the potential to transiently inhibit in vivo UGT to enhance therapeutic efficacy.
Inhibition of MPA glucuronidation by LS180 cells and mouse duodenal microsomes following in situ treatment with curcumin.
A, time course for curcumin inhibition of MPA glucuronidation in LS180 cells. Control MPA activity was 976 ± 42 pmol/mg protein/h and not detectable without substrate. Experiments were repeated three times; standard error was from ±1 to 5%. B, dose-response of mouse duodenal MPA glucuronidation after in vivo treatment with curcumin. Two hours after receiving curcumin (mg/kg body weight) orally, animals (3–5/group) were sacrificed, and duodena were collected. Microsomes were incubated with 200 μM MPA at 37°C for 2 h at pH 6.4. Activity is expressed as pmol glucuronide/mg protein/h.
Because our preliminary evidence, including T73A/G or T202A/G mutants at protein kinase C sites in 1A7 and 1A10, also led to null glucuronidation of MPA, indicating that both 1A10 and 1A7 require phosphorylation for activity (Basu et al., 2003) and that the former is 2-fold more resistant to dephosphorylation than 1A7, 1A8, and 1A9 (unpublished data), we do not know the phosphorylation and, thus, activity status of UGTs in “normal” tissue.
Since curcumin is a natural dietary constituent found in turmeric, which is consumed in large quantities (Chuang et al., 2000; Okada et al., 2001) without evidence of toxicity, it is possible to adapt it for pretreatment of animal models to inhibit MPA glucuronidation and increase bioavailability of free drug and immunosuppression, as well as diminish its glucuronidation. Evidence indicates that curcumin pretreatment of antigen-treated mice enhances immunosuppression by MPA to a substantial degree (N. K. Basu and I. S. Owens, manuscript in preparation).
A comparison of two previous studies on MPA glucuronidation with recombinant UGTs expressed in COS-7 (Mackenzie, 2000) and Spodoptera frugiperda (Sf)-9 cells (Shipkova et al., 2001) shows similarities (Mackenzie, 2000; Shipkova et al., 2001) and differences (Shipkova et al., 2001). Whereas substantial activity by 1A7 (Shipkova et al., 2001), 1A8 (Mackenzie, 2000), 1A9 (Shipkova et al., 2001), and 1A10 (Mackenzie, 2000; Shipkova et al., 2001) agrees with our findings, we found little to barely detectable MPA conversion by 1A1, 1A3, 1A4, 1A6, 2B7, or 2B15 at pH 6.4 or 7.6. Additionally, the difference in glucuronidation rates for 1A10:1A8: 1A9:1A7 is 1:1:2:4 at Vmax using optimal conditions compared with approximately equal rates (Shipkova et al., 2001). It should be pointed out, however, that the 10 members of the UGT1A and UGT2B families tested were inhibited by curcumin and calphostin-C (unpublished data).
In summary, the four major metabolizers, 1A7, 1A8, 1A9, and 1A10, of MPA exhibit in vitro stimulation of activity by MPA concentrations up to 1.6 to 2.4 mM before reaching saturation kinetics. Such expansive effects of 2- to 3-g doses of MPA on UGTs, no doubt, occur in vivo, resulting in high glucuronide production and adverse pharmacokinetics for free MPA. More importantly, further investigations could lead to the adaptation of the natural dietary constituent, curcumin, for use as a physiologically acceptable pretreatment agent to reversibly inhibit MPA glucuronidation, to increase efficacy of immunosuppression.
Footnotes
-
Abbreviations: UGT, UDP-glucuronosyltransferase; MPA, mycophenolic acid; GI, gastrointestinal; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium; DMSO, dimethyl sulfoxide; AcMPAG, acylmycophenolic acid-glucuronide; TLC, thin-layer chromatography; MPAG, mycophenolic acid-glucuronide.
- Received November 18, 2003.
- Accepted April 14, 2004.
- The American Society for Pharmacology and Experimental Therapeutics