Abstract
Previous studies in our laboratory showed that among cDNA-expressed human cytochrome P450 (P450) supersomes, CYP2C19 was the most active in methoxychlor-O-demethylation (Hu et al., 2004). However, based on the lack of inhibition of methoxychlor-O-demethylation by monoclonal anti-CYP2C19 antibodies in human liver microsomes (HLM), CYP2C19 did not seem to catalyze that reaction in HLM. By contrast, CYP2C9, much less active than CYP2C19 in supersomes, was the most active in HLM. The current study examines whether the lack of methoxychlor-O-demethylation by CYP2C19 in HLM was due to CYP2C19 exhibiting inferior competition for the NADPH-cytochrome P450 reductase (CPR) versus CYP2C9 and explores the interactions between CYP2C9 and CYP2C19 in a singular and binary complex of a reconstituted system. When reconstituted with CPR, cytochrome b5, and lipid, purified CYP2C19 and CYP2C9 catalyzed methoxychlor-O-demethylation. However, whereas equimolar CPR to CYP2C9 supported maximal rates of methoxychlor demethylation and diclofenac hydroxylation, the rate of methoxychlor demethylation by CYP2C19 was not fully saturated, even with a 9-fold molar excess of CPR over CYP2C19. This behavior of CYP2C19 was also observed with S-mephenytoin as the substrate. When a binary reconstitution system was prepared by mixing CYP2C9 and CYP2C19 enzymes, methoxychlor-O-demethylation and S-mephenytoin hydroxylation by CYP2C19 were dramatically inhibited. Inhibition depended on the amount of CPR and substrate used. By contrast, in the incubation containing CYP2C9, diclofenac hydroxylation was activated by the presence of CYP2C19. These results show that interactions among P450 enzymes can modulate their catalytic rates, which depend on the substrate undergoing metabolism.
Cytochromes P450 (P450s) are a superfamily of heme proteins that catalyze the oxidative metabolism of a wide range of endogenous compounds and xenobiotics. For most drugs, biotransformation facilitates their elimination by producing metabolites of higher polarity than the parent compounds, and metabolism by P450 enzymes is a common pathway (Rendic, 2002). It is commonly assumed that the ability of a particular cytochrome P450 to elicit its catalytic effect in liver microsomes could be predicted by its behavior in simple reconstituted systems or in cDNA-expressed supersomes. Therefore, recombinant P450 enzymes from different sources, e.g., from human lymphoblastoid cells and yeast and insect cells, have been widely used in drug metabolism research (Yamazaki et al., 2002). In reconstituted systems, NADPH-cytochrome P450 reductase (CPR) is generally supplied in excess of the single respective human P450. However, it is not possible to detect interactions among different P450s when reactions are studied with only a single expressed P450 enzyme in a reconstituted system. Therefore, studies to determine potential interactions among P450s are usually conducted with multiple P450s, because P450s can interact with each other and such an interaction could significantly alter their respective catalytic activity (Kaminsky and Guengerich, 1985; Alston et al., 1991; Cawley et al., 1995, 2001; Tan et al., 1997; Backes et al., 1998). There are several aspects of hepatic microsomal metabolism that could alter the catalytic activity of a particular P450 enzyme in microsomes compared with a single enzyme in a reconstituted system. 1) Microsomal cytochrome P450 enzymes require CPR, molecular oxygen, and, in some cases, cytochrome b5 to function as competent monooxygenases (Lu et al., 1969; Vatsis et al., 1982). For efficient catalysis, the heme iron of cytochrome P450 has to be reduced by the passage of electrons from NADPH via CPR, apparently forming a 1:1 functional complex of the CPR with P450 (Miwa et al., 1979). However, in hepatic microsomes, cytochromes P450 exist in a large excess over the CPR molar concentrations, ranging from 10:1 to 25:1, indicating that the amount of CPR present may be a limiting factor in metabolism (Estabrook et al., 1971; Peterson et al., 1976). In addition, unlike cytochromes P450, CPR levels are not readily elevated by inducers of the P450 systems. Typical P450 inducers, such as phenobarbital or pregnenolone-16α-carbonitrile, produce only a 2- to 3-fold increase in CPR levels, whereas other P450 inducers, such as 3-methylcholanthrene, have little or no effect on CPR concentrations (Gonzalez and Kasper, 1980; Taira et al., 1980; Hardwick et al., 1983; Simmons et al., 1987). Therefore, during the course of P450 induction, the cytochrome P450 to CPR ratio becomes even less favorable for optimal catalytic activity. Because CPR is normally limiting in tissue microsomes, different P450s must compete for the available CPR molecules. 2) In hepatic microsomal preparations, a number of cytochromes P450 coexist, and their mutual interaction could influence their respective individual function. For example, Alston et al. (1991) provided evidence that CYP1A1 and CYP3A2 but not CYP1A2 or CYP2E1 form complexes in microsomal membranes.
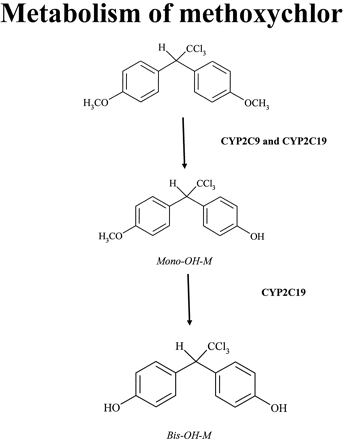
The P450-catalyzed metabolism of methoxychlor (Fig. 1), a currently used pesticide, was investigated in our laboratory (Hu and Kupfer, 2002). That study demonstrated that, in cDNA-expressed P450 supersomes (i.e., containing single P450s), CYP1A2 and CYP2C19 enzymes were the most active in mediating methoxychlor metabolism, with CYP2A6, CYP2B6, and CYP2C9 being only one-third to one-half as active in yielding oxidative metabolites of methoxychlor (Hu and Kupfer, 2002). However, in HLM, monospecific monoclonal anti-CYP2C19 antibodies did not inhibit methoxychlor monodemethylation, although the same antibodies extensively inhibited CYP2C19 activity in supersomes. The possibility that the lack of inhibitory activity of the monoclonal antibodies against CYP2C19 in HLM was due to inactivity of these antibodies when exposed to the HLM environment was excluded, because in HLM, these antibodies inhibited (by 96-98%) the hydroxylation of S-mephenytoin (a reaction probe used as positive control for CYP2C19 activity). By contrast, monoclonal anti-CYP2C9 antibodies were efficient in inhibiting methoxychlor-O-demethylation in HLM (Hu et al., 2004). Therefore, the focus of the present study was to investigate the possible interaction between CYP2C9 and CYP2C19 enzymes using a binary reconstituted system and to determine whether CYP2C9 can markedly modulate the catalytic activity of CYP2C19, and vice versa. Conventional chromatographic methods with UV and radiometric detections of metabolic transformations were used to measure cytochrome P450 activities to investigate the interactions among CYP2C9 and CYP2C19 enzymes. It was anticipated that such an investigation might help explain the reason for the pronounced CYP2C19 catalytic activity toward methoxychlor when present as a single catalyst but lack of CYP2C19 catalytic activity toward methoxychlor in the hepatic microsomal system.
Primary oxidative metabolites of methoxychlor formed by human liver P450s.
Materials and Methods
NADPH, 1,2-Didodecanoyl-rac-glycero-3-phosphocholine (DLPC), sodium phosphate, magnesium chloride, and [ring-UL-14C]methoxychlor (9.6 mCi/mmol) were purchased from Sigma-Aldrich (St. Louis, MO). Ultima Flow-M scintillation cocktail was obtained from Canberra Industries (Meriden, CT). Nonradiolabeled methoxychlor was obtained from Chem Service, Inc. (West Chester, PA). An HPLC Prodigy column (250 × 4.6 mm, 5 μm) was obtained from Phenomenex (Torrance, CA). Recombinant human CYP2C9 and CYP2C19 from overexpressed plasmids in Escherichia coli, purified human cytochrome b5, and recombinant human NADPH-CPR were purchased from Invitrogen (Carlsbad, CA). NADPH-cytochrome P450 reductase was quantified by UV spectrophotometry using an extinction coefficient of 21.4 mM-1 · cm-1 at 456 nm for the oxidized flavoprotein (Vermilion and Coon, 1978). Spectroscopic measurements were carried out using a Beckman DU 640B spectrophotometer (Beckman Coulter, Inc., Fullerton, CA).
Preparation of P450 Reconstituted System and Incubations with P450 Enzymes. Incubations were conducted in 4-ml vials under an atmosphere of air in a Dubnoff metabolic shaking incubator. The incubation contained 10 mM magnesium chloride in 50 mM sodium phosphate buffer (pH 7.4), substrate (methoxychlor, S-mephenytoin, or diclofenac at a concentration specified below), cytochrome P450, cytochrome b5, and CPR (amounts specified below). The molar ratio of P450/cytochrome b5 was kept at 1:1. This ratio was found to be optimal for CYP2C9 and CYP2C19-dependent catalytic activities (data not shown). The procedure for reconstitution of the monooxygenase system was very similar to that described previously (Vatsis et al., 1982). DLPC (2 mg) was dissolved in 1 ml of chloroform. The solution was evaporated to dryness at room temperature under a stream of nitrogen gas and subsequently suspended in 2 ml of water, yielding a final concentration of 1 mg of DLPC per milliliter of water. The resulting suspension was chilled in an ice-water bath and sonicated until clarification was observed.
The purified CYP2C9 and CYP2C19 enzymes were mixed at room temperature, followed by the addition of CPR and cytochrome b5, respectively. Then sonicated DLPC (30 μg) was added, followed by the addition of the phosphate buffer. This incubation mixture was preincubated on ice for 5 min. Then the substrate was added, the incubation mixture was kept at 37°C for 3 min, and the reaction was initiated by the addition of 2 mM NADPH in 100 μl of phosphate buffer. Control incubations were conducted without NADPH. In all cases, preliminary experiments were performed to verify that product formation was proportional to incubation time and cytochrome P450 concentration.
Methoxychlor Demethylation. The final incubation volume was 1 ml. The incubation mixture contained 50.0 μM methoxychlor (final concentration) added in 10 μl of ethanol, 25 pmol of CYP2C19, and/or 125 pmol of CYP2C9, cytochrome b5, and varying concentrations of CPR. Nonradioactive and radioactive methoxychlor were mixed to obtain a solution with appropriate methoxychlor concentration and selected specific radioactivity. The amount of radioactivity of the methoxychlor substrate was 15,000 dpm/incubation when using CYP2C19 and 130,000 dpm/incubation when using CYP2C9 or when both P450s were present. After 5 min of incubation time, reactions were terminated by the addition of 3 ml of diethyl ether and 1 ml of water with mixing and extraction. This was followed by a second extraction with 3 ml of diethyl ether. The extracts were combined (ca. 6 ml), and the solvent was evaporated under a stream of nitrogen gas at room temperature. The residue was dissolved in 300 μl of methanol and filtered through a 0.2-μm polytetrafluoroethylene (PTFE) filter, and the filtrate was subjected to HPLC analysis and quantified by the amount of radioactive metabolites formed.
S-Mephenytoin 4-Hydroxylase. The final incubation volume was 500 μl. The incubation mixture contained 100 μM S-mephenytoin (final concentration) added in 10 μl of ethanol, 25 pmol of CYP2C19, and/or 125 pmol of CYP2C9, cytochrome b5, and varying concentrations of CPR. After 15 min of incubation, reactions were terminated by the addition of 100 μl of ice-cold acetonitrile. The incubation mixture was centrifuged for 10 min in an Eppendorf 5415C centrifuge at 14,000 rpm. The supernatant was passed through a 0.20-μm PTFE filter, and the filtrate was subjected to HPLC analysis to identify the metabolites and determine the rates of product formation.
Diclofenac Hydroxylase. The incubation volume was 500 μl. The incubation mixture contained 100 μM diclofenac (final concentration) added in 50 μl of 10% ethanol/water, 25 pmol of CYP2C9, cytochrome b5, and varying concentrations of CPR in the reconstituted systems. After 20 min of incubation, reactions were terminated by the addition of 100 μl of ice-cold acetonitrile. The incubation mixture was centrifuged in an Eppendorf 5415C centrifuge at 14,000 rpm. The aliquots were passed through a 0.20-μm PTFE filter, and the filtrate was subjected to HPLC analysis to identify the metabolites and determine the rates of product formation.
Metabolite Analysis. Analyses of the metabolites generated by the reconstitution systems were conducted in the following HPLC apparatus: Waters 1525 binary HPLC pump, 2996 diode array UV detector, and 717 plus Autosampler (Waters, Milford, MA), and a Packard 500 TR flow scintillation radioactivity detector (Canberra Industries). The eluent/scintillation cocktail ratio was 1:3 (v/v) in all measurements containing radioactive substances. Analyses used a 5-μm Phenomenex Prodigy ODS3 column (250 × 4.6 mm). Radiolabeled methoxychlor oxidative metabolites were detected and quantified by their 14C radioactivity and measured as area under the curve of the metabolite peaks. 4-Hydroxymephenytoin and 4-hydroxydiclofenac were detected and quantified by their UV absorption at a wavelength specified below, comparing their retention times and UV spectrum with authentic compounds. Rates of product formation were determined by measuring the area under the curve of the metabolite peaks and comparing it to a standard curve prepared by measuring the area under the curve of various amounts of authentic standard. All data were analyzed using the mean of at least triplicate determinations; the deviation in each value was less than 15% of the mean.
The following chromatographic condition was used for the detection of methoxychlor metabolites (Fig. 1). The flow rate was 1 ml/min; the eluates were monitored by their UV absorption at 230 nm and radioactivity [modification of a method described by Hu and Kupfer (2002)]. The following HPLC conditions were used: pump A = 50% acetonitrile/water and pump B = 90% acetonitrile water. The following stepwise gradient was applied: 100% pump A from 0 to 4 min followed by 30% pump A until 8 min. From 8 min, a linear gradient of 100% pump B was used over 6 min. The retention times were 9 min for bis-OH-M, 14 min for mono-OH-M, and 17 min for methoxychlor.
The following chromatographic condition was used for the detection of 4-hydroxymephenytoin [modification of a method described by Hu et al. (2004)]. The flow rate was 1 ml/min; the metabolites were detected at 204 nm (determined to be the λmax). The HPLC solvent system consisted of the following: pump A = 20% acetonitrile/water and pump B = 40% acetonitrile/water. The following stepwise gradient program was used: 100% pump A for 0 to 5 min, 75% pump A and 25% pump B from 5 to 8 min, and 50% pump A and 50% pump B until 11 min, followed by 100% pump B. Retention times were 12 min for 4-OH mephenytoin and 18 min for S-mephenytoin.
The following chromatographic condition was used for the detection of 4-hydroxy-diclofenac [modification of a method described on the BD Gentest (Woburn, MA) Website]. The flow rate was 1 ml/min; the metabolite was detected at 267 nm (determined to be the λmax). The HPLC solvent system consisted of the following: mobile phase A = 20% acetonitrile, 79.9% water, and 0.1% HCl; and mobile phase B = 100% acetonitrile. Initial conditions were 100% A with a linear gradient to 100% B over 12 min. Retention times were 11 min for 4′-hydroxy-diclofenac and 13 min for diclofenac.
Results
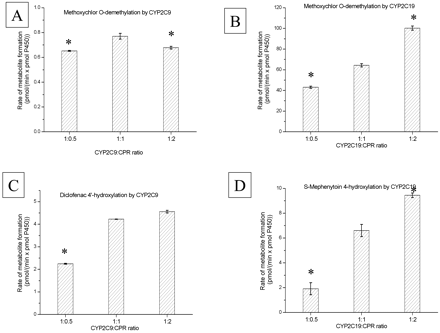
Characteristics of Single P450 Reconstituted Systems. The rate of methoxychlor-O-demethylation catalyzed by purified CYP2C9 in a reconstituted system increased as the concentration of CPR was increased up to a ratio of 1:1 (Fig. 2A). A further increased concentration of CPR caused a slight decline in CYP2C9-dependent methoxychlor-O-demethylation. In contrast, the rate of methoxychlor-O-demethylation catalyzed by CYP2C19 could not be fully saturated, even when the CYP2C19/CPR ratio was 1:2 (Fig. 2B). To determine whether this effect was merely due to the particular incubation conditions we used, experiments were carried out with various lipid concentrations; i.e., instead of using the usual 30 μg of lipid, we used 10 and 3 μg of lipid with CYP2C19 and 15 and 10 μg of lipid with CYP2C9 and employed a different (20 mM) Mg2+ concentration. The formation of O-demethylated methoxychlor metabolites was diminished as we decreased the lipid concentration and increased the ionic strength with either CYP2C9- or CYP2C19-dependent catalysis. Nevertheless, in all the systems we examined, methoxychlor-O-demethylation by CYP2C9 was saturated at a CYP2C9/CPR ratio of 1:1, followed by a decline in the rate of demethylation at a CYP2C9/CPR ratio of 1:2, whereas CYP2C19 activity could not be saturated, even at a 9-fold molar excess of CPR (data not shown). Likewise, saturation of CYP2C19 activity by a molar excess of CPR could not be achieved when CYP2C19 activity was examined using S-mephenytoin as a substrate. Indeed, the S-mephenytoin 4-hydroxylase activity (Fig. 2D) was augmented as increasing amounts of CPR were added to the reconstituted system. CYP2C9 was also able to catalyze the 4-hydroxylation of S-mephenytoin, yielding 0.06 pmol of product/(min × pmol P450) compared with 7.4 pmol of product/(min × pmol of P450) by CYP2C19 at a P450/CPR ratio of 1:1. Diclofenac 4′-hydroxylation by CYP2C9 showed a similar pattern, as observed in the CYP2C9-dependent O-demethylation of methoxychlor. The rate of diclofenac 4′-hydroxylation reached saturation at a CYP2C9/CPR ratio of 1:1 (Fig. 2C). At this P450/CPR ratio, CYP2C19 also exhibited diclofenac 4-hydroxylation activity (not shown), although to a much lower extent than did CYP2C9 [i.e., 4.2 pmol/(min × pmol of CYP2C9) compared with 0.6 pmol/(min × pmol of CYP2C19)]
Effect of various molar ratios of CYP2C9 and CYP2C19 to CPR on the metabolic activity of reconstituted systems. See Materials and Methods for incubation conditions. Values represent the mean ± S.E. of at least three determinations. *, statistically significant (p < 0.05) difference between the enzymatic activities (compared with the enzymatic activity measured at a P450/CPR ratio of 1:1). A, methoxychlor-O-demethylation by CYP2C9 (125 nM CYP2C9 per incubation). B, methoxychlor-O-demethylation by CYP2C19 (25 nM CYP2C19 per incubation). C, diclofenac 4′-hydroxylation by CYP2C9 (50 nM CYP2C9 per incubation). D, S-mephenytoin 4′-hydroxylation by CYP2C19 (50 nM CYP2C19 per incubation).
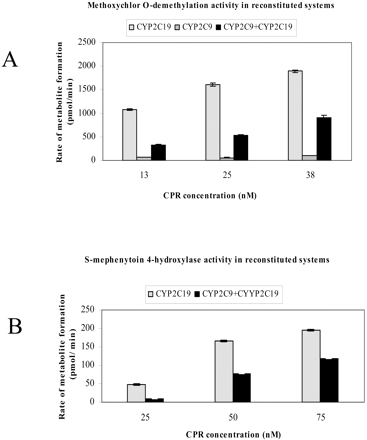
Effect of CYP2C9 on CYP2C19-Catalyzed Methoxychlor-O-demethylation and S-Mephenytoin 4′-Hydroxylation. To determine whether the presence of CYP2C9 alters the catalytic activity of CYP2C19, methoxychlor-O-demethylation (Fig. 3A) and S-mephenytoin 4-hydroxylation (Fig. 3B) were examined in both single and binary reconstituted systems. Because the level of CYP2C9 in human liver was reported to be approximately 5-fold that of CYP2C19 (Gibson and Skett, 2001), a CYP2C9/CYP2C19 ratio of 5:1 was used in the current study. Methoxychlor metabolism by CYP2C19 was greatly inhibited in the presence of CYP2C9 (Fig. 3A). The degree of this inhibition depended on both the substrate used and the amount of CPR added to the reconstituted system; in fact, the extent of inhibition was smaller with S-mephenytoin than with methoxychlor (Fig. 3B).
Effect of CYP2C9 on CYP2C19-mediated metabolism of methoxychlor and S-mephenytoin as a function of NADPH-CPR concentration. A, methoxychlor-O-demethylation. Methoxychlor-O-demethylation was determined in both single (CYP2C9 or CYP2C19) and binary (CYP2C9 + CYP2C19) reconstituted systems. The concentration of CYP2C9 was 125 nM; the concentration of CYP2C19 was 25 nM. B, S-mephenytoin 4′-hydroxylation. S-Mephenytoin 4′-hydroxylation was determined in single (CYP2C19) and binary (CYP2C9 and CYP2C19) reconstituted systems. The concentration of CYP2C9 was 250 nM; the concentration of CYP2C19 was 50 nM.
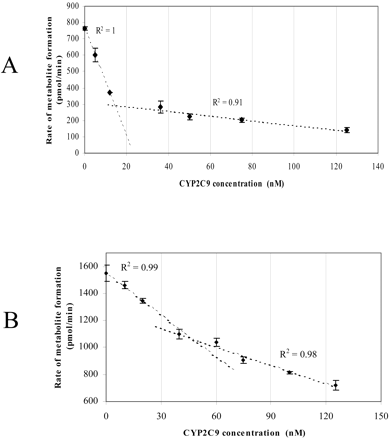
To distinguish between the two possibilities of 1) direct interactions of CYP2C9 and CYP2C19 with each other and 2) competition between the two P450s for the available CPR, the effect of varying CYP2C9 concentrations while maintaining fixed concentrations of CYP2C19 and CPR on the catalytic activity was examined (Fig. 4, A and B). A biphasic response was observed, with a steep decrease in methoxychlor-O-demethylation activity caused by the addition of small amounts of CYP2C9; ca. 50% inhibition occurred by adding 12 pmol (12 nM) of CYP2C9, and ca. 30% inhibition occurred by adding 40 pmol (40 nM) of CYP2C9 at a CYP2C19/CPR ratio of 2:1 (Fig. 4A) and CYP2C19/CPR ratio of 1:2 (Fig. 4B), respectively, compared with the activity in the absence of CYP2C9. This was followed by a segment in the graph that was much less affected by the further addition of CYP2C9 (there was a further 30 and 24% inhibition at 125 pmol (125 nM) of CYP2C9 concentration at a CYP2C19/CPR ratio of 2:1 (Fig. 4A) and CYP2C19/CPR ratio of 1:2 (Fig. 4B), respectively. Linear regression was fitted to the data points where steep decrease and less effect were observed. Extrapolation of these two lines resulted in an intersection at a concentration equal to the P450 reductase in the sample at both the CYP2C19/CPR 2:1 and CYP2C19/CPR 1:2 ratios (Fig. 4, A and B).
The effect of CYP2C9 on CYP2C19-dependent methoxychlor metabolism. Values represent the mean ± S.E. of at least three determinations. See Materials and Methods for incubation conditions. A, effects at a CYP2C19/CPR ratio of 2:1. B, effects at a CYP2C19/CPR ratio of 1:2. The amount of CYP2C19 added was 25 pmol/1 ml of incubation (i.e., 25 nM).
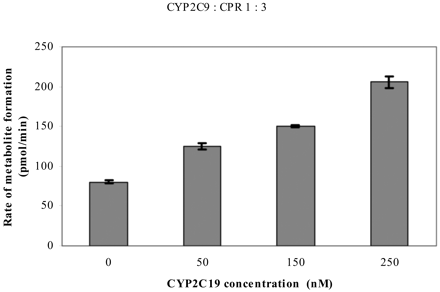
Effect of CYP2C19 on the CYP2C9-Catalyzed Reaction. To determine whether the catalytic activity of CYP2C9 enzyme is also influenced by the presence of CYP2C19, diclofenac 4′-hydroxylase activity was examined in the presence of CYP2C19 using a CYP2C9/CPR ratio of 1:3 (Fig. 5). It should be noted that—because the CYP2C9/CPR ratio was kept constant throughout the experiment—an increasing amount of CYP2C19 enzyme resulted in an increase in the total P450/CPR ratio. In spite of this, activation of diclofenac 4′-hydroxylation activity was observed. This activation continued to increase with increasing amount of CYP2C19 enzyme.
Effect of CYP2C19 on CYP2C9-dependent diclofenac 4′-hydroxylation. Values represent the mean ± S.E. of at least three determinations. Effects at a CYP2C9/CPR ratio of 1:3. See Materials and Methods for incubation conditions. The amount of CYP2C9 added was 25 pmol/0.5 ml of incubation (i.e., 50 nM).
Discussion
Our previous findings in supersomes demonstrated that among cDNA-expressed human P450s, CYP2C19 was the most active in methoxychlor-O-demethylation, whereas in HLM, CYP2C19 was found to contribute only to a minimal extent, if at all, to methoxychlor demethylation (Hu et al., 2004). This observation provides a noteworthy example that a single-enzyme model of metabolism could lead to erroneous conclusions when trying to extrapolate the data obtained in that model to the in vivo situation. Although our data and that of other investigators (Kaminsky and Guengerich, 1985; Tan et al., 1997; Backes et al., 1998; Li et al., 1999) established that the presence of multiple P450 enzymes can influence the catalytic properties of a particular P450, the mechanistic details of how one P450 affects the catalytic activity of another remained to be clarified. Consequently, in the current study we attempted to determine whether the inhibition of methoxychlor-O-demethylation by CYP2C19 in the presence of other P450 enzymes was due to direct interaction of the P450 enzymes with each other or to a competition among the P450s for the limited amount of CPR present in liver microsomes. Because monoclonal anti-CYP2C9 antibodies were the most effective in inhibiting the oxidative metabolism of methoxychlor in HLM, the possible interactions between CYP2C9 and CYP2C19 were investigated. The level of expression of CYP2C9 in human liver microsomes seems to be approximately 5-fold that of CYP2C19 (Gibson and Skett, 2001) or possibly even higher (Venkatakrishnan et al., 1998); therefore, to approximate that relationship in a binary reconstituted system, a 5:1 ratio of CYP2C9/CYP2C19 was usually employed.
Several factors seem to influence the oxidative metabolism in a reconstituted system, including the particular cytochrome P450 enzyme, the ionic strength of the medium, the concentration of the lipid, and the substrate used (Causey et al., 1990; Voznesensky and Schenkmann, 1992; Backes and Kelley, 2003). Therefore, in an attempt to determine whether some of the observations in the current study could have been due to an artifact of the incubation conditions, experiments were carried out under a variety of conditions, including different lipid and Mg2+ concentration. The effects of increasing CPR concentration on methoxychlor-O-demethylation by CYP2C9 and CYP2C19 were not unique for an individual set of conditions, but the same tendency on catalytic activity was observed under all experimental conditions examined (data not shown). In addition, the duration of preincubation of the reconstituted system for up to 2 h was previously shown to affect enzymatic activities when the lipid/cytochrome P450 ratio was 300:1 (Causey et al., 1990); however, when the lipid concentration was more than 800 M excess with respect to the cytochrome P450, there was no effect on the enzymatic activity of varying the preincubation time. In the current studies, which compared the 2-h preincubation time of the reconstituted system (and a more than 800-fold molar excess of the lipid) with a brief preincubation time, there was no alteration in the enzymatic activities of CYP2C9-dependent diclofenac 4′-hydroxylation and CYP2C19-dependent S-mephenytoin 4-hydroxylation. Consequently, brief preincubation times were used in our experiments.
Methoxychlor metabolism as a function of the P450/CPR ratio by CYP2C9 and CYP2C19 showed a different pattern (Fig. 2, A and B); CYP2C9-mediated methoxychlor-O-demethylation activity declined at a P450 reductase concentration higher than the equimolar concentration with P450, whereas the methoxychlor-O-demethylation activity of CYP2C19 continued to increase and was not fully saturated, even at a cytochrome P450/CPR ratio of 1:9. Although the mechanism for the decline in CYP2C9 activity by excess P450 reductase concentration is not understood, similar results were observed by Dutton and Parkinson (1989); in their study, a molar excess of CPR to P450 inhibited the O-dealkylation of 7-pentoxy- and 7-benzoxy-resorufin catalyzed by rodent CYP2B1 and P450e. In addition, the rate of 7-ethoxyresorufin O-dealkylation could not be fully saturated, even with a 50-fold molar excess of CPR over CYP1A1. Interestingly, the behavior of the single-enzyme-containing reconstituted system as a function of the P450/CPR ratio depended more on the P450 enzyme than on the substrate used (Fig. 2, A-D). Namely, both CYP2C19-dependent S-mephenytoin and methoxychlor metabolism could not be saturated by a molar excess of CPR, whereas CYP2C9-dependent metabolism of both methoxychlor and diclofenac demonstrated saturation at about a CYP2C9/CPR ratio of 1:1. A possible explanation for these observations is that CYP2C19 exhibits markedly lower affinity for CPR than CYP2C9. Indeed, earlier studies demonstrated that different cytochromes P450 display diverse affinities toward CPR (in the absence of a substrate), e.g., with rabbit liver CYP2B4 showing the lowest affinity for CPR compared with several rat liver P450s (Tamburini at al., 1986). In accordance with that finding, CYP2B4-dependent benzphetamine demethylation and 7-pentoxyresorufin O-dealkylation continued to increase up to a P450/CPR ratio of 1:3 with the addition of the P450 reductase (Cawley et al., 1995). It is also possible that substrate binding to CYP2C19 only minimally influences the affinity for CPR. Substrate binding was previously shown to increase the affinity of certain P450s toward CPR; however, benzphetamine binding to CYP2C11 did not alter CYP2C11 affinity toward CPR (Tamburini et al., 1986), although benzphetamine is metabolized by CYP2C11 (Yamada et al., 1997). Our results that CYP2C19-dependent methoxychlor and S-mephenytoin metabolism was inhibited by the presence of CYP2C9 (Fig. 3, A and B) also demonstrate that ligand characteristics that can enhance the affinity of a particular P450 enzyme for CPR could be different from the characteristics that are most favorable for their catalysis. It should be emphasized that our results observed in the reconstituted systems were also observed in human liver microsomes. As mentioned before, although among cDNA-expressed P450s, CYP2C19 possessed the highest activity of methoxychlor metabolism in supersomes but was unable to compete with CYP2C9 for the same reaction in the hepatic microsomes (Hu et al., 2004). In addition, CYP2C19 in the cDNA expression system was capable of S-mephenytoin hydroxylation at a rate that is at least 10-fold higher than the rate in human liver microsomes (Goldstein et al., 1994). The question of why various P450s markedly differ in their affinities for the P450 reductase has not been hitherto extensively addressed. There is the possibility that the amino acid sequences of the CPR binding sites in the various P450s are not well conserved and that certain P450 sequences are much better suited for P450 reductase binding. In addition, with respect to the substrate-mediated enhancement of P450 reductase binding, there is a possibility of certain substrates eliciting conformational changes in the P450s that are more favorable for P450 reductase binding. These are important questions that would require further studies to determine the factors and causes that lead to these important interactions.
Our studies of methoxychlor and mephenytoin metabolism by CYP2C19 in a single reconstituted system and in a binary system containing both CYP2C19 and CYP2C9 enzymes (Fig. 3, A and B) clearly showed that these P450s modulate each other's functions in a complex system. Using a similar approach as in the studies of Cawley et al. (1995) and Backes et al. (1998), two possible mechanisms were proposed: 1) there is a simple competition between CYP2C9 and CYP2C19 for the CPR, and CYP2C9 exhibits a much higher affinity toward CPR than CYP2C19 and is therefore capable of preferentially sequestering the CPR; or 2) CYP2C19 and CYP2C9 form a binary complex in the presence of the substrate, with altered affinity toward CPR rather than in the CYP2C19-CPR or CYP2C9-CPR individual complexes. The CYP2C9 moiety of this binary complex exhibits higher—although altered—affinity for the CPR than the CYP2C19 moiety; therefore, it apparently draws CPR away from the CYP2C19 moiety, especially at subsaturating CPR concentrations. Both models would help explain our findings that the extent of inhibition of methoxychlor and S-mephenytoin metabolism by the binary (CYP2C19 + CYP2C9) reconstitution system compared with a single CYP2C19 reconstitution system depended on the CPR concentration, with a decreasing extent of inhibition as CPR concentration was being increased (Fig. 3, A and B). At a fixed CPR concentration (12.5 or 50 nM CPR and 25 nM CYP2C19), the inhibitory effect of increasing the amount of CYP2C9 on methoxychlor metabolism was found to depend on the amount of CPR rather than that of CYP2C19 (Fig. 4, A and B). These findings again can be explained by both models, because they both assume that CYP2C9 preferentially binds the CPR at the expense of CYP2C19 either by forming a simple complex with CPR with a much higher affinity than does CYP2C19 or that the CYP2C9 moiety of the CYP2C19-CYP2C9 binary complex exhibits higher affinity toward CPR. Although the affinity for CPR is apparently altered in a CYP2C9-CYP2C19 complex, in both cases it is CYP2C9 that exhibits the higher affinity toward the CPR. Therefore, the rate of metabolism depends primarily on the CPR concentration and less on the available CYP2C9 forming the complex with CYP2C19. However, if CYP2C9 and CYP2C19 were merely involved in a simple competition for the P450 reductase (first model), then the CYP2C9-dependent diclofenac hydroxylase activity should not have been significantly influenced by the presence of CYP2C19, because CYP2C19 has a much lower affinity for CPR. However, diclofenac hydroxylase activity was actually activated in the presence of CYP2C19 (Fig. 5), and the extent of activation increased with increasing CYP2C19 concentration. This finding cannot be explained by the model of a simple competition between CYP2C9 and CYP2C19 enzymes for the CPR. Rather, a possible explanation will involve a direct interaction between CYP2C9-CYP2C19 enzymes forming CYP2C9-CYP2C19 complexes (i.e., the second model), with the CYP2C9 moiety of this complex possessing a higher activity toward the CPR than the CYP2C9-CPR complex.
In conclusion, our results demonstrate that, even if a substrate is efficiently metabolized by a particular P450 enzyme, it is not the sole factor affecting the affinity of that P450 for the CPR. Thus, our results provide evidence that interactions among P450 enzymes can modulate their catalysis in either direction, e.g., by decreasing or increasing their catalytic reaction rates, these depending on the substrate undergoing metabolism.
Footnotes
-
doi:10.1124/dmd.104.001578.
-
ABBREVIATIONS: P450, cytochrome P450; CPR, cytochrome P450 reductase; HLM, human liver microsome(s); DLPC, 1,2-didodecanoyl-racglycero-3-phosphocholine; HPLC, high-performance liquid chromatography; PTFE, polytetrafluoroethylene; mono-OH-M, 1,1,1-trichloro-2-(4-hydroxyphenyl)-2-(4-methoxyphenyl)ethane; bis-OH-M, 1,1,1-trichloro-2,2-bis(4-hydroxyphenyl)ethane.
- Received July 28, 2004.
- Accepted October 8, 2004.
- The American Society for Pharmacology and Experimental Therapeutics