Abstract
Clinical use of the nonsteroidal anti-inflammatory drug diclofenac (DF) is associated with an incidence of idiosyncratic hepatoxicity. The formation of reactive metabolites of DF in vivo has been proposed to be responsible for such toxicity. One type of reactive metabolite, a benzoquinone imine of DF formed through oxidation by cytochromes P450, can be trapped by glutathione in vitro in liver microsomes to form glutathione (GS) adducts. Three GS adducts from DF were reported in the literature, namely, 5-hydroxy (OH)-4-glutathione-DF, 4′-OH-3′-glutathione-DF and 5-OH-6-glutathione-DF, and they all have the same molecular weight of 616. Recently, we developed a sensitive and high throughput method for the detection of GS adducts from liver microsome incubation. This method uses a constant neutral loss scan of m/z 129, a “structure-characteristic” fragment for GS adduct, on an automated chip-based nanoelectrospray (Advion NanoMate 100) attached to a tandem mass spectrometer (Sciex API 3000). The analysis of GS adducts from human liver microsome incubation with DF by the NanoMate 100-API 3000 method unambiguously revealed a new adduct ion with m/z 583 (MH+), in addition to the known adduct peak with m/z 617 (MH+). This new adduct was further confirmed to be 4′-OH-2′-glutathion-deschloro-diclofenac by liquid chromatography (LC) tandem mass spectrometry (MS), LC/MS-NMR, and comparison to a synthetic standard.
Diclofenac (DF) is a nonsteroidal anti-inflammatory drug that is widely used for the treatment of osteoarthritis and rheumatoid arthritis (Small, 1989). Chemically reactive metabolites of DF have been proposed to explain the idiosyncratic hepatoxicity associated with the clinical use of the drug (Banks et al., 1995; Miyamoto et al., 1997; Shen et al., 1999; Tang et al., 1999b; Tang, 2003). One type of reactive metabolite associated with the formation of protein adducts (Maggs et al., 1995) is a benzoquinone imine intermediate generated via oxidation by cytochromes P450 (Shen et al., 1999; Poon et al., 2001). Metabolism of DF has been studied extensively (Corcoran et al., 2000), and it was proposed that P450-catalyzed oxidation of DF resulted in the formation of DF-2,5-quinone imine and DF-1′,4′-quinone imine intermediates through initial formation of 5-OH-DF and 4′-OH-DF, respectively (Tang et al., 1999a). These reactive intermediates can be trapped by glutathione (GSH) to form GS adducts (Baillie and Slatter, 1991) in vitro (in liver microsomes or hepatocytes), as well as in vivo (in bile and urine; often detected as degradation products, N-acetylcysteine, of the initially formed GS adduct). Three GS adducts from DF were reported in the literature, namely, 5-OH-4-GS-DF, 4′-OH-3′-GS-DF, and 5-OH-6-GS-DF (Tang et al., 1999a), and they all have the same molecular weight of 616.
A neutral loss (NL) scan of m/z 129, a “structure-characteristic” fragment (loss of glutamate) for glutathione adducts, by liquid chromatography-tandem mass spectrometry (LC/MS/MS) was developed to detect reactive metabolite formation (Baillie and Davis, 1993) from a GSH trapping assay in liver microsomes. This methodology has the potential to detect unknown GS adducts in a drug biotransformation study. However, the relatively poor sensitivity associated with a NL scan coupled with a long LC run time (5–35 min per sample) has limited its broad use as a high-throughput screen for drug discovery. To develop a more sensitive NL scan method with higher throughput capacity for the detection of GS adducts, we investigated an automated chip-based nanoelectrospray ion source (Advion NanoMate 100) attached to a tandem mass spectrometer (Sciex API 3000). The NanoMate 100-API 3000 NL scan of m/z 129 with run time < 1 min was found to be ∼100-fold more sensitive than the conventional LC-API 3000 method by direct comparison with four authentic standards of GS adducts (Chen et al., 2004). The analysis using the NanoMate 100-API 3000 for GS adducts in human liver microsome incubated with DF unambiguously revealed a new adduct ion with m/z 583 (MH+), in addition to the known adduct peak with m/z 617 (MH+). This new adduct was confirmed to be 4′-OH-2′-glutathion-deschloro-diclofenac (4′-OH-2′-GS-DDF) by LC/MS/MS, LC/MS-NMR, and comparison to a synthetic standard.
Materials and Methods
Materials. Diclofenac, GSH, and NADPH were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals and solvents were obtained from Aldrich Chemical Co. (Milwaukee, WI). Pooled human liver microsomes (n = 59) were obtained from an in-house bank of liver microsomes maintained at Pfizer Global Research and Development (Groton, CT). Microsomal P450 content was 0.33 nmol/mg protein. 4′-OH-DF was obtained from the Pfizer sample bank, and the identify and purity were confirmed by LC/MS, m/z 312 (MH+), and 1H NMR: δ3.68 (s, CH2COOH); 6.23 (d, J = 8 Hz, 3-CH), 6.80 (t, J = 8.0 Hz, 5-CH); 6.93 (s, 3′- and 5′-CH); 7.02 (t, J = 8 Hz, 4-CH); 7.15 (d, J = 8 Hz, 6-CH).
Instrumentation and Analytical Methods. LC/MS/MS was carried out with a Sciex API-3000 tandem mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA) interfaced to a high-performance liquid chromatography system consisting of two LC-10Avp pumps and a static-bed mixer (Shimadzu, Kyoto, Japan). LC/MS/MS experiments were performed with an ion spray interface with positive ion detection at a voltage of 2.5 kV. The orifice potential was 30 V, and the collision energy was 35 eV. Chromatography was performed on a Synergi Max-RP 80A column (4.60 × 150 mm, 4 μm; Phenomenex, Torrance, CA), and samples were delivered at a flow rate of 0.75 ml/min. The mobile phase consisted of MeOH (solvent A) and 0.1 mM aqueous solution of ammonium acetate with 1% of 2-propanol (solvent B) and was programmed by a linear increase from 5% to 50% during a 20-min period.
NanoESI-MS/MS was carried out with a NanoMate 100 (Advion BioSciences, Ithaca, NY), which is a combined autosampler (a rack of 96 disposable and conductive pipette tips and a 96-well microtiter plate containing samples to be analyzed) and a nanoESI source (a chip-based nanoelectrospray device with 100 nozzles), mounted to a mass spectrometer (Sciex API-3000). The details of this system can be found elsewhere (Kapron et al., 2003; Van Pelt et al., 2003). A sample solution (5 μl) was delivered at a flow rate ≤300 nl/ml to the nanoESI source for up to 2 min. The positive ion model electropspray ionization process occurred from individual nozzles initiated by a 1400 V potential applied through the pipette tip. The ion source temperature was 40°C, and the orifice potential was 30 V. The collision energy was 35 eV.
The LC/MS-NMR system consisted of an Agilent 1100 binary pump (Agilent Technologies, CA), an Agilent 1100 auto injector, a Bruker BioSpin BSFU-0 column oven (Bruker BioSpin, Billerica, MA), a Bruker BioSpin photodiode array detector, a Bruker BioSpin BNMI interface using a 100:1 split, a Bruker Daltonics Esquire 3000 ion trap MS (Bruker Daltonics, Billerica, MA) equipped with an electropsray source, a Bruker BioSpin BPSU-36 peak storage unit, and a Bruker BioSpin 600MHz Avance DRX Spectrometer equipped with a 4-mm 1H-13C inverse z-gradient LC flow probe. The NMR to MS split ratio was 100:1. Chromatographic separation was achieved on a Synergi Polar RP 4-μm 3.0 × 150 mm column (Phenomenex) at ambient temperature. A mobile phase gradient of (0.1% trifluoroacetic acid-D in D2O)-(acetonitrile-D3), 90:10 to 60:40 over 45 min, at a flow rate of 0.5 ml/min was used to separate the metabolites. One percent of the effluent was split off postcolumn and diluted with 9:1 acetonitrile-D3:D2O containing 0.2% acetic acid-D4 at a flow rate of 20 μl/min prior to entering the mass spectrometer. The remaining 99% passed through the diode array detector, and the peaks of interest were stored in the BPSU-36 unit using the loop storage technique for subsequent introduction into the NMR spectrometer. 1H NMR spectra were obtained at 305 K on the LC peaks using one-dimensional nuclear Overhauser effect spectroscopy-type double presaturation of solvent NMR resonances. All chemical shifts are reported in ppm downfield from tetramethylsilane as referenced to acetonitrile-D3 at 1.93 ppm.
Synthesis of Diclofenac GS Adducts (4′-OH-3′-GS-DF and 4′-OH-2′-GS-DDF). Synthetic glutathione adducts were prepared by first oxidizing 4′-OH-DF to the quinone imine with MnO2 in a mixture of ethyl acetate and acetone. The dark purple product was purified by passing through a plug of silica using 5% methanol/dichloromethane eluent. The pure quinone imine was concentrated to dryness and then treated with GSH in either water or pH 7 phosphate buffer solvent. The reaction gave a mixture of 4′-OH-3′-GS-DF and 4′-OH-2′-GS-DDF, with the ratio being pH-dependent.
Incubations with Human Liver Microsomes. DF (10, 30, or 100 μM) and GSH (10 mM) in 0.1 M phosphate buffer (pH 7.4) were mixed with human liver microsomes (1 μM P450s). The reaction was initiated by addition of NADPH (5 mM final concentration) and incubated at 37°C for 1 h. After 30 min of incubation, additional NADPH (5 mM final concentration) was added to the mixture. The negative controls with either no NADPH or no DF were incubated as described above. The reaction was quenched with 2 volumes of MeOH to precipitate proteins, followed by centrifugation at 3500 rpm for 5 min. The supernatant was removed to a new vial and dried down by a stream of nitrogen at 37°C. The resulting residue was reconstituted with 5% MeOH in 1% HCl-water solution. The reconstituted solution was loaded three times on an HLB μElution plate (Waters, Milford, MA) under a vacuum of 5 mm Hg, and then washed three times with water. The metabolites were eluted from the HLB μElution plate with MeOH. After evaporating the solvent, the residue was dissolved in MeOH-H2O (75:25) containing 1% acetic acid and analyzed directly by NanoMate 100-API 3000, or LC/MS/MS or LC/MS-1H NMR.
Results and Discussion
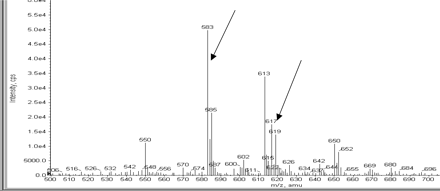
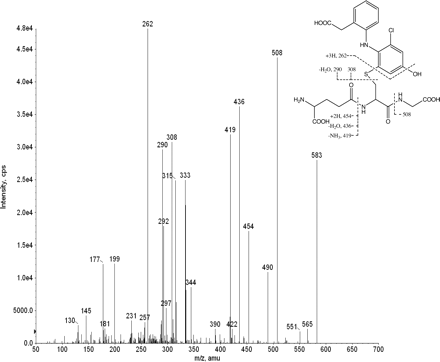
Figure 1 shows a NL scan of m/z 129 on the NanoMate 100-API 3000 for the GS adducts of DF from the microsomal incubation mixture. The ions (MH+) with m/z 583 and 617 were detected from the incubation mixture containing DF, human liver microsomes, NADPH, and GSH, but not from the negative control samples (in the absence of either DF or NADPH). The abundant signal at m/z 613 corresponds with protonated glutathione dimer, GSH-glutathione disulfide. The ion with m/z 583 exhibited the characteristic one-chlorine isotope cluster (m/z 583:585 at a 1:0.3 ratio) and has not been previously reported. The ion with m/z 617 gave the characteristic two-chlorine isotopes cluster (m/z 617:619 at ∼1:0.7 ratio), consistent with the known GH adducts of DF in the literature (Miyamoto et al., 1997; Tang et al., 1999a,b). Subsequent collision-induced dissociation of the ion at m/z 583 with the NanoMate 100-API 3000 produced product ions at m/z 508, 454, and 436, consistent with neutral losses of glycine (75 Da), pyroglutamate (129 Da), and ammoniated pyroglutamic acid (147 Da), respectively, suggesting that this ion at m/z 583 is a GS adduct of DF (Fig. 2). The structure of this new adduct is proposed to be 4′-OH-2′-GS-DDF (Fig. 3).
NL scan of m/z 129 on NanoMate 100-API 3000 for detection of GS adducts of DF from an incubation containing DF, human liver microsomes, NADPH, and GSH. amu, atomic mass units.
Collision-induced dissociation spectrum of product ion m/z 583 on NanoMate 100-API 3000. amu, atomic mass units.
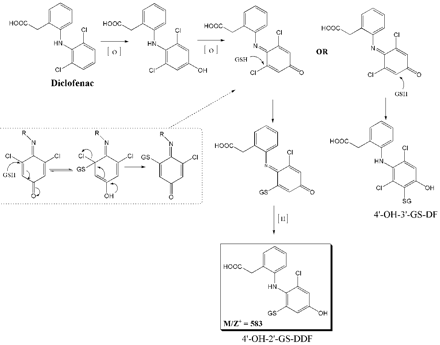
Proposed metabolic pathways leading to the formation of 4′-OH-2′-GS-DDF and 4′-OH-3′-GS-DF through cytochrome P450-mediated biotransformation.
To rule out the possibility that the formation of 4′-OH-2′-GS-DDF was an artifact caused by the nanoelectrospray system (no LC separation), the microsomal incubation mixture was analyzed by LC/MS/MS using a product ion scan of m/z 583 and m/z 617. The metabolite adduct with m/z 583 (4′-OH-2′-GS-DDF) was easily detected at the retention time of 18.4 min, in addition to the other expected GS adducts at m/z 617 (data not shown). The product ion spectrum of m/z 583 obtained by LC/MS/MS was almost identical to that obtained with the NanoMate 100-API 3000 (Fig. 2).
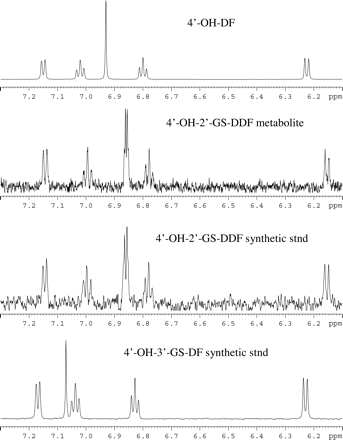
To further confirm the structure of 4′-OH-2′-GS-DDF, the microsomal incubation mixture was analyzed by LC/MS-1H NMR. Figure 4 shows a comparison of the 1H NMR aromatic region from the analysis of 4′-OH-DF, 4′OH-2′-DS-DDF metabolite in the microsomal incubation mixture, 4′-OH-2′-GS-DDF synthetic standard, and 4′-OH-3′GS-DF synthetic standard. The metabolite with retention time of 30.4 min and m/z consistent with 583 plus partial exchanged deuterium from the mobile phase shows two strongly coupled doublet peaks at δ 6.85 and δ 6.86 ppm, with total integration of two protons and a coupling constant of 2.4 Hz. This indicates two asymmetric protons from the phenol ring meta-positioned to each other. The spectral pattern of the other four aromatic protons is similar to those in the 4′-OH-DF standard. In contrast, the spectrum of 4′-OH-3′-GS-DF from the synthetic standard, analyzed under the same LC/MS-NMR conditions, has a singlet peak at δ 7.07 ppm, which integrates to one proton. This peak corresponds to the 5′-CH, which is downfield shifted compared with 4′-OH-DF and 4′-OH-2′-GS-DDF. Therefore, the LC/MS-1H NMR spectra of 4′-OH-2′-GS-DDF is in complete agreement with the proposed structure.
1H NMR spectra of 4′-OH DF and the GSH adduct of DF metabolites. stnd, standard.
The metabolic pathway leading to the formation of 4′-OH-2′-GS-DDF is proposed in Fig. 3. Following formation of the DF-1′,4′-quinone imine intermediate through initial formation of 4′-OH-DF, GSH may attack either at the 3′-position to give the known 4′-OH-3′-GS-DF or at the chlorine position by an ipso substitution, resulting in 4′-OH-2′-GS-DDF (Liu and Kurth, 2002). To support this hypothesis, both 4′-OH-3′-GS-DF and 4′-OH-2′-GS-DDF were independently synthesized by the addition of GSH to purified DF-1′,4′-quinone imine. This relatively stable quinone imine was prepared by a chemical (MnO2) oxidation of 4′-OH DF. The MS/MS fragmentation patterns, high-performance liquid chromatography retention times, and 1H NMR spectra were identical for the synthetic adducts and the ones from the microsomal incubation mixture.
The ipso substitution of a halogen from a hydroquinone derivative by GSH has been previously reported (Erve et al., 2004). Formation of 4′-OH-2′-GS-DDF through a similar ipso substitution is mechanistically feasible, as evidenced by the chemical synthesis. In addition, 4′-OH-2′-GS-DDF appeared to be more abundant than the known adduct, 4′-OH-3′-GS-DF, based on the NL scan signal intensity (Fig. 1), suggesting that 4′-OH-2′-GS-DDF may be an important adduct. However, to the best of our knowledge, no 4′-OH-2′-GS-DDF has ever been reported. The reasons why this adduct was not identified from human liver microsomal incubation by other investigators may be due to different analytical methods, use of a different human microsome source (ours is pooled from 59 individual human liver microsomes), or an oversight. The quinone imine intermediate of the ipso GS adduct may be subjected to further conjugation in a similar way. In fact, the mass spectrum of the NL scan of m/z 129 detected ions consistent with double GS adducts containing one (m/z 888) or no (m/z 854) chlorine atom.
In conclusion, the present study identified a novel GS adduct of DF, namely, 4′-OH-2′-GS-DDF, formed from a human liver microsome incubation. The discovery of this novel GS adduct of DF demonstrated the power of using a highly sensitive NL scan of m/z 129 with the NanoMate 100-API 3000 to screen for GS adducts and the utility of LC-MS-NMR for structure determination of a metabolite. Additional studies are required to determine whether 4′-OH-2′-GS-DDF or its degradation products, such as cysteinylglycine and cysteine derivatives, are formed in vivo.
Acknowledgments
We thank Dr. Amit Kalgutkar for valuable discussion and intellectual input. We also acknowledge Advion BioSciences for technical assistance.
Footnotes
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.104.002840.
-
ABBREVIATIONS: DF, diclofenac; P450, cytochrome P450; GSH, glutathione; GS, glutathione radical; DF, diclofenac; DDF, deschloro-diclofenac; NL, neutral loss; LC/MS/MS, liquid chromatography-tandem mass spectrometry; nanoESI, nano-electrospray ionization; MeOH, methanol.
- Received November 7, 2004.
- Accepted January 4, 2005.
- The American Society for Pharmacology and Experimental Therapeutics