Abstract
7-Ethyl-10-hydroxy-camptothecin (SN-38), the active metabolite of the anti-cancer agent irinotecan, contains a lactone ring that equilibrates with a carboxylate form. Since SN-38 lactone is the active and toxic form, it is prudent to examine whether the more soluble carboxylate is a surrogate for SN-38 lactone conjugation. Therefore, relative rates of glucuronidation and isoform specificity of SN-38 lactone and carboxylate were characterized. The stability of SN-38 lactone and carboxylate in incubation mixtures of microsomes and UDP-glucuronosyltransferase (UGT) isoforms was used to determine optimal incubation times. Microsomal incubations were conducted using rat and human intestinal and hepatic microsomes and human and rat recombinant UGT1A isoforms. Where estimates of lactone and carboxylate glucuronidation rates could not be established due to short incubation times and detection limits, kinetic modeling was used to recover these rate constants. The stability experiments revealed that the lactone was stabilized by rat microsomes, however, the opposite was observed in human microsomes and recombinant isoforms. For all tissues and most UGT isoforms examined, the lactone consistently had catalytic rates up to 6-fold greater than the carboxylate. The rank order of glucuronidation for both SN-38 lactone and carboxylate was 1A7 > 1A1 > 1A9 > 1A8 and 1A7 > 1A8 > 1A1 for human and rat isoforms, respectively. This study provides further support that SN-38 lactone and carboxylate may be considered pharmacokinetically distinct agents. The in vivo impact of this conjugation difference is unknown, since variations in protein binding and transport proteins may affect intracellular concentrations of the lactone or carboxylate.
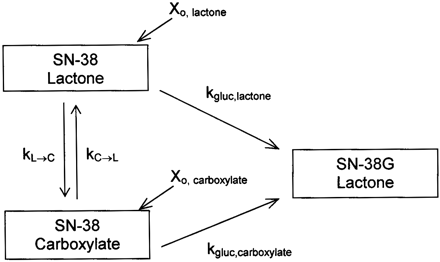
The topoisomerase I inhibitor, irinotecan [manufactured as irinotecan hydrochloride trihydrate (Camptosar; Pfizer Inc., New York, NY)], in combination with fluorouracil and leucovorin, is approved in the treatment of metastatic colorectal cancer. Irinotecan is increasingly being used in chemotherapy regimens for many other malignancies, including pancreatic and lung cancers (Rothenberg, 2001). The cytotoxicity of irinotecan is achieved largely through a metabolite, 7-ethyl-10-hydroxy-camptothecin (SN-38), which is at least 100-fold more potent in inducing cell death in vitro (Kawato et al., 1991). Irinotecan is a prodrug, since SN-38 is not directly administered to patients due to its poor aqueous solubility and unacceptable toxicity (Rajkumar and Adjei, 1998). SN-38 is formed by the metabolism of irinotecan by carboxylesterases in various tissues (Khanna et al., 2000; Mathijssen et al., 2001). SN-38 is then glucuronidated by UDP-glucuronosyltransferase (UGT) 1A1, UGT1A7, and UGT1A9, with similar reported catalytic efficiencies, to form SN-38 glucuronide (SN-38G) (Gagne et al., 2002) (Fig. 1).
The lactone ring of SN-38, like all camptothecin derivatives, can undergo reversible, pH-dependent hydrolysis to yield the corresponding carboxylate (Fig. 1). The equilibrium favors the poorly soluble lactone form at lower pH values, and at neutral and basic pH values, the more soluble carboxylate form predominates. Both forms exist considerably in vivo, with the lactone accounting for 54 to 64% of the total SN-38 area under the plasma concentration versus time curve (Rivory et al., 1994; Sasaki et al., 1995). The lactone and carboxylate are considered two pharmacologically distinct compounds, as the intact lactone is necessary for topoisomerase binding and the carboxylate is only weakly cytotoxic (Hertzberg et al., 1990). Additionally, SN-38 lactone and carboxylate possess different affinities for transporters and exhibit differences in pharmacokinetics in animals and humans. In Eisai hyperbilirubinemic rats (rats deficient in Mrp2), SN-38 carboxylate biliary clearance was reduced, whereas for the lactone form it was unchanged, suggesting a role of Mrp2 in the hepatic transport of only the carboxylate (Chu et al., 1997). SN-38 lactone has a greater volume of distribution and binds more tightly to albumin when compared with SN-38 carboxylate (Burke and Mi, 1993; Kaneda et al., 1997; Xie et al., 2002). An intact lactone may also be favored for substrate-enzyme binding or catalysis, as irinotecan lactone is metabolized to SN-38 at a greater rate than irinotecan carboxylate (Haaz et al., 1997b). It is unknown whether or not this phenomenon occurs for SN-38 with the UGTs, and there is contradictory data suggesting metabolic differences between SN-38 lactone and carboxylate. Haaz et al. (1997a) assessed rates of lactone and carboxylate glucuronidation from a 15-min microsomal incubation of SN-38 in human liver microsomes. The authors estimated that by 15 min, 70% of the lactone form was hydrolyzed to the carboxylate, and no significant differences in glucuronidation were noted. However, it did appear that the lactone form was metabolized more quickly (Haaz et al., 1997a). SN-38 lactone administered by intravenous bolus to rats was cleared 2-fold faster by metabolism and excretion when compared with administration of SN-38 carboxylate (Kaneda et al., 1997). In contrast, a population pharmacokinetic study in humans predicted via extensive modeling that SN-38 lactone and carboxylate had similar total clearance values (Xie et al., 2002).
Glucuronidation of SN-38 after esterase cleavage of irinotecan. The scheme depicts the lactone and carboxylate species of each compound.
The purpose of these studies was to assess whether SN-38 lactone and carboxylate are glucuronidated by human and rat tissues and recombinant isoforms at different rates and whether the lactone and carboxylate differed in their specificity for individual UGT isoforms. Deciphering the latter is important, as the more soluble carboxylate is often used as a surrogate for the active lactone form in microsomal metabolism studies (Ciotti et al., 1999; Hanioka et al., 2001a,b; Gagne et al., 2002). Knowledge of differences in glucuronidation rates or isoform specificity between SN-38 lactone and carboxylate may be instrumental in understanding cellular toxicity or resistance to the chemotherapeutic agent.
Materials and Methods
Chemicals and Reagents. Irinotecan and SN-38G methyl ester were a gift from Dr. Robert Kelly at Pfizer (Kalamazoo, MI), previously Pharmacia. SN-38 was cleaved from irinotecan by hydrolysis with base according to a procedure provided by Dr. Kelly. The purity of SN-38 obtained was confirmed by high performance liquid chromatography (HPLC) with UV detection and by liquid chromatography/mass spectrometry. SN-38G methyl ester was also hydrolyzed by base to produce SN-38G. The concentration of SN-38G diluted in methanol was determined by β-glucuronidase treatment, and the resultant SN-38 generated was analyzed by HPLC using SN-38 standards. Chemicals used in the microsomal preparations and reactions were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals and solvents used were of reagent grade and obtained through commercial vendors.
Microsome and Recombinant Isoform Preparation. Human recombinant UGT isoforms and human liver microsomes were obtained commercially from BD Gentest (Woburn, MA). Human intestinal microsomes were purchased from Xenotech LLC (Lenexa, KS). Rat recombinant UGT isoforms were prepared from human embryonic kidney cell membranes as previously described (Kessler et al., 2002). Rat hepatic and intestinal microsomes were prepared and pooled from four male Sprague-Dawley rats, weighing 250 g (Charles River Breeding Laboratories, Portage, MI). Mucosal scrapings of the enterocytes or portions of the liver were subject to homogenization in 250 mM sucrose with 1 mM EDTA, 0.1 mM dithiothreitol, and 0.25 mM phenylmethylsulfonyl fluoride (PMSF) and centrifuged at 10,000g ×20 min. The supernatant was then spun at 100,000g ×1 h. The resulting pellet was reconstituted in 250 mM sucrose containing 0.25 mM PMSF and 10 mM leupeptin. Protein concentrations were determined by the Bradford method, using albumin as a standard.
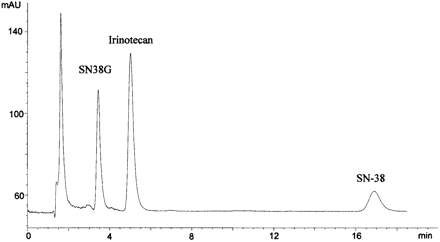
HPLC Analysis. The HPLC method to analyze SN-38 and SN-38G was modified from a published method (Sparreboom et al., 1998). The HPLC system consisted of an LC-600 isocratic pump (Shimadzu, Tokyo, Japan), AS-100 autosampler (Bio-Rad, Hercules, CA), and HP1046A fluorescence detector (Hewlett Packard, Palo Alto, CA). A Hypersil BDS C-18 column (5 uM, 150 × 4.6 mm) was used to separate analytes (Thermo Electron Corporation, Waltham, MA). For the SN-38G glucuronidation assay, the mobile phase consisted of methanol: 10 mM tetrabutylammonium sulfate and 100 mM ammonium acetate, pH 4.9 (37:63). For the purposes of quantitating SN-38G in microsomal reactions, 200 to 300 μl microsomal aliquots were precipitated with acetonitrile (800 μl) containing irinotecan (70 ng) as internal standard and perchloric acid (5%, 5 μl). SN-38 (30 μg) was added to prevent adsorption of irinotecan and SN-38G to the reaction tubes. The acetonitrile was evaporated under a stream of nitrogen. The residue was then reconstituted in 120 μl of weak mobile phase, pH 4.9 (30% methanol). The pH of this solution ensures that all SN-38G is in the lactone form. SN-38G was detected by fluorescence using excitation and emission wavelengths of 229 and 420 nm, respectively, which provided optimal sensitivity for SN-38G, but not SN-38. The chromatographic separation of SN-38G (3.5-min retention time), irinotecan (5 min), and SN-38 (17 min) is shown in Fig. 2. Standard curves for SN-38G were linear between 1 ng/ml to 1000 ng/ml.
Sample chromatogram of rat recombinant 1A1 glucuronidation of SN-38 lactone over a 10-min incubation period. SN-38G has a retention time of 3.5 min, irinotecan (internal standard) elutes at 5 min, and SN-38 is detected at 17 min. Ordinate is arbitrary units of fluorescence output.
When assessing SN-38 lactone and carboxylate stability, the mobile phase pH was not adjusted, and a pH of approximately 5.6 was employed. Also, to elute SN-38 carboxylate and lactone more quickly, the mobile phase strength was increased to 42% methanol. SN-38 lactone and carboxylate were detected using optimized excitation and emission wavelengths of 229 and 543 nm, respectively. Under these conditions, the retention times were approximately 3.0 and 6.0 min for the carboxylate and lactone, respectively. To assess whether a similar response was obtained when SN-38 carboxylate and lactone were injected onto the HPLC in equal amounts, the relative response ratio was determined. Aliquots (20 μl) from three separate solutions of SN-38 carboxylate and lactone (5 μM) were analyzed, and SN-38 lactone consistently had a 1.78-fold greater response than the carboxylate. Therefore, when relating the peak areas of SN-38 lactone to carboxylate and vice versa in assessing their stability, the area of the carboxylate was multiplied by 1.78 to account for the lower fluorescence response.
Stability Studies. Solutions of SN-38 lactone and carboxylate were made by dissolving SN-38 in dimethyl sulfoxide/0.1M sodium acetate (50:50, pH 4.5) and dimethyl sulfoxide/1 × 10–5 M sodium hydroxide (50:50, pH 9.5), respectively, and incubated for 24 h at 37°C. This procedure produced lactone or carboxylate, respectively, at approximately 99% content, as verified by HPLC. SN-38 lactone or carboxylate (5 μM, final concentration in solution) was added to either buffer or the reaction mixture containing recombinant isoforms or microsomes described below at 37°C without uridine diphosphate glucuronic acid (UDPGA). At various times, 20 μl of the buffer or incubation mixture was directly injected onto HPLC. First-order rate constants for conversion (kL→C or kC→L) were generated by using nonlinear regression analysis software (WinNonlin; Pharsight, Mountain View, CA). These rate constants were used to determine the length of incubation where <5% conversion from the lactone to carboxylate (and vice versa) occurred or were used as constants in kinetic modeling.
Microsomal Reaction Conditions. Incubation mixtures contained Brij 35 (0.5 mg/mg protein), d-Saccharic acid 1,4-lactone (10 mM), magnesium chloride (10 mM), UDPGA (2 mM), and microsomal or recombinant protein (0.1 mg/ml for human recombinant UGTs, 0.25 mg/ml for rat recombinant UGTs, or 0.5 mg/ml for human and rat hepatic and intestinal microsomes) in a final volume of 500 to 1600 μl of 0.1M Tris, pH 7.0. The microsomes as well as the recombinant isoforms were found to be activated between 2.5- to 4-fold using Brij 35 at the above concentration (data not shown). The concentration of dimethyl sulfoxide in the incubations was consistently held at 1%. The protein concentrations for each matrix were fixed and used previously to determine the stability of SN-38 lactone and carboxylate. The reaction was initiated by spiking in SN-38 lactone or carboxylate (5 μM, final concentration in solution) and terminated at times predetermined by the stability studies to minimize lactone and carboxylate interconversion (<5%). For incubations where quantitation of SN-38G from relatively pure SN-38 lactone or carboxylate was not possible due to rapid conversion, SN-38G concentrations from at least five time points from 5 to 60 min were analyzed to allow glucuronidation rate constants for SN-38 lactone and carboxylate to be recovered from kinetic modeling.
Kinetic Modeling. Compartmental modeling using WinNonlin provided estimates of the glucuronidation rate constants of SN-38 lactone and carboxylate by various microsomes and isoforms. Data used in this model (Fig. 3) to generate the glucuronidation rate constants, kgluc, lactone and kgluc, carboxylate, was expressed as cumulative formation of SN-38G over time. Both SN-38 lactone and carboxylate are depicted to form SN-38 lactone due to the acidified solution injected onto the HPLC, which converts any SN-38G carboxylate to SN-38G lactone (see “HPLC Analysis” under Materials and Methods). Simultaneous modeling of the cumulative SN-38G formed when the carboxylate and lactone were spiked (Xo, carboxylate or Xo, lactone) in separate experiments allowed the rate constants to be recovered. Interconversion rate constants between SN-38 lactone and carboxylate (kL→C or kC→L) found in the stability studies were held as constants. Initial iterative values for the glucuronidation rate constants were set as the slope of the percentage of SN-38 remaining versus time. An assumption was made that glucuronidation proceeded under nonsaturable conditions. It was also assumed that the initial conversion rates found in the stability studies were unidirectional conversion rates, since the stability profiles (Fig. 4) were log-linear, reflective of a first-order process.
Kinetic model used to determine glucuronidation rate constants of SN-38 lactone or carboxylate (kgluc, lactone and kgluc, carboxylate). This model uses SN-38G lactone concentrations generated from spiking either SN-38 lactone or carboxylate (Xo, lactone, or Xo, carboxylate) in two separate experiments. Any SN-38G carboxylate formed is converted to SN-38G lactone in the sample workup, as an acidified solution was used to reconstitute each sample (see “HPLC Analysis” under Materials and Methods). Hence, both SN-38 lactone and carboxylate are depicted to form SN-38G lactone. The hydrolysis and lactonization rate constants, kL→C or kC→L, respectively, were generated from studies assessing the stability of each form in microsomal or recombinant isoform matrix.
Representative plots of the stability of SN-38 lactone (A) or carboxylate (B) as determined by HPLC in 0.1 M sodium acetate, pH 4.5 (▴); 0.1 M sodium phosphate, pH 6.0 (•); 0.1 M Tris, pH 7.0 (▪); or 1 × 10–5M sodium hydroxide, pH 9.5 (▾) over time at 37°C. Data shown are from up to three incubations per pH studied.
Results
The stability of SN-38 lactone and carboxylate in various buffers is shown in Fig. 4. The experimentally derived rate constants were similar to what was expected and previously reported, since the half-life of SN-38 lactone at pH 7.0 and 37°C is 95 min versus 96 min as calculated from rate constants published by Akimoto et al. (1994). These values were also in rough agreement with Chourpa et al. (1998), who reported a half-life of approximately 32 min at a higher pH (7.3), were lactonolysis is increasingly favored (Akimoto et al., 1994; Chourpa et al., 1998). Consistently, the lactone remained intact at low pH values, and the carboxylate was more stable in neutral and basic aqueous buffers. These findings gave confidence that the HPLC method used to determine the first-order rate constants for hydrolysis and lactonization in microsomes and recombinant isoforms was accurate with no conversion on the column during the analysis (Table 1). The stability of SN-38 lactone and carboxylate was initially assessed in buffers with recombinant human UGT1A1 and UGT1A8, with virtually identical hydrolysis and lactonization rates. Therefore, these values were employed for these and other human UGT isoforms. The same was observed for recombinant rat UGT1A1, UGT1A8, and UGT2B2. The data in Table 1 indicate that hydrolysis (i.e., kL→C) is facilitated by the addition of human microsomes or human or rat recombinant isoforms relative to Tris buffer. Conversely, the presence of rat microsomes stabilized the lactone moiety. SN-38 carboxylate was cyclized to the lactone (i.e., kC→L) at a faster rate in rat microsomes relative to Tris buffer. However, both SN-38 lactone and carboxylate were sufficiently stable in rat hepatic and intestinal microsomes to allow SN-38G quantitation from a microsomal reaction with little conversion to the other form (less than 2.5%).
First-order rate constants for lactonization and hydrolysis of SN-38 carboxylate and lactone, respectively, in Tris buffer and biological matrices/Tris buffer at 37°C, pH 7.0
Incubations contained 0.5 mg protein/ml for human and rat hepatic and intestinal microsomes, 0.1 mg/ml for human recombinant UGTs, and 0.25 mg/ml for rat recombinant UGTs and were conducted without cofactor UDPGA. Subsequent glucuronidation experiments were conducted using these fixed protein concentrations.
Once the stability of the lactone/carboxylate forms was established, catalytic rates of formation of SN-38G from each form was estimated in vitro with the presence of UDPGA. In both rat intestinal and hepatic microsomes, SN-38 lactone was glucuronidated more quickly compared with the carboxylate (Table 2), where these reactions could be conducted in 6.0 to 15.3 min with insignificant conversion to the carboxylate (<2.5%). However, a 1.5-min or less reaction time would have been required to estimate such values for the lactone in human microsomes and recombinant isoforms due to rapid hydrolysis in these matrices (Table 1). Since these constraints were experimentally unfeasible for the lactone, kinetic modeling was used to recover glucuronidation rates for SN-38 carboxylate and lactone in human microsomes and human and rat recombinant isoforms (Fig. 5 and Table 3). Although glucuronidation rates (picomoles per minute per milligram protein) could be recovered for pure carboxylate without modeling, modeling was used so that a comparison could be made between SN-38 lactone and carboxylate by using first-order glucuronidation rate constants. SN-38 lactone and carboxylate had glucuronidation rates in the same rank order, with UGT1A7 being the most active, followed by UGT1A1, UGT1A9, and UGT1A8. Barely quantifiable levels of SN-38G were produced with UGT1A6 and UGT1A10 at 60 min, and UGTs 1A3, 1A4, 2B4, 2B7, 2B15, and 2B17 were inactive toward both forms of SN-38. Rat UGT1A7 glucuronidated SN-38 carboxylate and lactone at the fastest rate, with rat UGT1A8 and UGT1A1 also having activity. Rat UGT1A2, UGT1A3, UGT1A5, UGT1A6, UGT2B2, UGT2B3, and UGT2B18 did not glucuronidate SN-38. Virtually every isoform or tissue that had measurable catalysis glucuronidated SN-38 lactone at a greater rate compared with the carboxylate. This difference was typically a 3.6-fold increase in glucuronidation; however, up to a 6-fold difference was observed with UGT1A1. UGT1A8 was the lone exception, with the carboxylate being metabolized approximately twice as quickly compared with the lactone.
SN-38 lactone and carboxylate glucuronidation rates in rat hepatic and intestinal microsomes
Study performed with an incubation time that only permitted 2.5% conversion of lactone to carboxylate or carboxylate to lactone. Values in parentheses indicate the standard deviation between the averages of two different experiments (each with three replicates).
First-order rate constants for glucuronidation of SN-38 lactone and carboxylate in microsomes and recombinant isoforms
Numbers in parentheses indicate the parameter coefficient of variance (%).
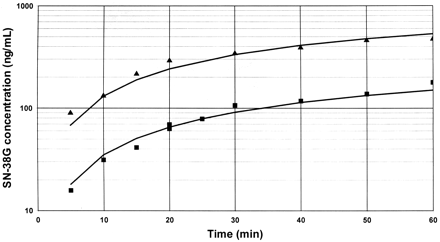
Representative plots of the formation of SN-38G in human hepatic microsomes when SN-38 carboxylate (-▪-) or SN-38 lactone (-▴-) was the substrate. SN-38G concentration is represented in SN-38 equivalents in nanograms per milliliter. The points on each line shown represent data from three different incubations, since more than one incubation was needed to provide this number of data points.
Discussion
It is well established that SN-38 lactone and carboxylate exhibit pharmacodynamic and pharmacokinetic differences. Here, we have verified that these differences also extend to rates of glucuronidation. For all the rat and human isoforms that appreciably catalyze SN-38G formation, the lactone is almost always conjugated at a faster rate compared with the carboxylate. The faster SN-38 lactone turnover relative to the carboxylate by individual isoforms should translate into higher glucuronidation rates for the lactone by tissues, which was found for microsomes prepared from human and rat liver and intestine. A potential explanation for this difference may be an increased ability of the lactone to gain access to the catalytic site of the UGT in the relatively lipophilic environment of the microsome. Although a detergent (Brij 35) was used to expose the active site, the carboxylate may still not partition well due to its negative charge. It is likely that in vivo, the lactone would more easily access the active site of the UGT if passive processes are operative, since the enzyme exists in the inner lumen of the endoplasmic reticulum (Yokota et al., 1992; Meech and Mackenzie, 1997). Alternatively, the UGTs may simply preferentially recognize and have a higher binding affinity (discussed below) for the intact SN-38 lactone compared with the acid functional group.
The extrahepatic isoform UGT1A8 does metabolize SN-38 carboxylate faster than the lactone. An in vivo effect of this unusual behavior is not likely, as the rate of glucuronidation by this isoform is approximately 150-, 40-, and 4-fold less than UGT1A7, UGT1A1, and UGT1A9, respectively (Table 2). It is possible that a preference for SN-38 carboxylate conjugation may be observed in tissues where UGT1A8 is highly expressed compared with UGT1A7, UGT1A1, and UGT1A9. UGT1A8 has not been reported to be in the liver and small intestine but is found in the colon and esophagus (Strassburg et al., 1998).
Although intrinsic clearance determinations may be a more relevant in vivo value to obtain compared with a velocity at a certain concentration, the latter approach was used to rank active isoforms and compare the efficiency of lactone and carboxylate glucuronidation, since solubility limitations of SN-38 lactone preclude estimation of Michaelis-Menten enzyme kinetics. The solubility of SN-38 lactone in aqueous buffers published by Zhang et al. (2004) is approximately 18 μM (7.2 μg/ml), which is a value similar to that obtained in our laboratory. Michaelis-Menten studies were initially conducted using SN-38 lactone and did yield the characteristic concentration versus velocity curve, and the apparent Vmax was obtained at approximately the solubility limit (data not shown). Therefore, it is not clear whether a true Vmax was reached or that the plateau was due to solubility. For this reason, we conducted studies at 5 μM, a concentration at which both SN-38 lactone and carboxylate are soluble and does not saturate the enzyme (Haaz et al., 1997a) (data not shown). Elucidation of a Km for SN-38 in various tissues and UGT isoforms was not attempted in this study due to the solubility limitations of the lactone as previously discussed. Although Km values are helpful for in vitro-in vivo predictions, knowledge of these values for SN-38 lactone or carboxylate in tissues or UGT isoforms would not be expected to be helpful. Peak plasma concentrations of SN-38 are in the nanomolar range after a standard irinotecan dose, which is significantly below any literature-reported Km in the micromolar range (Haaz et al., 1997a; Hanioka et al., 2001a; Gagne et al., 2002). Hence, the rank order of active isoforms and the relative differences observed between SN-38 lactone and carboxylate that are reported here should mimic the true in vivo situation. Since SN-38 concentrations in vivo and in the experiments presented here are below the Km, Michaelis-Menten kinetics are operative, and the rates of glucuronidation both in vivo and in vitro are proportional to SN-38 concentrations.
The results of this study substantiate those of previous publications identifying UGT1A7, UGT1A1, and UGT1A9 as having the highest relative glucuronidation activity toward SN-38 (Ciotti et al., 1999; Hanioka et al., 2001b; Gagne et al., 2002). In these studies, either the authors state that SN-38 carboxylate was used as the substrate (Hanioka et al., 2001b), or the upper concentrations of SN-38 used in the microsomal reactions implies that the carboxylate was used due to solubility limitations with the lactone form, allowing a larger concentration range over which to conduct Km and Vmax studies (Ciotti et al., 1999; Gagne et al., 2002). Should SN-38 lactone and carboxylate be preferentially glucuronidated by different UGT isoforms, SN-38 carboxylate would not be a suitable surrogate for assessing the glucuronidation profiles of the more cytotoxic lactone form. From this study, it is clear that investigators may continue to compare relative glucuronidation patterns of cells and tissues using SN-38 carboxylate in in vitro reactions. However, investigators should not rely upon Vmax and intrinsic clearance values generated for SN-38 carboxylate, since they may underestimate the true values for the active species, the lactone.
Interestingly, rat intestinal and hepatic microsomes were the only matrices in which the lactone was relatively stable to hydrolysis and were the only matrices prepared with the protease inhibitors PMSF and leupeptin. It was then theorized that proteases in the recombinant isoforms and human microsomes were catalyzing SN-38 lactonolysis. To test this theory, human hepatic microsomes were treated with PMSF and leupeptin in concentrations equal to that in rat microsomes prior to the stability study with SN-38 lactone. The addition of these protease inhibitors did not have an effect on the conversion rate of SN-38 lactone to carboxylate (data not shown). Therefore, it is unknown why lactonolysis proceeds so rapidly in recombinant isoforms and human microsomes compared with rat microsomes or buffer alone.
The in vivo impact of the differential catalysis of SN-38 lactone and carboxylate is unknown. The predominance of SN-38 lactone observed in the plasma of patients may not be observed intracellularly at the site of action, if SN-38 lactone is more rapidly glucuronidated. Since cellular damage is presumably mediated by intracellular SN-38 lactone, one might propose that reducing intracellular levels of SN-38 lactone by glucuronidation may increase tumor resistance or reduce the toxicity profile of irinotecan/SN-38 (Gupta et al., 1994; Tukey et al., 2002). Although glucuronidation may reduce SN-38 lactone relative to carboxylate, other factors may exist that would impact intracellular levels, such as pH or differential protein binding, uptake, or efflux.
To summarize, SN-38 lactone and carboxylate are glucuronidated in the same rank order by both rat and human UGT1A isoforms. However, the lactone was almost always consistently glucuronidated at a faster rate by these isoforms, which translated into faster overall catalysis of SN-38 lactone in rat and human hepatic and intestinal microsomes. Due to the solubility limitations of SN-38 lactone, investigators are unable to elucidate Km values for this form in various matrices. Therefore, using SN-38 carboxylate as a more soluble surrogate will allow investigators to obtain an in vitro Km, Vmax, and intrinsic clearance. However, these values should be cautiously interpreted, since the Vmax is likely to be underestimated and the Km values for the two forms are not necessarily equivalent.
Acknowledgments
We thank Dr. Robert Kelly of Pharmacia for irinotecan supplies and Brendan Bender for assistance with the kinetic modeling.
Footnotes
-
Supported by National Institutes of Health Grant GM 61188.
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.104.003491.
-
ABBREVIATIONS: SN-38, 7-ethyl-10-hydroxy-camptothecin; UGT, UDP-glucuronosyltransferase; SN-38G, SN-38 glucuronide; HPLC, high performance liquid chromatography; PMSF, phenylmethylsulfonyl fluoride; UDPGA, uridine diphosphate glucuronic acid.
- Received December 23, 2004.
- Accepted April 14, 2005.
- The American Society for Pharmacology and Experimental Therapeutics