Abstract
Relative expression factors (REFs) are used to scale in vitro transporter kinetic data via in vitro–in vivo extrapolation linked to physiologically based pharmacokinetic (IVIVE-PBPK) models to clinical observations. Primarily two techniques to quantify transporter protein expression are available, immunoblotting and liquid chromatography–tandem mass spectrometry. Literature-collated REFs ranged from 0.4 to 5.1 and 1.1 to 90 for intestinal P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), respectively. The impact of using human jejunum–Caco-2 REFs for P-gp (REFiP-gp) and BCRP (REFiBCRP), generated from the same samples and using different proteomic methodologies from independent laboratories, on PBPK outcomes was assessed. A 5-fold decrease in REFiP-gp for a single oral dose of digoxin resulted in a 1.19- and 1.31-fold higher plasma area under the curve and Cmax, respectively. All generated REFiP-gp values led to simulated digoxin Cmax values within observed ranges; however, combining kinetic data generated from a different laboratory with the 5-fold lower REFiP-gp could not recover a digoxin-rifampicin drug-drug interaction, emphasizing the necessity to obtain transporter-specific kinetic estimates and REFs from the same in vitro system. For a theoretical BCRP compound, with absorption taking place primarily in the jejunum, a decrease in the REFiBCRP from 2.22 (University of Manchester) to 1.11 (Bertin Pharma) promoted proximal intestinal absorption while delaying tmax 1.44-fold. Laboratory-specific differences in REF may lead to different IVIVE-PBPK outcomes. To understand the mechanisms underlying projected pharmacokinetic liabilities, it is important to assess the potential impact of bias on the generation of REFs on an interindividual basis within a target population.
Introduction
In vitro–in vivo extrapolation linked to physiologically based pharmacokinetic (IVIVE-PBPK) models aim to predict profiles of drug disposition dynamically. This is accomplished by incorporating “drug” data, generated in vitro, and physicochemical knowledge together with “systems” data in a population (Rostami-Hodjegan, 2012). Kinetic data [i.e., maximal flux capacity of the transporter protein (Jmax) and Km] describing the active transport processes generated from cell systems can also be included in IVIVE-PBPK models. To scale these data to in vivo, human and in vitro system transporter protein expression or activity data are also required in combination with physiologic, demographic, and genetic information (Rostami-Hodjegan, 2012). To date, intestinal transporter IVIVE scaling factors (Neuhoff et al., 2013a) have been generated based on Western blotting, a relative quantitative technique to quantify transport expression (Troutman and Thakker, 2003b). Yet, absolute transporter protein abundances quantified by liquid chromatography–tandem mass spectrometry (LC-MS/MS) have recently been explored for hepatic application in IVIVE-PBPK (Vildhede et al., 2014).
In this study, we provide a systematic analysis of the mRNA and protein expression data available in the literature for generating the relative expression factor (REF), an IVIVE scalar that describes the ratio of in vivo to in vitro systems transporter expression for human jejunum and Caco-2 monolayer P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP). We also evaluate the impact of intestinal P-gp and BCRP REFs generated by different laboratories and methodologies on drug absorption in a PBPK model.
Materials and Methods
Literature Review of the Intestinal Expression Data for P-gp and BCRP.
Starting with a previously reported meta-analysis that established human intestinal P-gp and BCRP region-specific protein expression (Harwood et al., 2013), a new search for the relevant published data quantifying P-gp and BCRP mRNA or protein expression in human jejunum and filter-grown Caco-2 monolayers using PubMed (http://www.ncbi.nlm.nih.gov/pubmed) was undertaken. The following keyword combinations were used in PubMed: human; jejunum; Caco-2; P-gp; MDR1; BCRP; ABCB1; ABCG2; mRNA; protein; expression; absolute; abundance; proteomics. Graphical data were extracted, where required, by GetData Graph Digitizer (http://getdata-graph-digitizer.com).
Generation of the Relative Expression Factors.
A REF for P-gp or BCRP was generated, where P-gp or BCRP mRNA/protein expression for human jejunum and Caco-2 monolayers was available using the same technique within a laboratory, including reference genes. These were compared with REFs generated from two different LC-MS/MS workflows (two independent laboratories; matching samples) for P-gp and BCRP in human jejunum and 21-day-cultivated Caco-2 monolayers. Methodological details and individual values are provided in the companion study (Harwood et al., 2016).
Incorporating Intestinal REFs into IVIVE-PBPK Models.
The impact of the laboratory-specific intestinal relative expression factor for P-glycoprotein (REFiP-gp) and intestinal relative expression factor for breast cancer resistance protein (REFiBCRP) in virtual healthy Caucasian volunteers (HVs) was assessed in a PBPK model (version 14.1; Simcyp, a Certara company, Sheffield, UK) containing the regional distribution of intestinal P-gp and BCRP and their population variability (Harwood et al., 2013). P-gp and BCRP transport in the model is driven by the unbound intracellular enterocyte concentration and is multiplied by REF and the regional-specific transporter expression to yield effective permeability (Yang et al., 2007; Neuhoff et al., 2013a).
The Impact of P-gp and BCRP REF in IVIVE-PBPK.
The impact of REFiP-gp values generated by the LC-MS/MS [University of Manchester (UoM), Manchester, UK; Harwood et al., 2016) compared with the immunoblotting approach (Troutman and Thakker, 2003b) on digoxin Cmax was investigated using identical digoxin parameter inputs as the previously reported digoxin IVIVE-PBPK model (Neuhoff et al., 2013a). Caco-2–derived Jmax and Km data (Troutman and Thakker, 2003a) were applied to intestinal and hepatic P-gp, assuming P-gp activity in vitro in healthy individuals corresponds to that in vivo, and that Jmax is related to P-gp protein expression. Simulations were run with a single oral digoxin dose of 0.5 mg in 100 HV individuals to evaluate if kinetic data for digoxin generated in a Caco-2 system from another laboratory (Troutman and Thakker, 2003a) to the REFiP-gp from the UoM Caco-2 system, could capture the observed digoxin-rifampicin drug-drug interaction (DDI) via induction of intestinal P-gp (Greiner et al., 1999), thus verifying the correct contribution of the active transport built into the digoxin PBPK model.
The impact of laboratory-specific differences for REFiBCRP on pharmacokinetic parameter predictions was evaluated using the Simcyp simulator. A permeable theoretical BCRP compound (TC); see (Supplemental Materials, Technical Note with limited gut metabolism and a specific BCRP activity was administered orally (10 mg in solution) to 100 HV individuals, with the default region-specific BCRP expression within the PBPK model, as published by Harwood et al. (2013).
Results and Discussion
REFiP-gp and REFiBCRP Generation from Different Laboratories.
According to our literature analysis, human jejunum mRNA and protein expression was identified in 19 studies for P-gp and nine studies for BCRP [(Supplemental Materials; (Supplemental Table 1)]. Expression data for Caco-2 P-gp and BCRP from the same laboratory using the same protocol to generate an REFiPgp or REFiBCRP were found for five and four studies, respectively (Table 1). For P-gp, relative mRNA expression analysis (reverse-transcription polymerase chain reaction) enabled the generation of REFiP-gp from two laboratories in three studies (Taipalensuu et al., 2001; Seithel et al., 2006; Hilgendorf et al., 2007), as the data from Seithel et al. (2006) and Hilgendorf et al. (2007) used Caco-2 monolayers cultivated in the same laboratory for 23 and 16 days, respectively. An REFiP-gp from two independent laboratories that used Western blotting (Troutman and Thakker, 2003b; von Richter et al., 2009) is available, but not for LC-MS/MS quantification for either P-gp or BCRP, as the Caco-2 cell abundances reported by Oswald et al. (2013) were from plastic, not filter-grown cells (Dr. Stefan Oswald, personal communication). The REFiP-gp based on mRNA expression ranged 7.1-fold, and for LC-MS/MS quantification, 2.6-fold. The REFiBCRP of 90 (Taipalensuu et al., 2001) may result from low BCRP levels or variability in the housekeeping gene used (Seithel et al., 2006). The REFiP-gp and REFiBCRP generated from P-gp and BCRP quantification by LC-MS/MS from two different laboratories, UoM and Bertin Pharma (BPh), for the same samples are provided in Table 1 (Harwood et al., 2016). The REFiP-gp (Troutman and Thakker, 2003b) from independent samples quantified by Western blotting was 5-fold higher than the REFiP-gp generated by the UoM (UoM-REFiP-gp), whereas the UoM-REFiBCRP was approximately 2-fold higher than BPh (LC-MS/MS) and Altana AG (Western blot; Wesel, Germany) (von Richter et al., 2009).
Generation of REFiP-gp and REFiBCRP from human jejunum and Caco-2 from relative mRNA expression and protein abundance (relative and absolute) data available in the literature for P-gp (MDR1) and BCRP
Assessing the Sensitivity of REFiP-gp in IVIVE-PBPK.
Both the UoM-REFiP-gp of 0.4 (Harwood et al., 2016) and the REFiP-gp of 2 (Troutman and Thakker, 2003b) led to digoxin Cmax values within observed ranges after a single oral dose of 0.5 mg of digoxin (Fig. 1A), implying both REFiP-gp reflect realistic contributions of P-gp in estimating observed Cmax values when using the Jmax and Km for P-gp reported by Troutman and Thakker (2003a). Using the UoM-REFiP-gp of 0.4 compared with the REFiP-gp of 2 led to a modest 1.31- and 1.19-fold lower mean Cmax and area under the curve, respectively, in 100 HV individuals (Fig. 1B). A previous study showed that the observed digoxin-rifampicin DDI, which was attributed to a 3.5-fold increase in intestinal P-gp expression (Greiner et al., 1999), could be recovered using an IVIVE-PBPK strategy, in which the REFiP-gp of 2.0 was increased 3.5-fold to 7 after (Fig. 1C) induction (Neuhoff et al., 2013b). A 3.5-fold increase in the UoM-REFiP-gp of 0.4 gave an REFiP-gp of 1.4, leading to a simulated underprediction in the observed DDI (Fig. 1D). This indicates that the lower UoM-REFiP-gp (that is not derived from the same Caco-2 system in which the apparent kinetic data were generated) is not sufficient to recover the contribution of P-gp induction by rifampicin to digoxin plasma concentration in HVs. The inability to recover the observed DDI when using the UoM-REFiP-gp may result from lower P-gp expression and hence lower activity in the UoM Caco-2 systems. This can be due to laboratory differences in methods of expression quantification, Caco-2 cell cultivation, and the variability in jejunum expression. To recover the activity shortfall when using the UoM-REFiP-gp, a 4.3-fold increase in Jmax (1874 pmol/min/cm2) was required to recover the observed DDI (Fig. 1E) after using the Nelder-Mead minimization method and weighted least-squares algorithm in the simulators parameter estimation module (Jamei et al., 2014).
(A) Sensitivity of Cmax to REFiP-gp for digoxin (single oral dose, 0.5 mg) in 100 HV individuals. The ranges of observed Cmax values are given between the dashed lines, and the REFiP-gp of 2.0 from Troutman and Thakker (2003b) (gray arrows) and the UoM-REFiP-gp (Harwood et al., 2016) (dashed arrows) are shown. (B) The mean digoxin plasma concentration when using the REFiP-gp of 2.0 (Troutman and Thakker, 2003b) and the UoM REFiP-gp of 0.4 in 100 HVs. The observed and predicted plasma concentrations for digoxin (single oral dose, 1 mg) in 80 HV individuals after the dosing of rifampicin (600 mg, 11 doses, once daily) using an REFiP-gp of 2 (Troutman and Thakker, 2003b) (C), REFiP-gp of 0.4 (Harwood et al., 2016) (D), and REFiP-gp of 0.4 (E) after optimizing the Jmax of P-gp by parameter estimation. The thin gray lines represent mean values for 10 individual virtual trials of eight individuals, males aged 21–37 years; the thick black lines are the overall means of the virtual population (n = 80); and the dashed gray lines are the 95th and 5th percentiles of the confidence interval. Open circles mark the observed digoxin concentrations when coadministered with multiple doses of rifampicin (Greiner et al., 1999).
Assessing the Sensitivity of REFiBCRP in IVIVE-PBPK.
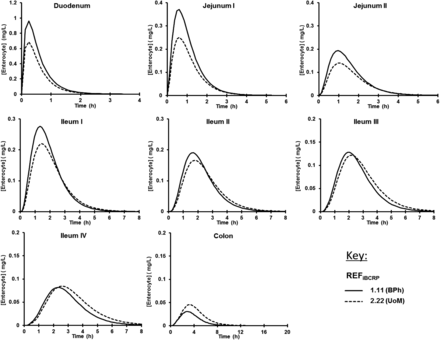
The sensitivity of the region-specific fraction of dose absorbed and enterocyte concentrations to REFiBCRP generated by BPh (1.11) and the UoM (2.22) (Table 1; Harwood et al., 2016) was assessed for the BCRP test compound TC. Figure 2 shows the free segmental enterocyte concentration for TC, used as the driving force for apical efflux transporters. As expected, the lower BPh-REFiBCRP leads to higher TC enterocyte concentrations in proximal regions than the higher UoM-REFiBCRP, whereas an increasing importance of intestinal BCRP UoM-(REFiBCRP) results in higher TC absorption and higher enterocyte concentration in the distal intestine due to the efflux activity promoting TC retention in the gut lumen and transit to the colon, a region with 7.7-fold lower BCRP levels (Harwood et al., 2013). The higher REFiBCRP has a limited impact on lowering Cmax and area under the curve (1.22- and 1.03-fold, respectively), but increases tmax 1.44-fold to 2.8 hours and is in line with clinical observations, where an inhibition of intestinal BCRP leads to a decrease in tmax (Schneck et al., 2004). Alongside differences in BCRP expression, interindividual variability in system parameters, such as the small intestinal transit time (range 0.5–10 hours; Yu et al., 1996), also contributes to region-specific fraction of dose absorbed [Supplemental Fig. 1; (Supplemental Materials)]. Acidic BCRP substrates, such as rosuvastatin, are expected to possess higher enterocyte concentrations, as limited metabolism, low passive permeation, and apical uptake transporters operate (Li et al., 2012; Jamei et al., 2014). Therefore, increased BCRP expression alters tmax and regional absorption, while not limiting overall absorption and bioavailability. This is dissimilar to the cooperation of P-gp and CYP3A4 activities that facilitate a drug’s repeated exposure to intestinal CYP3A4, increasing overall gut metabolism and reducing bioavailability (Wacher et al., 1998). To our knowledge, the current study is the first highlighting this difference of the colocalized transporters P-gp and BCRP.
Simulated enterocyte concentration profiles for all intestinal segments of the model for the BCRP compound TC (10 mg oral, single dose, in solution) in 100 HVs using the BPh REFiBCRP (1.11) or UoM-REFiBCRP (2.22) with a mean small intestinal transit time of 3.34 hours, a passive apparent permeability of 115.2 × 10−6 cm/s, and an intrinsic clearance for BCRP of 17 µl/min/cm2.
Combining IVIVE scalars and activity data generated from different laboratories for ATP-dependent transporters may not lead to successful IVIVE, whereas we postulate that laboratory-specific differences in REF may impact the mechanistic understanding of projected pharmacokinetic liabilities (efficacy/toxicity). This is due to in vitro activity, reproducibility of in vitro assays, culture conditions, and proteomic workflows. As discussed previously, direct translation of protein expression to activity may not always occur; therefore, accounting for deviations in this linear relationship via activity-abundance scalars will be required (Harwood et al., 2013). Ideally, scaling factors should be defined on a laboratory-specific basis against a common reference and combined with activity data from the same system. However, it is improbable within an industrial setting that groups will possess a bank of human intestinal tissues by which to obtain the in vivo abundance for in-house intestinal REF generation. It is therefore advocated that commercially available pooled human intestinal microsomes (constituting ≥20 intestines) are used to generate an REF using the same proteomic methods as those used for quantifying in vitro system abundances used for determining activity. Alternatively, a link between human liver microsomes, intestinal microsomes, and Caco-2 cells can be approached.
Authorship Contributions
Participated in research design: Harwood, Neuhoff, Warhurst, Rostami-Hodjegan.
Conducted experiments: Harwood, Achour.
Contributed new reagents or analytic tools: Russell, Carlson.
Performed data analysis: Harwood, Achour, Neuhoff.
Wrote or contributed to the writing of the manuscript: Harwood, Achour, Neuhoff, Warhurst, Rostami-Hodjegan.
Footnotes
- Received October 9, 2015.
- Accepted February 1, 2016.
↵
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
Abbreviations
- BCRP
- breast cancer resistance protein
- BPh
- Bertin Pharma
- DDI
- drug-drug interaction
- HV
- healthy Caucasian volunteer population
- IVIVE
- in vitro–in vivo extrapolation
- LC-MS/MS
- liquid chromatography–tandem mass spectrometry
- PBPK
- physiologically based pharmacokinetic
- P-gp
- P-glycoprotein
- REF
- relative expression factor
- REFiBCRP
- intestinal relative expression factor for breast cancer resistance protein
- REFiP-gp
- intestinal relative expression factor for P-glycoprotein
- TC
- theoretical BCRP compound
- UoM
- University of Manchester
- Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics