Abstract
Membrane transporters can play a clinically important role in drug absorption and disposition; Caco-2 and Madin-Darby canine kidney (MDCK) cells are the most widely used in vitro models for studying the functions of these transporters and associated drug interactions. Transport studies using these cell models are mostly focused on apical transporters, whereas basolateral drug transport processes are largely ignored. However, for some hydrophilic drugs, a basolateral uptake transporter may be required for drugs to enter cells before they can interact with apical efflux transporters. The objective of this study was to evaluate potential differences in drug transport across Caco-2 and MDCK basolateral membrane that could cause discrepancy in the identification of efflux transporter substrates and to elucidate the underlying factors that may cause such differences, using rosuvastatin as a model substrate. Bidirectional transport results in Caco-2 and breast cancer resistance protein-MDCK cells demonstrated the necessity of an uptake transporter at the basolateral membrane for rosuvastatin. Kinetic study revealed saturable and nonsaturable processes for rosuvastatin uptake across the Caco-2 basolateral membrane, with the saturable process encompassing >75% of overall rosuvastatin basolateral uptake at concentrations below the Km (4.2 μM). Furthermore, rosuvastatin basolateral transport exhibited cis-inhibition and trans-stimulation phenomena, indicating a facilitated diffusion mechanism. This basolateral transporter appeared to be a prerequisite for rosuvastatin and perhaps for other hydrophilic substrates to interact with apical efflux transporters. Deficit of such a basolateral transporter in certain cell models may lead to false-negative results when screening drug interactions with apical efflux transporters.
Introduction
Drug transporters play important roles in drug disposition, drug targeting, and, particularly transporter-mediated drug-drug interactions (DDIs) (Giacomini and Sugiyama, 2006). Clinical pharmacokinetic DDI studies have suggested that transporters often work together in drug intestinal absorption and hepatic/renal elimination. Uptake and efflux transporters can interact dynamically to mediate drug entry and exit from cells. For example, drug molecules can be taken up across the basolateral membrane of hepatocytes or proximal tubule cells by uptake transporters and subsequently be extruded across the apical membrane into bile or urine by efflux transporters. Given that large numbers of individuals undergo concurrent, prescription or nonprescription, multidrug therapy, the potential for transporter-mediated interactions between coadministered drugs is a cause of concern for physicians, the pharmaceutical industry, and regulatory agencies (Giacomini et al., 2010). Furthermore, the realization that clinical adverse events involving drug transporters are poorly understood and difficult to predict is fueling an increasing demand for the development of in vitro cell systems for DDI prediction.
One of the most extensively used in vitro cell models for drug transporter assessment has been the human epithelial colorectal adenocarcinoma (Caco-2) cells, which possess many characteristics of intestinal epithelial cells, including intercellular junctional complexes and various uptake and efflux transporters (Artursson and Karlsson, 1991; Hidalgo, 2001; Elsby et al., 2008). Another commonly used in vitro cell model is the Madin-Darby canine kidney (MDCK) cells (Polli et al., 2001; Xiao et al., 2006; Kitamura et al., 2008). The latest U.S. Food and Drug Administration draft guidance for drug interaction studies (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM292362.pdf) recommends bidirectional permeability assays in either Caco-2 cells or polarized epithelial cell lines overexpressing membrane transporters as a preferred method for in vitro determination of whether investigational drugs are substrates of P-gp and/or BCRP. Because drug molecules may interact with multiple uptake and efflux transporters naturally expressed in vivo, the missing interplay between apical and basolateral transporters in cell lines expressing a single transporter, by not reflecting the in vivo situation, may lead to false-negative results. In this regard, Caco-2 cells, a primary-like cell culture that expresses multiple uptake and efflux transporters, seems to be a more appropriate choice of model for this type of study, because it avoids the limitations of singly transfected cells that cannot recapitulate all the mechanisms involved in transcellular transport. Because drug transport studies with Caco-2 cells mostly focus on apical transporters, the mechanism of drug translocation across the basolateral membrane is largely ignored. Although a recent publication indicated that organic solute transporter α (OSTα)-OSTβ may be involved in the basolateral uptake of taurocholic acid and estrone 3-sulfate in Caco-2 cells (Grandvuinet and Steffansen, 2011), the role of the basolateral transporter(s) in Caco-2 cells is not widely appreciated.
The current study explored the basolateral uptake route of drug transport in two different cell systems, such as Caco-2 and MDCK cells, using rosuvastatin as a model compound. Rosuvastatin is a member of HMG-CoA reductase inhibitors (statins), which are among the most widely used drugs worldwide for the treatment and prevention of ischemic heart disease. Rosuvastatin is avidly taken up into hepatocytes, the site of statin action, by multiple active uptake transporters, including organic anion transporting polypeptide (OATP) 1B1, 1B3, 2B1, 1A2, and Na+-taurocholate cotransporting polypeptide (NTCP) transporters at the hepatic sinusoidal membrane (Ho et al., 2006; Choi et al., 2011; Varma et al., 2011). Owing to its hydrophilicity, and ensuing slow passive diffusion across cell membranes, rosuvastatin is liver selective and has a limited distribution into nonhepatic cells. It has been shown that the absolute oral bioavailability of rosuvastatin in humans was ∼20%, and hepatobiliary excretion seemed to be the predominant elimination route, representing ∼70% of total clearance (Martin et al., 2003a). Although two metabolites, rosuvastatin-5S-lactone and N-desmethyl rosuvastatin, have been found, the vast majority of rosuvastatin was excreted unchanged, indicating a minor role of metabolism that presumably involves CYP2C9 (Martin et al., 2003b). The clinical significance, organ distribution (primarily governed by multiple transporters), slow biotransformation, and passive membrane diffusion of rosuvastatin make it an ideal model compound for drug transporter studies.
In the present study, we examined the bidirectional transport of rosuvastatin across polarized Caco-2 and BCRP-MDCK cell monolayers. The distinct profiles of rosuvastatin transport revealed the existence of the basolateral uptake transporter(s) in Caco-2 cells, which is deficient in BCRP-MDCK cells. Then we investigated the kinetics and mechanism of rosuvastatin basolateral transport and interplay between the putative basolateral transporter and apical efflux transporter BCRP in Caco-2 cells.
Materials and Methods
Chemicals.
Rosuvastatin calcium was purchased from Toronto Research Chemicals Inc. (North York, Ontario, Canada). Dulbecco's modified Eagle's medium was purchased from Invitrogen (Carlsbad, CA). Hanks' balanced salt solution (HBSS) and fetal bovine serum were obtained from Omega (Tarzana, CA). Penicillin-streptomycin, nonessential amino acids, sodium pyruvate, and neomycin (G418) were obtained from CellGro (Herndon, VA). Rat tail collagen was purchased from BD Biosciences Discovery Labware (Bedford, MD). Acetonitrile was purchased from EMD Chemicals (Gibbstown, NJ). Radioimmunoprecipitation assay buffer was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The bicinchoninic acid protein assay kit was purchased from Thermo Fisher Scientific (Waltham, MA). Prazosin and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture.
Caco-2 cells and MDCK cells were obtained from American Type Culture Collection (Manassas, VA). The MDCK cells were transfected with pEZ-Lv151 vector (Genecopoeia, Rockville, MD) containing the full-length cDNA clone of human ABCG2 wild-type gene (R482) using the electroporation method, and stably transfected cells were selected with 800 μg/ml G418. For transport study, cells were seeded onto rat tail collagen-coated, microporous, polycarbonate membranes in 12-well Transwell plates (1.13-cm2 insert area, 0.4-μm pore size; Corning Life Sciences, Lowell, MA) at 60,000 cells/cm2. Cells were maintained in Dulbecco's modified Eagle's medium containing 100 μM nonessential amino acid, 1 mM sodium pyruvate, 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. G418 (800 μg/ml) was supplemented to maintain selective pressure for BCRP-MDCK cells. All cells were maintained at 37°C in a humidified atmosphere of 5% CO2 in air for 1 week to form confluent monolayers. The culture medium was changed 24 h after seeding to remove cell debris and dead cells; afterward, the medium was changed every other day. Monolayers were used for transport assays between 21 and 28 days for Caco-2 cells and 7 and 11 days for BCRP-MDCK cells. Cell monolayers were certified based on our internal criteria on transepithelial electrical resistance values, and only monolayers that met the acceptance criteria (transepithelial electrical resistance >450 Ω · cm2 for Caco-2 cells and >1200 Ω · cm2 for BCRP-MDCK cells) were selected for transport studies.
Transport Assays.
The transport assay buffer was HBSS containing 15 mM glucose, supplemented with 10 mM MES, pH 5.5, or 10 mM HEPES, pH 7.4. Transport experiments were conducted at apical and basolateral pH 7.4, unless noted otherwise. Bidirectional transport assays in Caco-2 and MDCK cells were carried out by dosing 2 μM rosuvastatin in assay buffer to either the apical compartment (A-to-B) or the basolateral compartment (B-to-A); cells were then incubated at 37°C, and samples were collected at 60 and 120 min. A time-course study of rosuvastatin B-to-A transport in Caco-2 cells was performed by dosing 10 or 200 μM rosuvastatin to the basolateral compartment at 37°C; at preselected time points, samples were collected from the apical compartment, and cell monolayers were washed twice with ice-cold assay buffer and lysed with acetonitrile on ice for 10 min. Concentration dependence of rosuvastatin basolateral uptake was evaluated by dosing rosuvastatin at various concentrations to the basolateral compartment, cells were incubated at 37°C for 30 min, and samples from the apical compartment and cell lysates were collected. The inhibition study was carried out by measuring 2 μM rosuvastatin B-to-A transport in the absence (control) or presence of the indicated test compounds. trans-stimulation of rosuvastatin basolateral efflux by extracellular taurolithocholate was evaluated by dosing 50 μM rosuvastatin and 10 μM atenolol (low-permeability marker) in assay buffer, pH 5.5, to the apical chamber and compound-free (control) or taurolithocholate-containing buffer, pH 7.4, to the basolateral compartment. This high concentration (50 μM) of rosuvastatin was dosed into the apical compartment to overwhelm apical efflux processes as to allow rosuvastatin to reach the intracellular compartment from the apical side. Also, the apical compartment was acidic (pH 5.5), whereas the basolateral compartment was close to neutral (pH 7.4) to maintain the proton-dependent apical uptake activity of OATP2B1 (Varma et al., 2011). After dosing, monolayers were incubated at 37°C, and samples were collected at 15, 30, and 60 min from the basolateral compartment. In all cases, each determination was performed in triplicate. For protein analysis, cells were lysed with radioimmunoprecipitation assay buffer, and protein concentrations were determined by bicinchoninic acid protein assay.
Transporter Gene Expression by Real-Time Polymerase Chain Reaction.
Real-time quantitative polymerase chain reaction (qPCR) was performed using the LightCycler 480 system (Roche Diagnostics, Mannheim, Germany). Total RNA was isolated from mature cell monolayers using the RNeasy Mini kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer's protocol. Synthesis of cDNA was carried out from 1 μg of total RNA using the QuantiTect RT kit (QIAGEN GmbH) for reverse transcription-PCR with random hexamer primers according to the manufacturer's protocol. Primer and probes were designed using the Universal Probe Library (Roche Diagnostics, Basel, Switzerland). qPCR analysis was performed in 20 μl of reaction mixture containing 10 ng of cDNA, the LightCycler 480 probes master kit with 0.1 μM probe, and 0.5 μM primers. The PCR was run at 95°C for 10 min, followed by 45 amplification cycles of 95°C for 10 s, 60°C for 15 s, and 72°C for 1 s. Amplification curves were analyzed using LightCycler 480 Basic software (version 1.2), which produced threshold cycle time (CT) values at where fluorescence is higher than a defined threshold level. Relative gene expression levels of the target genes were normalized to the expression level of the housekeeping gene β-actin using the formula of 2−ΔCT, where ΔCT = CT (target gene) − CT (β-actin).
Liquid Chromatography with Triple Quadruple Tandem Mass Spectrometry Sample Analyses.
Rosuvastatin and prazosin concentrations were determined using reverse-phase liquid chromatography with triple quadruple tandem mass spectrometry methods. The high-performance liquid chromatography equipment consisted of a LEAP CTC HTS PAL autosampler (LEAP Technologies, Carrboro, NC) and Agilent 1100 pumps (Agilent Technologies, Santa Clara, CA). Chromatography was performed at an ambient temperature using a 30 × 2.1 mm i.d., 3 μm Thermo Hypersil BDS C18 column (Thermo Fisher Scientific) with a guard column. The mobile phase buffer was 25 mM ammonium formate buffer, pH 3.5; the aqueous reservoir was 90% deionized water and 10% buffer (v/v); the organic reservoir was 90% acetonitrile and 10% buffer. The gradient started at 5% organic and changed linearly over 1.5 min to 100% organic phase at a flow rate of 250 μl/min. The injection volume was 20 μl, and the total run time was 3.5 min. Mass spectrometry was performed on a Sciex API3000 triple quadruple mass spectrometer in the multiple reaction monitoring modes using a Turbo IonSpray interface (Applied Biosystems, Foster City, CA). The Q1/Q3 settings were +482.1/258.3 and +384.5/247.1 for rosuvastatin and prazosin, respectively.
Data Analysis.
The apparent permeability coefficient (Papp) was calculated using the following equation:
 where dQr/dt is the cumulative drug amount transported into the receiver compartment (Qr) over time (t) during the transport experiment, A is the area of cell monolayer, and C0 is the initial concentration in the donor compartment. The efflux ratio (ER) was calculated as the ratio of the Papp measured in the B-to-A direction divided by the Papp in the A-to-B direction.
where dQr/dt is the cumulative drug amount transported into the receiver compartment (Qr) over time (t) during the transport experiment, A is the area of cell monolayer, and C0 is the initial concentration in the donor compartment. The efflux ratio (ER) was calculated as the ratio of the Papp measured in the B-to-A direction divided by the Papp in the A-to-B direction.
Kinetic analysis of rosuvastatin basolateral uptake in Caco-2 at 37°C was performed by nonlinear regression analysis using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA). The data were fit to a model containing a saturable and a nonsaturable term (Ming et al., 2011):
 where J is the uptake rate normalized to protein, Jmax is the maximal uptake rate, Km is the Michaelis constant for the saturable uptake, Kd is a constant for the nonsaturable uptake, and C is the initial concentration in the donor compartment.
where J is the uptake rate normalized to protein, Jmax is the maximal uptake rate, Km is the Michaelis constant for the saturable uptake, Kd is a constant for the nonsaturable uptake, and C is the initial concentration in the donor compartment.
Statistical Analysis.
Statistical analysis was performed using GraphPad Prism (version 5.04 for Windows; GraphPad Software Inc.). Statistical significance was evaluated using the Student's t test (unpaired, two-tailed) for two-group comparison or one-way analysis of variance (ANOVA) followed by post hoc Tukey's tests for more than two group comparisons. Differences with p < 0.05 were considered statistically significant.
Results
Different Transport Profiles of Rosuvastatin in Caco-2 and BCRP-MDCK Cells.
Rosuvastatin exhibited high efflux in Caco-2 cells, as evidenced by an ER of 83, and moderate efflux in BCRP-MDCK cells, which had an ER of 5.8 (Table 1). Although rosuvastatin transport in the A-to-B direction was low in both Caco-2 and BCRP-MDCK cells (0.25 and 0.28 × 10−6 cm/s), B-to-A transport in Caco-2 cells was 12-fold higher than B-to-A transport in BCRP-MDCK cells (20.66 versus 1.63 × 10−6 cm/s). To confirm the BCRP functionality in BCRP-MDCK cells, we determined the bidirectional permeability of prazosin, a prototypical BCRP probe substrate (Xiao et al., 2006; Giri et al., 2009; Mittapalli et al., 2012). Prazosin was highly efflux in both cell lines (Table 1), but differences in transport patterns were observed. B-to-A transport rates were comparable (45 × 10−6 cm/s in BCRP-MDCK versus 41 × 10−6 cm/s in Caco-2); however, because of a lower A-to-B transport in BCRP-MDCK cells, the ER was 3 times higher in BCRP-MDCK (34.5 in BCRP-MDCK versus 10.5 in Caco-2).
Permeabilities of rosuvastatin and prazosin in Caco-2, MDCK, and BCRP-MDCK cells
Data are expressed as mean ± S.D. (n = 3).
Transporter-Mediated Basolateral Uptake of Rosuvastatin in Caco-2 Cells.
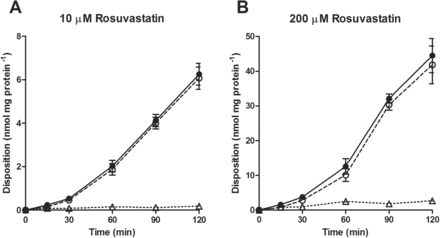
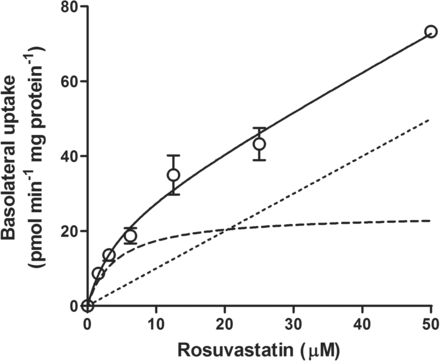
To determine whether the discrepancy in rosuvastatin transport between Caco-2 and BCRP-MDCK could be explained by the presence in Caco-2 cells, and absence in BCRP-MDCK cells, of a basolateral uptake transporter, we undertook the characterization of this putative transporter in Caco-2 monolayers. The rate of B-to-A transport and extent of cellular accumulation of rosuvastatin were roughly linear between 10 and 200 μM (Fig. 1). At preselected time points, the amount of rosuvastatin associated with the cells and the amount released into the apical compartment were added up to determine the total amount of rosuvastatin taken up across the basolateral membrane. Considering that the transport rate was linear at 30 min and that the lower receiver chamber concentrations at earlier time points would increase analytical variability, we decided to measure total rosuvastatin basolateral uptake rates at 30 min. The rosuvastatin basolateral uptake rates were concentration dependent (Fig. 2) and best fit to a modified Michaelis-Menten model with saturable and nonsaturable processes. The apparent Michaelis-Menten constant (Km), the saturable maximal flux (Jmax), and the passive diffusion coefficient (Kd) were estimated to be 4.212 ± 2.130 μM, 24.64 ± 6.10 pmol · min−1 · mg protein−1, and 0.9985 ± 0.1162 μl · min−1 · mg protein−1, respectively.
Time course of rosuvastatin B-to-A transport in Caco-2 cells. Open triangle, drug amount retained inside the cell; open circle, drug released into the apical compartment; filled circle, sum of the drug retained inside the cell and released into the apical compartment. Each point represents the mean ± S.D.; n = 3.
Concentration-dependent uptake of rosuvastatin across Caco-2 basolateral membrane. Results are represented by the fitted uptake data (solid line) and the saturable (dashed line) and nonsaturable (dotted line) transport components. Values are expressed as the mean ± S.D.; n = 3.
Expression of Organic Anion Transporter mRNA in Caco-2 Cells.
Rosuvastatin has an estimated pKa of 4.2 to 4.6 (Varma et al., 2011) and exists primarily as an organic anion at physiological pH. OATP1A2, OATP1B1, OATP1B3, and OATP2B1 have been shown to mediate the uptake of rosuvastatin (Ho et al., 2006; Varma et al., 2011). The heteromeric OSTα-OSTβ, identified as a major basolateral bile acid and steroid transporter in human intestinal epithelia (Ballatori et al., 2005), is the only basolateral exchanger known to transport organic anions (Grandvuinet and Steffansen, 2011). Thus, through the use of real-time PCR, we determined the mRNA expression levels in Caco-2 cells of the transporters commonly associated with the transport of organic anionic molecules. As shown in Table 2, Caco-2 cells express the genes of OATP1A2, OATP2B1, OSTα, and OSTβ but not OATP1B1 and OATP1B3.
Relative mRNA expression of organic anion transporters in Caco-2 cells
Data are expressed as mean ± S.D. (n = 3).
cis-Inhibition and trans-Stimulation of Rosuvastatin Basolateral Transport in Caco-2 Cells.
To further investigate the possibility of OSTα-OSTβ involvement in rosuvastatin basolateral transport, inhibition of rosuvastatin B-to-A transport was evaluated in the presence of a series of compounds known to interact with OSTα-OSTβ (Seward et al., 2003; Ballatori et al., 2005). When codosed with rosuvastatin into the basolateral compartment, all the tested bile salts, steroid conjugates, and organic anions caused significant cis-inhibition of the basolateral uptake of rosuvastatin (Table 3). The basolateral efflux of rosuvastatin from cells was measured in the absence (control) and presence of taurolithocholate in the basolateral compartment. In the absence of taurolithocholate, rosuvastatin basolateral efflux was 14.24 ± 0.34 pmol · min−1 · mg protein−1; when taurolithocholate was present in the basolateral compartment, rosuvastatin basolateral efflux increased by more than 3-fold (Table 4). The transport of a low-permeability marker, atenolol, codosed along with rosuvastatin to ensure the integrity of Caco-2 monolayers, showed no significant differences under these assay conditions, indicating that the barrier properties of the Caco-2 monolayers were not compromised.
cis-inhibition of rosuvastatin basolateral uptake in Caco-2 cells
Rosuvastatin (2 μM) was codosed with the indicated compounds into the basolateral compartment at pH 7.4, and intracellular uptake was measured. Data are expressed as mean ± S.D. (n = 3).
trans-stimulation of rosuvastatin basolateral efflux in Caco-2 cells
Rosuvastatin (50 μM) and atenolol (10 μM) were codosed into the apical compartment at pH 5.5; rosuvastatin release in the basolateral compartment at pH 7.4 was measured in the absence (control) or the presence of taurolithocholate. Data are expressed as mean ± S.D. (n = 3).
Cooperative Action of Basolateral Uptake and Apical Efflux Transporters in Rosuvastatin B-to-A Transport.
Our concept of the B-to-A transport process of rosuvastatin across Caco-2 cell monolayers is portrayed in a simplified schematic model (Fig. 3), in which rosuvastatin is taken up into the cell by a basolateral uptake transporter and excreted across the apical membrane by an apical efflux transporter. On the basis of this model, inhibition of the basolateral transporter would reduce the B-to-A transport and intracellular accumulation of rosuvastatin, whereas inhibition of the apical efflux transporter would also decrease rosuvastatin B-to-A transport but would increase its intracellular accumulation. To test the hypothesis, we used estrone 3-sulfate and rifamycin SV to inhibit the basolateral transporter (OSTα-OSTβ) and 3-(6-isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12α-octahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester (Ko143) and novobiocin to inhibit BCRP-mediated apical efflux in Caco-2 cells. Although all four compounds significantly reduced rosuvastatin B-to-A transport (Fig. 4A), estrone 3-sulfate and rifamycin SV decreased the intracellular accumulation of rosuvastatin, whereas Ko143 and novobiocin increased it (Fig. 4B).
Model for the proposed rosuvastatin B-to-A transport across Caco-2 cell monolayers. Left, rosuvastatin is taken up by a basolateral transporter from the basal membrane and excreted by apical efflux transporters at the apical membrane; middle, blocking the basolateral transporter reduces rosuvastatin B-to-A transport and cellular accumulation; right, blocking the apical efflux transporter reduces rosuvastatin B-to-A transport but increases its intracellular accumulation.
Effects of selected chemicals on rosuvastatin B-to-A transport in Caco-2 cells. B-to-A transport of 2 μM rosuvastatin was conducted in the absence (control) or the presence of selected inhibitors: 50 μM estrone 3-sulfate (E3S), 50 μM rifamycin SV (Rif SV), 2 μM Ko143, or 10 μM novobiocin (Novo). A, B-to-A transepithelial flux. B, B-to-A intracellular accumulation. Each bar represents the mean ± S.D.; n = 3. *, P < 0.05, **, P < 0.01, ***, P < 0.001, significant level of the difference from the control.
Discussion
Rosuvastatin is well known to be transported by BCRP in vitro (Huang et al., 2006; Deng et al., 2008; Li et al., 2011) and in vivo (Zhang et al., 2006; Keskitalo et al., 2009). In the current study, rosuvastatin was effectively effluxed in Caco-2 cells, with an ER of 83, but only mildly effluxed in BCRP-MDCK, with an ER of 5.8 (Table 1). Although one possible reason for the reduced rosuvastatin transport in BCRP-MDCK cells could be low BCRP functionality in BCRP-MDCK cells, the transport data of BCRP substrate prazosin rule out this possibility. Rosuvastatin, with an estimated pKa of 4.2 to 4.6 (Varma et al., 2011), exists primarily in the anionic form at physiological pH, and its pH partition-driven permeability component can be considered negligible. Indeed, rosuvastatin transport in wild-type MDCK cells was extremely low in both directions. Because MDCK cells express little transport activity, these values reflect the low intrinsic membrane permeability of rosuvastatin. These observations suggest that low apical efflux of rosuvastatin in BCRP-MDCK is not due to the lack of BCRP function but, rather, reflects deficient basolateral uptake, and rosuvastatin efflux in Caco-2 is likely enhanced by a concerted action of the basolateral uptake transporter(s) and apical efflux transporters. One implication of this difference is that the use of BCRP-MDCK cells alone to study drug interactions with BCRP could lead to erroneous results for hydrophilic molecules, which require a basolateral uptake transporter (Kitamura et al., 2008). Thus, this study was set forth to investigate the transport mechanism(s) in Caco-2 cells responsible for rosuvastatin basolateral uptake. Although rosuvastatin was used as a model compound, this type of study would have application to other drugs and other transporters that may be involved in carrier-mediated transepithelial drug transport.
First, we examined rosuvastatin transport across Caco-2 cell monolayers in the B-to-A direction (Fig. 1). The B-to-A transport of rosuvastatin dosed at 10 and 200 μM was initially linear up to 30 min, after which it further increased. The nonlinear increase in rosuvastatin B-to-A transport after 30 min may be due to progressive engagement of the apical efflux transporters (P-gp, BCRP, or multidrug resistance-associated protein 2) when the increasing amount of intracellular rosuvastatin reached the apical membrane. The amount of rosuvastatin released into the apical compartment, in a 2-h experiment, was much higher than the amount retained inside the cell. Such results indicate that the apical efflux transporters efficiently vacuumed the drug from the intracellular compartment, and it can be concluded that basolateral uptake is the rate-limiting step in the B-to-A rosuvastatin transport. Concentration-dependence experiments showed that rosuvastatin basolateral uptake consisted of saturable and nonsaturable processes (Fig. 2), and kinetic analysis revealed that at concentrations below Km, the saturable process accounts for more than 75% of the total uptake. These data provided direct experimental evidence that a transporter was involved in rosuvastatin basolateral uptake.
Although these data indicated that a basolateral uptake transporter was involved, the identity of this transporter was unclear. Multiple active uptake transporters expressed at the hepatic sinusoidal membrane have been shown to mediate the hepatic uptake of rosuvastatin, including OATP1B1, OATP1B3, OATP2B1, OATP1A2, and NTCP (Ho et al., 2006; Choi et al., 2011; Varma et al., 2011). OATP1B1 and OATP1B3, believed to be liver specific, are primarily expressed on the sinusoidal membrane of human hepatocytes (Abe et al., 1999; König et al., 2000). In addition, our qPCR results (Table 2) and a previous study (Hayeshi et al., 2008) showed that Caco-2 cells do not express OATP1B1 or OATP1B3 mRNAs. Although NTCP mRNAs were detected in Caco-2 cells from certain laboratories, the expression levels were low and even absent in some cell sources (Hilgendorf et al., 2007; Hayeshi et al., 2008). Furthermore, in contrast to MDCK cells, Caco-2 failed to sort recombinant NTCP-GFP protein into polarized surface expression on the plasma membrane and showed no polarity of taurocholate uptake (Sun et al., 2001), thus suggesting a lack of meaningful NTCP protein expression and function in Caco-2 systems. Both OATP1A2 and OATP2B1 are expressed at the apical membrane of enterocytes or Caco-2 cells (Sai et al., 2006; Glaeser et al., 2007), where they can contribute to the absorption of drugs such as statins, fexofenadine, levofloxacin, methotrexate, and talinolol (Badagnani et al., 2006; Ho et al., 2006; Maeda et al., 2007; Kitamura et al., 2008; Shirasaka et al., 2010; Ming et al., 2011). For the aforementioned reasons, it is highly unlikely that OATP1B1, OATP1B3, OATP2B1, OATP1A2, or NTCP could be involved in the basolateral uptake of rosuvastatin in Caco-2 cells. As a result, basolateral OSTα-OSTβ transporter was singled out as the most likely candidate among known transporters for facilitating rosuvastatin penetration across the basolateral membrane. OSTα-OSTβ heteromeric exchanger transports substrates in both directions across the cell membrane (i.e., uptake and efflux) via a facilitated diffusion mechanism (Ballatori et al., 2005). Challenging rosuvastatin basolateral uptake with OSTα-OSTβ inhibitors (Seward et al., 2003) caused significant cis-inhibition, which indicates the likelihood of OSTα-OSTβ involvement. Next, we investigated the directionality of rosuvastatin basolateral transport to further characterize the mechanism of this basolateral transport system. Because OSTα-OSTβ is a membrane exchanger (Ballatori et al., 2005), if rosuvastatin basolateral transport is indeed mediated by OSTα-OSTβ, this process is expected to be bidirectional and susceptible to trans-stimulation. A large increase in basolateral efflux when taurolithocholate was present in the opposite side of cell membrane (i.e., trans-stimulation) supports the involvement of OSTα-OSTβ in rosuvastatin basolateral transport in Caco-2 cells.
The finding that rosuvastatin is transported across basolateral membrane by a facilitated diffusion in Caco-2 cells could shed light on rosuvastatin intestinal absorption in vivo. Given the hydrophilic nature and limited membrane permeability of rosuvastatin, it is likely that rosuvastatin intestinal absorption, estimated to be about 50% (Martin et al., 2003a), involves apical uptake and basolateral efflux (or exchange) transporters. A recent report showed that rosuvastatin underwent OATP2B1-mediated apical uptake in Caco-2 cells (Varma et al., 2011), it is conceivable that the intestinal absorption of rosuvastatin in vivo involves the apical uptake transporter(s) and the basolateral exchange/efflux transporter(s). After a usual dose of 20 mg, the maximum plasma concentration (Cmax) of rosuvastatin ranged from 0.006 to 0.03 μM in healthy male volunteers (Zhang et al., 2006), which is well below the Km value of 4.2 μM determined for the basolateral transporter in Caco-2 cells; thus, this transporter can undertake the facilitated basolateral transport of rosuvastatin in vivo. However, the intestinal disposition of rosuvastatin has not been fully delineated yet; additional studies are needed to clarify the roles of OATP2B1 and OSTα-OSTβ in rosuvastatin transport across the intestinal epithelium. One approach that could furnish unambiguous identification of transporter involvement is the use of RNA interference to knock down the expression of relevant transporters. This technique has already been used to selectively knock down the expression of BCRP, P-gp, and multidrug resistance-associated protein 2 in Caco-2 cells (Zhang et al., 2009), which proved valuable in elucidating the role of efflux transporters in the biliary efflux of ximelagatran (Darnell et al., 2010) and several statin drugs (Li et al., 2011).
Finally, we investigated the cooperative action of this basolateral uptake transporter with apical BCRP involved in rosuvastatin B-to-A transport. On the basis of our conceptual model (Fig. 3), it is possible to tease out the role of the basolateral transporter from the apical efflux transporter. The model predicts that inhibition of the apical efflux transporter would increase the intracellular accumulation of rosuvastatin, whereas inhibition of the basolateral uptake transporter would reduce the intracellular accumulation. Thus, to test the prediction of a cooperative interaction between the basolateral uptake transporter and apical BCRP, we determined rosuvastatin B-to-A transport in the presence of estrone 3-sulfate or rifamycin SV (inhibitors of basolateral OSTα-OSTβ) and in the presence of Ko143 or novobiocin (inhibitors of apical BCRP). Results show that all four compounds inhibited rosuvastatin overall B-to-A transport, but estrone 3-sulfate and rifamycin SV reduced intracellular accumulation whereas Ko143 and novobiocin increased it, demonstrating a functional interaction between the basolateral transporter and apical BCRP for rosuvastatin B-to-A transport in Caco-2 cells. It was noticed that although novobiocin produced greater inhibition on rosuvastatin B-to-A flux than Ko143, it caused less intracellular accumulation. One possible explanation is that novobiocin also partially inhibits the basolateral uptake transporter, because cross-inhibition is a common difficulty associated with transporter studies using chemical inhibitors (Watanabe et al., 2005; Wang et al., 2008).
In conclusion, this study sought to investigate the distinct rosuvastatin transport profiles: robust apical efflux in Caco-2 cells but much reduced efflux in BCRP-MDCK cells. Through a series of transport experiments, the role of a basolateral transporter facilitating the basolateral penetration of rosuvastatin to access apical efflux transporters was revealed in Caco-2 cells, and our data suggest that the OSTα-OSTβ heteromeric exchanger is likely involved. Additional investigations are needed to achieve a definitive characterization of this basolateral transporter with respect to molecular identity and substrate selectivity. Such research is of value because the use of cell monolayer systems lacking such a transporter may lead to false-negative recognition of potentially important drug-transporter interactions.
Authorship Contributions
Participated in research design: Li, Wang, Zhang, and Hidalgo.
Conducted experiments: Wang and Hein.
Contributed new reagents or analytic tools: Huang.
Performed data analysis: Wang and Li.
Wrote or contributed to the writing of the manuscript: Li, Wang, and Hidalgo.
Acknowledgments
We thank Jennifer Winans and Samantha M. Allen for technical assistance.
Footnotes
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
ABBREVIATIONS:
- DDI
- drug-drug interaction
- A
- apical
- B
- basolateral
- BCRP
- breast cancer resistance protein
- Caco-2
- human epithelial colorectal adenocarcinoma cells
- HBSS
- Hanks' balanced salt solution
- MDCK
- Madin-Darby canine kidney cells
- OATP
- organic anion-transporting polypeptide
- OST
- organic solute transporter
- Papp
- apparent permeability coefficient
- P-gp
- P-glycoprotein
- qPCR
- real-time quantitative polymerase chain reaction
- NTCP
- Na+-taurocholate cotransporting polypeptide
- ER
- efflux ratio
- Ko143
- 3-(6-isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12α-octahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester.
- Received March 10, 2012.
- Accepted August 1, 2012.
- Copyright © 2012 by The American Society for Pharmacology and Experimental Therapeutics