Abstract
Organic anion–transporting polypeptide (OATP)1B1, OATP1B3, and OATP2B1 transporters play an important role in hepatic drug disposition. Recently, an increasing number of studies have reported proteomic expression data for OATP transporters. However, systematic analysis and understanding of the actual differences in OATP expression between liver tissue and commonly used cellular systems is lacking. In the current study, meta-analysis was performed to assess the protein expression of OATP transporters reported in hepatocytes relative to liver tissue and to identify any potential correlations in transporter expression levels in the same individual. OATP1B1 was identified as the most abundant uptake transporter at 5.9 ± 8.3, 5.8 ± 3.3, and 4.2 ± 1.7 fmol/μg protein in liver tissue, sandwich-cultured human hepatocytes (SCHH), and cryopreserved suspended hepatocytes, respectively. The rank order in average expression in liver tissue and cellular systems was OATP1B1 > OATP1B3 ≈ OATP2B1. Abundance levels of the OATP transporters investigated were not significantly different between liver and cellular systems, with the exception of OATP2B1 expression in SCHH relative to liver tissue. Analysis of OATP1B1, OATP1B3, and OATP2B1 liver expression data in the same individuals (n = 86) identified weak (OATP1B1-OATP2B1) to moderately (OATP1B3-OATP2B1) significant correlations. A significant weak correlation was noted between OATP1B1 abundance and age of human donors, whereas expression of the OATPs investigated was independent of sex. Implications of the current analysis on the in vitro–in vivo extrapolation of transporter-mediated drug disposition using physiologically based pharmacokinetic models are discussed.
Introduction
Organic anion–transporting polypeptides (OATP)1B1, OATP1B3, and OATP2B1 belong to the superfamily of solute carrier transporters and mediate the membrane uptake of a wide range of clinically relevant drugs from the portal blood into hepatocytes (Giacomini et al., 2010; Niemi et al., 2011). OATP1B1 has been highlighted as one of the most clinically relevant hepatic uptake transporters. Genetic polymorphism in the SLCO1B1 gene and drug-drug interactions (DDI) associated with this transporter, often coupled with metabolic interaction, raise efficacy and safety concerns (Gertz et al., 2013; Shitara et al., 2013; Gertz et al., 2014; Prueksaritanont et al., 2014; Tsamandouras et al., 2014).
Recommendations regarding in vitro transporter assays and modeling approaches for the prediction of transporter-mediated pharmacokinetics and corresponding DDIs have been recently reviewed by the International Transporter Consortium (Zamek-Gliszczynski et al., 2013). Despite advances in characterization of active uptake in vitro and consideration of mechanistic modeling of such data (Poirier et al., 2009; Menochet et al., 2012a), a tendency to under-predict transporter-mediated hepatic clearance is often observed. This under-prediction trend (on average 17- to 58-fold) was evident across different cellular systems used to generate in vitro transporter data for the comparable drug set (Poirier et al., 2009; Jones et al., 2012; Menochet et al., 2012b). To bridge this gap in the in vitro–in vivo extrapolation (IVIVE), clinical plasma concentration-time data have been used to optimize active uptake clearance in physiologically based pharmacokinetic (PBPK) models, requiring an empirical scaling factor (ESF) for this parameter (Zamek-Gliszczynski et al., 2013; Gertz et al., 2014). The issue of concern is that the extent of underprediction and reported ESFs show drug- and donor-dependent differences and large variability depending on the hepatocyte format used and assumptions made in the optimization routine (Poirier et al., 2009; Watanabe et al., 2009; Gardiner and Paine, 2011; Jones et al., 2012; Menochet et al., 2012b; Gertz et al., 2014). So far, transporter IVIVE of hepatocyte data generally does not account for any potential differences in the transporter expression between the tissue and the actual cellular system used, which may contribute to this underprediction trend observed. In the case of OATP-transfected cell lines, attempts have been made to account for expression differences in the form of relative expression factors (Hirano et al., 2004; Jamei et al., 2014). However, these are often based on Western blot measurements and account for expression differences between the transfected cell line and hepatocytes (based usually on a small number of donors) (Hirano et al., 2004), rather than the actual liver tissue.
Recently, an increasing number of studies have reported the quantitative expression data for OATP1B1, OATP1B3, and OATP2B1 in human liver tissue and in some of the most commonly used in vitro cellular systems. It is evident that protein extraction and quantification methods used to obtain abundance data vary across systems, together with procurement conditions, storage of tissue samples, and demographics of the subjects. Most of the proteomic transporter expression studies have as their basis a relatively small number of subjects, with the exception of the recent study (Prasad et al., 2014). However, systematic analysis of such data and understanding of the magnitude of differences in the OATP expression levels between liver tissue and cellular systems is currently lacking.
The aim of the current work was to collate reported OATP expression data in human liver tissue and cellular systems, namely sandwich-cultured human hepatocytes (SCHH) and cryopreserved hepatocytes in suspension. A meta-analysis approach was performed to address the issues raised above and overcome the limitations of the individual studies to provide a reliable assessment of differences in OATP expression levels between liver tissues and hepatocyte systems. On the basis of relevant eligible studies, critical outcomes have been defined, such as the average abundance and variability of OATP1B1, OATP1B3, and OATP2B1 in liver tissue and in in vitro cellular systems, as well as the heterogeneity between studies. In addition, correlations between individual transporter expression levels in liver tissue and the impact of demographic factors (including age and sex) were investigated. Implications of the integration of OATP abundance as relative expression factors in PBPK models to improve IVIVE of transporter-mediated drug disposition are discussed.
Materials and Methods
Literature Search Strategy and Study Eligibility.
Relevant published articles on the abundance data of OATP1B1, OATP1B3, and OATP2B1 transporters were searched through the electronic database MEDLINE using the following keyword combinations: hepatic/liver OATP transporter abundance, correlation of expression, quantification. The following criteria were applied for the inclusion of the studies in the analysis: 1) simultaneous measurement of OATP1B1, OATP1B3, and OATP2B1 abundance in the same individuals, 2) use of liquid chromatography–tandem mass spectrometry (LC-MS/MS) proteomic methods, 3) reported abundance levels for the transporters of interest in human liver tissue and/or in vitro cellular systems, and 4) the abundance data were based on quantification of total membrane protein (rather than plasma membrane) expression levels of the investigated OATP transporters. Individual abundance data were extracted from the collated studies. If data were not quoted, GetData Graph Digitizer version 2.25 (http://getdata-graph-digitizer.com/) was used to digitize and extract the data. Donor demographics (including age and sex) were also collated when available. Studies with reported abundance data for the investigated transporters based on immunoblotting techniques, reported as plasma membrane protein measurement and/or reported in relative or arbitrary units, were excluded.
Weighted Means and Coefficient of Variation Calculations.
Normality of distribution and homoscedasticity were assessed for expression data per individual transporter. The Kolmogorov-Smirnov test was conducted to determine the normality of distribution of abundance data. Brown-Forsythe Levene-type test was applied to examine the homogeneity of variances of collated expression data. Individual abundance levels expressed in liver tissue and in each in vitro cellular system were combined from the collected studies to generate weighted mean (W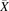 ) and weighted coefficient of variation (WCV) per individual transporter (eqs. 1 and 2).
) and weighted coefficient of variation (WCV) per individual transporter (eqs. 1 and 2).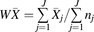 (1)
(1)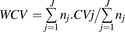 (2)where subscript j indicates the study j, nj the sample size of study j and
(2)where subscript j indicates the study j, nj the sample size of study j and 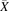 j the mean abundance of a singular OATP transporter in study j.
j the mean abundance of a singular OATP transporter in study j.
Assessment of Heterogeneity between Studies.
Cochran X2-based Q test and I2 statistic (Higgins and Thompson test) were performed to define the between-study heterogeneity (Cochran, 1954; Higgins and Thompson, 2002; Higgins et al., 2003), where a higher value of Cochran heterogeneity statistic Q of the collated data indicates greater heterogeneity. The I2 heterogeneity index is an indicator of the fraction of the variability attributable to true between-study differences. These calculations were based on eqs. 3, 4, and 5. I2 values of 25%, 50%, and 75% reflect evidence of low, moderate, and high heterogeneity, respectively. Probabilities were quoted based on X2 distribution of the Q value and the number of degrees of freedom, k-1. Probability values were symbolized by *P < 0.05, **P < 0.01, ***P < 0.001 and P values of P < 0.05 were considered statistically significant. (3)
(3)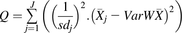 (4)
(4)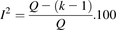 (5)where (VarW
(5)where (VarW ) represents the variance in the weighted mean of the collated data, sdj the standard deviation of study j, and k the number of studies.
) represents the variance in the weighted mean of the collated data, sdj the standard deviation of study j, and k the number of studies.
Comparison of Abundance Levels of OATP Transporters in In Vitro Cellular Systems Relative to Liver Tissue.
A nonparametric Kruskal-Wallis one-way analysis of variance (ANOVA) was conducted to assess any potential significant differences in abundance levels of OATP transporters between liver tissue and in vitro cellular systems. Ratio of the transporter expression in the liver tissue relative to the cellular system investigated defined the relative expression factor for the corresponding transporter and cellular system. If significant differences between groups were detected by the one-way ANOVA, Mann-Whitney rank-order U test multiple comparisons were carried out to determine which groups were significantly different. Bonferroni correction was applied to adjust the level of significance (P value) for the pairwise comparisons performed on a single set of data. Probability values were symbolized by *P < 0.025, **P < 0.005, and ***P < 0.0005, and P < 0.025 was considered statistically significant. Impact of any potential sample size effect (r) in the Mann-Whitney U test was performed as described by eq. 6. The reported absolute values of r represent small (0.1), moderate (0.3), and large (0.5) size effect.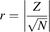 (6)where Z represents the test statistic value and N the total number of samples.
(6)where Z represents the test statistic value and N the total number of samples.
Analogously, hepatic tissue expression of OATPs was analyzed by performing a Kruskal-Wallis ANOVA followed by a Mann-Whitney rank order U test multiple comparisons to identify any potential interstudy differences. Bonferroni correction was applied to adjust the level of significance for the pairwise comparisons performed on a single set of data. Probability values are symbolized by *P < 0.0125, **P < 0.0025, and ***P < 0.00025, where P < 0.0125 was considered statistically significant.
Assessment of Potential Correlation in Expression Levels of OATP Transporters in the Same Individuals.
Correlations between the hepatic tissue expression levels of OATP1B1, OATP1B3, and OATP2B1 were investigated using the nonparametric Spearman rank correlation test (Armitage, 2001). The Spearman rank coefficient of correlation (Rs) and the probabilities (p) were calculated for each correlation test. Collated abundance data were normalized beforehand using the mean values of reference studies (eq. 7). Bonferroni correction was applied to adjust the level of significance for the pairwise comparisons performed on a single set of data. Probability values were symbolized by *P < 0.025, **P < 0.005, ***P < 0.0005, and P < 0.025 was considered statistically significant. (7)where xj,normalized represents the normalized abundance value of the abundance measurement x in study j;
(7)where xj,normalized represents the normalized abundance value of the abundance measurement x in study j;  j, the mean abundance value of study j; and
j, the mean abundance value of study j; and 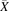 reference, the mean abundance in the reference study used for the normalization process. The weighted means and the reference abundance values used for data normalization are shown in Table 2.
reference, the mean abundance in the reference study used for the normalization process. The weighted means and the reference abundance values used for data normalization are shown in Table 2.
Investigation of Age- and Sex-Related Differences in Hepatic Tissue Expression Levels of OATP Transporters.
Age- and sex-related differences in hepatic tissue expression data were assessed per individual OATP transporter. A Mann-Whitney rank order U test was carried out to establish any potential differences between male and female subjects. Probability values were symbolized by *P < 0.05, **P < 0.01, ***P < 0.001, where P < 0.05 was considered statistically significant. In addition, a nonparametric Spearman rank correlation test was performed to investigate any potential correlation of transporter expression data with age of the human donors. The correlations were tested for the collated data. Probability values were symbolized by *P < 0.05, **P < 0.01, ***P < 0.001, where P < 0.05 was considered statistically significant.
Results
Eligible Studies for the Meta-Analysis.
In the preliminary analysis, 11 studies with reported OATP abundance data were identified. Out of those, three studies were excluded because the methods used were assessed as inappropriate (e.g., abundance measurement based on plasma membrane protein measurements), as detailed in Supplemental Table 1. A total of eight studies met the meta-analysis inclusion criteria. Abundance data of OATP1B1, OATP1B3, and OATP2B1 were obtained in five different laboratories using different LC-MS/MS methods to quantify the total membrane protein expression levels. Transporter abundance data were extracted in liver tissue (five studies), SCHH (two studies), and in cryopreserved human hepatocytes in suspension (five studies). Individual protein expression levels were not reported in some of the selected studies, and mean data were quoted in two studies. In five studies, the expression levels of OATP transporters were measured in only one sample, as outlined in Table 1. Details of sample preparation and LC-MS/MS quantification used in the studies included in the meta-analysis are shown in Supplemental Table 2.
Total membrane protein expression levels of OATP transporters in human liver tissue, SCHH, and human cryopreserved hepatocytes in suspension
Data represent the mean abundance ± S.D. expression levels of OATP transporters determined using the total membrane fraction. All values are fmol/μg protein.
Comparison of Abundance Levels of OATP Transporters in In Vitro Cellular Systems Relative to Liver Tissue.
OATP1B1 was identified as the most abundant uptake transporter with 5.9 ± 8.3, 5.8 ± 3.3, and 4.2 ± 1.7 fmol/μg protein in liver tissue, SCHH, and cryopreserved suspended human hepatocytes, respectively (Table 2). The rank order in average expression in liver tissue and in vitro cellular systems was OATP1B1 > OATP1B3 ≈ OATP2B1. Surprisingly, relative expression factors for all the OATPs investigated did not exceed 2.5. On average, OATP1B1 expression in the liver tissue was 1.4-fold greater in comparison with cryopreserved hepatocytes in suspension, whereas abundance of this transporter in SCHH (on the basis of n = 5) was comparable to the mean expression data in the liver tissue, suggesting no requirement for abundance corrections (relative expression factor = 1). Mean OATP1B1 expression in human liver tissue was 3-fold greater than OATP1B3 and OATP2B1. The relative OATP1B1/OATP1B3 expression was higher compared with data reported by the Western blot measurements in 46 livers (1.6-fold difference) (Michalski et al., 2002). Similar trends were seen in expression of OATP1B1 relative to other OATPs in cryopreserved hepatocytes. In contrast, expression of OATP1B1 was up to 7-fold higher in SCHH relative to OATP1B3 and OATP2B1 in the same system (Table 2). The average protein expression levels of OATP1B3 and OATP2B1 in cryopreserved hepatocytes in suspension were comparable to that observed in human liver tissue samples (1.8 versus 2.0 and 1.6 versus 1.9 fmol/μg protein). The expression of these transporters in SCHH was up to 2.5-fold lower in comparison with liver tissue and suspended hepatocytes (Table 2).
Heterogeneity analysis of the investigated OATP transporter abundance levels in human liver tissue
The weighted means, coefficients of variation (CV), range, and heterogeneity analysis of the analyzed hepatic tissue OATP transporter abundance levels data in liver tissue, SCHH, and cryopreserved hepatocytes in suspension
To assess the within-study variability in quantitative transporter expression data, the ratio of maximum and minimum abundance was calculated for each study and per individual transporter. OATP1B3 protein expression in the liver tissue exhibited the greatest variability, resulting in 1.5- to 32-fold difference across individual studies. The within-study variability in hepatic tissue expression ranged from 3.3- to 6.6-fold for OATP1B1 and from 1.5- to 7.5-fold for OATP2B1. A maximum of 4.7- and 3.6-fold difference was observed in the reported OATP expression in SCHH and cryopreserved hepatocytes, respectively (based on 4–13 donors, Table 1).
Collated OATP transporter abundance data reported for liver tissue and hepatocytes in different formats exhibited neither normal distribution nor equal variances. As a consequence, a nonparametric Kruskal-Wallis one way ANOVA was conducted followed by a multiple comparison Mann-Whitney U test as post-hoc analysis. The threshold level of significance was modified from 0.05 to 0.025 using Bonferroni correction owing to the two pairwise comparisons performed on the same dataset to reduce the likelihood of false-positive results. Transporter abundance data were not significantly different statistically between liver tissue and in vitro cellular systems, with the exception of OATP2B1 expression in SCHH relative to liver tissue (Mann-Whitney U test, P < 0.005) characterized with a moderate sample size effect (r = 0.29) (Fig. 1).
Individual abundance levels of OATP transporters in human liver tissue, SCHH, and human cryopreserved hepatocytes in suspension. The individual abundance levels of OATP1B1 (A), OATP1B3 (B), and OATP2B1 (C) were collated in human liver tissue(open symbols), sandwich-cultured human hepatocytes (gray symbols), human cryopreserved hepatocytes in suspension (black symbols). The symbols in the figure represent: heavy solid line, weighted mean of OATP transporter abundance; and dashed lines, 5th and 95th percentiles of hepatic tissue expression levels of OATP transporters. Differences were considered significant at the level of significance, **P < 0.005.
Interstudy Variability in OATP Abundance in Liver Tissue.
Heterogeneity tests were performed to investigate interstudy variability in reported abundance data. Heterogeneity tests detected moderate (OATP1B1, I2 ≈ 60%, P < 0.05) and high (OATP1B3 and OATP2B1, I2 > 80%, P < 0.001) heterogeneity between data reported for liver tissue, as outlined in Table 2. Heterogeneity tests were not achievable between studies reporting data in in vitro cellular systems, either because of the small number of studies or lack of individual data. Owing to non-normal distribution, hepatic tissue abundance data were compared applying the same nonparametric statistical analysis as was used for the analysis of transporter expression in cellular systems relative to liver tissue. The threshold level of significance was modified from 0.05 to 0.0125 because of the four pairwise comparisons performed on the same dataset (Bonferroni correction). Hepatic tissue expression data showed statistically significant differences between studies and was characterized by moderate (0.4) to high (0.7) sample-size effects; different levels of significance are detailed in Table 2 and Fig. 2.
Differences in expression of OATP transporters in liver tissue between studies. Differences in hepatic tissue expression levels between studies for OATP1B1 (A), OATP1B3 (B), and OATP2B1 (C) were considered significant applying a multiple comparison Mann-Whitney U test at the level of significance, **P < 0.0025, ***P < 0.00025. Individual expression levels are represented by black symbols and the mean abundance value is represented by the solid line.
Assessment of Potential Correlation in Expression Levels of OATP Transporters in the Same Individuals.
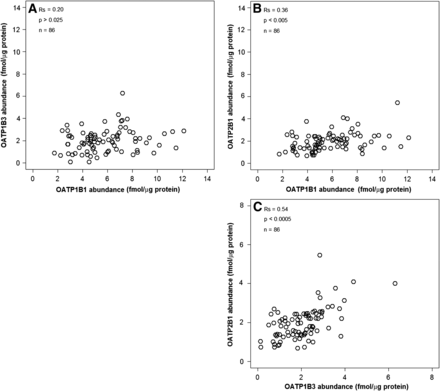
The distribution of hepatic tissue abundance data collated per individual transporter from five studies was not normal based on the Kolmogorov-Smirnov test. Therefore, nonparametric Spearman rank correlation test was conducted to assess potential correlations in expression levels between OATP transporters. Collated abundance data were normalized using the weighted mean values of reference studies (Table 2). The threshold level of significance was modified from 0.05 to 0.025 owing to the two pairwise comparisons performed on the same dataset (Bonferroni correction). Analysis of OATP1B1, OATP1B3, and OATP2B1 expression data in the liver tissue from the same individuals identified statistically significant weak (OATP1B1/OATP2B1, Spearman correlation test, Rs = 0.36, P < 0.005, n = 86) to moderate (OATP1B3/OATP2B1, Spearman correlation test, Rs = 0.54, P < 0.0005, n = 86) correlations (Fig. 3). No statistically significant correlation was established between OATP1B1/OATP1B3 (Spearman correlation test, Rs = 0.20, P > 0.025, n = 86). The correlation between genotype and protein expression of OATP1B1, OATP1B3, and OATP2B1 could not be assessed owing to lack of data for either hepatic liver tissue or the two cellular systems investigated.
Correlation in hepatic tissue expression levels of OATP transporters in the same individuals. No significant correlation was detected between OATP1B1 and OATP1B3 (A) (Spearman rank test, Rs = 0.20, P > 0.025, n = 86). Significant weak correlation between OATP1B1 and OATP2B1 (B) (Spearman rank test, Rs = 0.36, P < 0.005, n = 86) to moderate correlations between OATP1B3 and OATP2B1 (C) (Spearman rank test, Rs = 0.54, P < 0.0005, n = 86) were detected. Plot shows data normalized on the basis of weighted means.
Investigation of Age- and Sex-Related Differences in Hepatic Tissue Expression Levels of OATP Transporters.
The distribution of hepatic tissue abundance data collated from three studies was non-normal based on the Kolmogorov-Smirnov test. As a result, nonparametric tests were applied to assess differences in expression levels between male and female subjects and against age. Statistically significant weak correlation was detected between expression levels of OATP1B1 and age of the human donors (Spearman correlation test, Rs = 0.33, P < 0.01, n = 80) (Fig. 4A), whereas the correlation with age for OATP1B3 and OATP2B1 transporters was not significant (Spearman correlation test, Rs < 0.10, P > 0.05, n = 80, Supplemental Fig. 1). In addition, hepatic tissue expression levels were not significantly different between male (n = 44) and female (n = 36) subjects for any of the OATP transporters investigated (Mann-Whitney U test, P > 0.05) (Fig. 4B).
Age- and sex-related differences in hepatic tissue expression levels of OATP transporters. Significant correlation of OATP1B1 transporter expression levels and age of donors was detected (A) (Spearman rank test, Rs = 0.33, P < 0.01, n = 80). Differences in expression levels of OATP transporters were not considered significant between male (black symbols) and female (open symbols) subjects (B) (U test, P > 0.05). Bars represent the means and upper bounds of S.D. of transporter expression levels.
Discussion
PBPK modeling approaches are increasingly used to predict transporter-mediated pharmacokinetics and related DDIs (Poirier et al., 2009; Gertz et al., 2013; Varma et al., 2013; Gertz et al., 2014; Jamei et al., 2014); however, the abundance of OATPs and other transporters is not adequately incorporated in the PBPK models as yet, primarily because of to lack of data (Zamek-Gliszczynski et al., 2013; Galetin, 2014). Despite emerging proteomic data, so far no systematic analysis of the differences in total membrane protein expression of OATP1B1, OATP1B3, and OATP2B1 reported in the liver tissue and cryopreserved hepatocytes has been performed. Following the inclusion criteria, eight individual studies were considered herein; the number of individual samples used to quantify the transporters investigated ranged from 1 to 64. Significant moderate to high heterogeneity between studies was detected per individual transporter for the liver tissue abundance data. In contrast, the degree of heterogeneity could not be assessed in cellular systems as the number of studies was either too small and/or only the average expression data of investigated transporters were quoted.
The current analysis revealed marginal differences in OATP expression levels between liver tissue and cryopreserved hepatocytes in suspension, as the relative expression factors for all OATPs were <1.5-fold for this cellular system (Fig. 1, Table 2). In the case of SCHH, relative expression factor of up to 2.5-fold is required for OATP1B3 and OATP2B1, in contrast to OATP1B1, where the weighted mean protein expression of this transporter was comparable between liver tissue and SCHH (based on n = 5 samples). The current analysis revealed substantial differences in the extent of variability of OATP1B3 abundance data (1.5- to 32-fold within individual studies). This trend was also evident in the study with the largest sample size, in which OATP1B3 expression varied 8.8-fold in 64 subjects (Prasad et al., 2014), in agreement with variability in OATP1B1 and OATP2B1 expression within the same study (up to 6.6-fold). In most of the studies, the extent of variability in protein expression exceeds the differences associated with the well-recognized genetic variations in the SLCO1B1 and SCLO1B3 genes encoding OATP1B1 and OATP1B3, respectively (Link et al., 2008; Niemi et al., 2011; Gong and Kim, 2013). However, direct analysis of any potential link between transporter genotype versus protein expression and, subsequently, functional activity was not possible because of the lack of data.
The present meta-analysis investigated for the first time the correlation between OATP expression levels in the same individuals; this analysis was only performed for liver tissue due to availability of data. The low and moderate correlations observed (Fig. 3) suggests limited dynamic coregulation of OATP transporters, as opposed to metabolizing enzymes (Achour et al., 2014). By aggregating the abundance data reported in three studies, the analysis identified a significant but weak correlation of hepatic tissue expression levels of OATP1B1 with age of the human donors, as opposed to the findings reported by each individual selected study (Balogh et al., 2012; Prasad et al., 2014; Vildhede et al., 2014). In addition, no differences in abundance data were apparent between male and female subjects, consistent with the outcomes of individual studies. This finding was in agreement with a recent meta-analysis of abundance data of drug-metabolizing cytochrome P450 enzymes (Achour et al., 2014).
There are several limitations to the analysis presented here that merit consideration when interpreting the findings. The first limitation is the relatively small number of eligible studies and also their sample size, which is particularly evident for data reported in SCHH. In addition, individual expression data of the selected OATP transporters were not quoted or extractable in some of the studies. Hence, average abundance data were incorporated in the analysis alongside individual data, lowering the statistical power of the analysis. In addition, most of the studies did not quantify the transporter expression levels in the liver tissue and cellular systems from the same donor. One exception is the study by Kimoto et al. (2012), in which OATP abundance data were reported for the hepatocyte systems (SCHH and cryopreserved hepatocytes in suspension) prepared from the same donor; however, no corresponding expression data for the liver tissue were available. The mean expression of OATP1B1 and OATP2B1 from the five donors in the Kimoto et al. study did not show a statistical difference between the two hepatocyte formats, with the exception of OATP1B3, which was significantly decreased in SCHH. These findings were partially in agreement with the outcomes of the meta-analysis, as no significant differences in expression levels of all OATP transporters investigated were detected between SCHH and cryopreserved hepatocytes in suspension (n = 17) (Fig. 1). In addition, no expression data are currently available for cryopreserved hepatocytes in monolayer format, highlighting the need for larger datasets to fully clarify differences in transporter expression under differing conditions of culture, associated variability between in vitro systems, and impact of these factors on transporter-mediated IVIVE. Finally, demographic information for the human donors (reported for n = 86 in total) allowed the analysis of correlation of OATP liver tissue expression with age or sex. The corresponding analysis was not feasible for either of the cellular systems, as no demographic information was reported.
No published studies so far have reported protein expression, genotype, mRNA levels and activities of OATP1B1, OATP1B3, and OATP2B1 transporters in the same samples. Similarly, only two studies so far have investigated a potential correlation between expression of OATP transporters and metabolizing enzymes in liver tissue and SCHH (Ohtsuki et al., 2012; Schaefer et al., 2012). However, considering that the abundance data were determined using the plasma membrane fraction, these two studies were excluded from the current analysis. Ohtsuki et al. (2012) reported higher expression of an ion transporter protein Na/K-ATPase (considered to be a basal membrane marker) in the crude total membrane fraction compared with the plasma membrane fraction obtained from the same liver samples (n = 17). However, a higher abundance should be expected, at least theoretically, in the plasma membrane fraction, which is anticipated to be more transporter-enriched and functionally pertinent. Arguably, losses attributable to plasma membrane preparation methods could be responsible for this contradiction, as highlighted recently (Harwood et al., 2014). Plasma membrane may contain only a fraction of the total expressed transporter attributable to potential internalization reported for several transporter proteins (Bow et al., 2008; Kock et al., 2010; Choi et al., 2011). In addition, impact of post-translational modifications on cell surface trafficking and expression of OATPs is still not well defined. For these reasons, it seemed more appropriate to use studies that quantified transporters in crude total membrane as opposed to plasma membrane.
Heterogeneity between studies highlighted in this analysis may be a consequence of methodological diversity and raises questions of whether the differences in expression levels of OATP transporters may be attributable to the method of tissue procurement (organ donors versus resections from diseased livers), tissue storage conditions (frozen versus fresh tissue), and/or diversity in subject demographics. In addition, sample preparation, i.e., membrane fractionation methods, differences in LC-MS/MS protein quantification, selection of the standard peptide, variation in proteolytic digestion of membrane proteins, and technical recovery, may all contribute to the heterogeneity observed. Although there seemed to be an overall agreement between studies in the selection of internal standards and the use of proteolytic enzyme, differences were identified in the method of extraction of membrane fractions, the calibration process, proteolytic conditions, and the type of quantification (details in Supplemental Table 2). Interestingly, Karlgren et al. (2012) and Vildhede et al. (2014) used a label-free relative quantification method that does not use internal standards and requires no calibration. In all the studies, the calibration process was carried out using light peptide standards of known concentrations, with the exception of Ji et al. (2012), who used purified OATP proteins previously quantified by amino acid analysis. Calibration using protein standards is expected to improve the quality of the quantification process; however, this study only quantified one sample of cryopreserved hepatocytes, which did not permit comparison with other data. The discussed technical and analytical differences may account for some of the interstudy variability identified here. It is suggested, therefore, that the interpretation of the heterogeneity noted in the current analysis cannot be attributed solely to true biologic variability in OATP expression without any additional studies with respect to consistency of proteomic LC-MS/MS methodologies and cross-laboratory comparisons of abundance data using the same biologic samples. A recent report (Kumar et al., 2014) suggested that normalization of absolute abundance levels of OATPs with the abundance of the membrane protein marker Na/K-ATPase can reduce the effect of variability introduced by technical and analytical procedures. The authors demonstrated strong linear correlation between normalized OATP1B1 abundance and uptake of estradiol 17-β glucuronide in vitro, supporting further this concept.
In conclusion, quantitative protein levels of OATP transporters in different human hepatocyte formats relative to liver tissue were assessed in a systematic way, emphasizing caution against arbitrary use of transporter proteomic data reported in the literature. The analysis highlighted weak correlation between OATP1B1 expression and age and no direct relationship between sex and OATP expression levels. The current analysis reports for the first time weak to moderate correlation in expression of different transporters (e.g., OATP1B1 versus OATP1B3) in the same individual and highlights the need for corresponding data on transporter-enzyme expression (e.g., OATP1B1 versus CYP3A4). Differences in OATP expression between liver tissue and cellular systems should be accounted for in the IVIVE of transporter-mediated drug disposition in the form of relative expression factor. However, it is evident that minimal differences seen in the currently available OATP expression data cannot solely rationalize and improve underprediction of active uptake clearance reported so far. Additional studies with larger sample size and transporter and enzyme expression data reported in the same individuals are imperative to further refine our understanding of quantitative expression differences between the in vitro cellular systems and human liver tissue and their impact on PBPK-IVIVE of transporter kinetic data.
Authorship Contributions
Participated in research design: Badée, Achour, Rostami-Hodjegan, Galetin.
Performed data analysis: Badée, Achour.
Wrote or contributed to the writing of the manuscript: Badée, Achour, Rostami-Hodjegan, Galetin.
Footnotes
- Received November 7, 2014.
- Accepted January 7, 2015.
The work was funded by a consortium of pharmaceutical companies within the Centre for Applied Pharmacokinetic Research at the University of Manchester. J.B. is a recipient of a Ph.D. studentship from Biotechnology and Biological Sciences Research Council [BB/K501256/1] and AstraZeneca.
↵
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
Abbreviations
- DDI
- drug-drug interaction
- IVIVE
- in vitro–in vivo extrapolation
- LC-MS/MS
- liquid chromatography–tandem mass spectrometry
- OATP
- organic anion–transporting polypeptide
- PBPK
- physiologically based pharmacokinetic
- SCHH
- sandwich-cultured human hepatocyte
- Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics