Abstract
This study was designed to quantitatively assess the mRNA expression of 36 important drug transporters in human jejunum, colon, liver, and kidney. Expression of these transporters in human organs was compared with expression in commonly used cell lines (Caco-2, HepG2, and Caki-1) originating from these organs to assess their value as in vitro transporter system models, and was also compared with data obtained from the literature on expression in rat tissues to assess species differences. Transporters that were highly expressed in the intestine included HPT1, PEPT1, BCRP, MRP2, and MDR1, whereas, in the liver, OCT1, MRP2, OATP-C, NTCP and BSEP were the main transporters. In the kidney, OAT1 was expressed at the highest levels, followed by OAT3, OAT4, MCT5, MDR1, MRP2, OCT2, and OCTN2. The best agreement between human tissue and the representative cell line was observed for human jejunum and Caco-2 cells. Expression in liver and kidney ortholog cell lines was not correlated with that in the associated tissue. Comparisons with rat transporter gene expression revealed significant species differences. Our results allowed a comprehensive quantitative comparison of drug transporter expression in human intestine, liver, and kidney. We suggest that it would be beneficial for predictive pharmacokinetic research to focus on the most highly expressed transporters. We hope that our comparison of rat and human tissue will help to explain the observed species differences in in vivo models, increase understanding of the impact of active transport processes on pharmacokinetics and distribution, and improve the quality of predictions from animal studies to humans.
Numerous drug transporters have been identified as putative key determinants in the pharmaco- and toxicokinetics of a variety of drug compounds in the main pharmacokinetic organs: intestine, liver, and kidney (Sun et al., 2004; Treiber et al., 2004; Shitara et al., 2005). Literature data on transporter expression vary with varying methodology (Northern blot, Taqman-PCR, gene chip array analysis) and quality (quantitative, relative comparisons to untreated controls) and, importantly, also with respect to the transporter genes analyzed (Sun et al., 2002; Anderle et al., 2003; Landowski et al., 2003; Pfrunder et al., 2003; Zimmermann et al., 2005). To our knowledge there is no single study showing the relative expression of transporter proteins from important transporter subfamilies in any human organ. Transporter-transfected cell systems allow specific studies of drug transporter interactions, but extrapolation from the in vitro findings to the in vivo situation remains complex, particularly because of the scattered nature of available information on drug transporter expression.
Therefore, this study was designed to compare the gene expression of 36 important drug transporter genes in human tissues from intestine, liver, and kidney. We also investigated three commonly used organotypic human cell lines, Caco-2, HepG2, and Caki-1, which are utilized as in vitro surrogates for the respective organs, since no comprehensive description of drug transporter expression in these cell lines is available. The selection of transporters was based on their relevance as drug transporters; those selected comprised 10 ABC transporters, 25 transporters from SLC families, and the human peptide transporter 1 (HPT1)1 (Table 1).
List of all 36 transporter genes included in the analysis and the internal reference genes cyclophilin A and MVP
The table gives an overview of the assays used, systematic gene nomenclature according to the Human Genome Organisation consensus, and common gene symbols. The shaded coding in the center of the table depicts classified gene expression levels (white fields indicate absent genes, darker shades indicate higher expression) in human tissue samples from jejunal mucosa, colonic mucosa, liver, and kidney.
Our first aim was to generate quantitative information on the importance of the individual transporters to a particular organ's transporter profile. We reasoned that this information might provide new insight into the putative interplay between transporter proteins and improve in vitro/in vivo correlations and predictions to the human situation, particularly for compounds that are not the exclusive substrate for a specific transporter protein. We also wished to assess the extent to which transport in the cell lines reflects that in the organ of origin. Our final aim was to compare our data with recently published data from rat tissue, to obtain information on species differences in transporter expression profiles. This comparison was motivated by the fact that the rat is the most commonly used species in preclinical drug discovery pharmacokinetic studies and because there are numerous examples of discrepancies between humans and rats in this context (Augustine et al., 2005).
Materials and Methods
Tissues. The human tissues were obtained after written informed consent, in agreement with the local ethics committee. Fresh jejunum tissue was obtained from Sahlgrenska Hospital (Gothenburg, Sweden), from five healthy subjects of both genders (aged 49–79) undergoing gastric bypass surgery. Fresh colon samples were obtained from two male and two female subjects, 57 to 69 years of age, after cancer resections at East Hospital (Gothenburg, Sweden).
Immediately after resection, the mucosa was scraped off the underlying tissue with a glass microscope slide, and then weighed and snap-frozen in liquid nitrogen until isolation of mucosal mRNA. Human liver samples were obtained from the healthy regions of liver lobes from liver metastasis cancer resections at Sahlgrenska Hospital from three patients between 58 and 75 years of age; three additional frozen liver samples from patients without cancer were obtained from Medical Solutions (Nottingham, UK). Fresh frozen human kidney specimens were obtained from the kidneys of three male (51, 54, and 72 years of age) and one female (91 years) patient upon signed consent for research purposes from Organ Recovery Systems (Des Plaines, IL) and from Medical Solutions. All liver and kidney tissues were frozen in liquid nitrogen directly after resection and processed on ice for mRNA isolation.
Cell Cultures. Tissue culture media were obtained from Invitrogen Ltd. (Paisley, UK). Caco-2 cells were purchased from The American Type Culture Collection (Manassas, VA). The cells were maintained in Dulbecco's modified Eagle's medium containing 10% heat-inactivated fetal calf serum, 1% nonessential amino acids, and 1.5% l-glutamine, in an atmosphere of 95% air and 5% CO2 at 37°C. The cells were routinely subcultured by trypsinization using trypsin (0.05%)-EDTA (0.02%) solution, and were seeded at a density of 2.0 × 106 cells per 175-cm2 flask. The mRNA analysis was conducted with Caco-2 cells grown on Transwell filters (seeding 2.5 × 105 cells per 1.13-cm2 polycarbonate culture insert, pore size 0.4 mm; Corning Costar, Cambridge, MA) under conditions identical to those used for drug transport studies in our laboratories. Total RNA was isolated after 16 days in culture on filters, and the medium for the Transwell plates contained antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin).
Caki-1 cells were purchased from The American Type Culture Collection and maintained in McCoy's 5A medium supplemented with 10% heat-inactivated fetal calf serum. Cells were seeded into 75-cm2 T-flasks at 1.2 × 106 cells per flask, and grown for 1 week with medium change every second day. Cells were harvested by trypsinization, and the RNA from 10 million cells was isolated as described below. (Salisbury, UK)
HepG2 cells were purchased from the European Collection of Cell Cultures (Salisbury, UK) and cultures were grown in minimal essential medium supplemented with 10% heat-inactivated fetal calf serum, 1% nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 mM sodium pyruvate, in an atmosphere of 95% air and 5% CO2 at 37°C. Cells were allowed to grow for 48 h before the mRNA was isolated. mRNA Isolation and cDNA Synthesis. All mRNA isolation was carried out using RNA STAT-60 (Tel-Test, Friendswood, TX). For each Caco-2 monolayer sample, six filters were cut from the inserts and mixed with 400 μl of RNA STAT-60. For the other cell lines, which were grown adherent to plastic dishes, the cell layers were washed with PBS and lysed in an appropriate volume of RNA-STAT. The frozen human tissue samples were kept continuously on ice; all treatment was carried out on ice to prevent RNA degradation.
The scraped human intestinal mucosa was weighed and homogenized in 1 ml of RNA STAT-60 per 50 to 100 mg of tissue using a Polytron PT 1200 homogenizer (Kinematica, Luzern, Switzerland). Liver and kidney samples, obtained as frozen solid blocks, were cut under cryostat conditions into 200-μm slices that were weighed and homogenized using 1 ml of RNA-STAT per 50 mg of tissue.
The isolated RNAs were treated with DNase (DNA-free; Ambion, Huntingdon, UK) to digest genomic DNA remnants before the quantity and purity of the mRNA were determined spectrophotometrically using a GenQuant pro RNA/DNA calculator (Biochrom, Cambridge, UK). The integrity of the isolated mRNA was assessed by electrophoretic separation of 1 μg of total RNA on a 1% agarose gel run in Tris borate-EDTA buffer (0.09 M Tris borate, 2 mM EDTA, pH 7.8) at 80 mV for 1 h. The criteria for proceeding to cDNA synthesis included the presence of two sharp ribosomal RNA bands and an absence of RNA debris.
cDNA was prepared from 2 μg of total RNA by using the Superscript First-Strand Synthesis System (Invitrogen) with random hexamer primers according to the manufacturer's protocol. Incubation at 65°C for 5 min and 25°C for 2 min was followed by addition of 50 U of Superscript II reverse transcriptase, further incubation for 10 min at 25°C, and reverse transcription at 42°C for 50 min. The reaction was terminated by increasing the temperature to 70°C for 15 min to inactivate the enzyme. Negative controls without addition of reverse transcriptase were prepared for each sample.
RT-PCR. All quantitative PCR was carried out using an ABI PRISM 7900HT Sequence Detection System with custom designed 384-well cards loaded with Assay-on-Demand Gene Expression assays (Applied Biosystems, Foster City, CA).
The TaqMan analysis was performed in 1 μl of reaction mixture per gene containing 2 ng of RNA converted to cDNA, TaqMan Universal PCR Master Mix (consisting of AmpliTaq Gold DNA polymerase, deoxynucleoside-5′-triphosphates with dUTP, passive reference, and optimized buffer), and the Assay-on-Demand Gene Expression assay mixes containing specific primers and probes, purchased preloaded onto the plate (Table 1). The cycling conditions comprised 2 min at 50°C, 10 min of polymerase activation at 95°C, and 40 cycles alternating at 95°C for 15 s and 60°C for 1 min.
Relative Expression Analysis. Amplification curves were analyzed using SDS2.1 software (Applied Biosystems), setting baseline and threshold values for all samples, and extracting the Ct value (the Ct value is the cycle time when fluorescence is higher than a defined threshold level). Using a standard curve dilution method (Applied Biosystems, 1997), amplification efficiency was determined to be equal for most assays included in the analysis. Thirty-four genes showed equal amplification compared with the reference genes used for normalization, meeting the criteria for relative expression normalization. The two genes with lower efficiencies were MRP5 and OATP-A. Since preloaded cards with commercial premixes of primers and probes were used, no further optimization for these two genes could be performed. Quantification with RT-PCR technology uses housekeeping genes as a reference. There has recently been vigorous debate in the literature about how best to achieve this and about which housekeeping genes are suitable (Vandesompele et al., 2002; Pfaffl et al., 2004). The basic prerequisite for standardization of gene expression data is that the standard reflects all possible variables in sample handling (e.g., loading variability, RNA integrity, primer and enzyme performance in the assay), which is best reflected by the presence of an endogenous reference gene in all stages of the preparation and analysis procedure. The principle for choice of internal reference genes is that they should be unregulated in all samples of interest and their amplification behavior should be comparable to that of the genes of interest. If not all these prerequisites are taken into consideration, it could well be impossible to build a relative expression analysis on one single reference gene.
The optimal internal reference gene for the comparison over all samples was determined using the Excel-based tool BestKeeper (Pfaffl et al., 2004). A panel of six potentially suitable housekeeping genes was analyzed for each cDNA sample. This large initial number seemed appropriate since this study aimed to compare gene expression levels in tissue samples from three different organs and three cell lines. The reference genes were investigated for their stability compared with the genes of interest according to the BestKeeper method published by Pfaffl et al. (2004).
Using cyclophilin A (peptidylpropyl isomerase A) and MVP as internal reference genes, the analysis showed a strong correlation for both candidates (0.90 < r < 0.97). Although the parameters for the BestKeeper index looked best for combinations including 18S, it was decided to exclude 18S mRNA from the index, since it had Ct values around 14, making it clearly different from the target genes, which had Ct values between 25 and 35. The maximal -fold intrinsic variance in the sample set was low, which allows quantitative comparison between all samples included in the analysis. The method of relative quantification of the transporter genes used the geometric mean of the most stable housekeeping genes, cyclophilin A and MVP. Relative gene expression levels were calculated as 2–ΔCt (Applied Biosystems, 1997).
Data Analysis. Principal component analysis (SIMCA-P+ 10.5; Umetrics, Kinnelon, NJ) and Spearman rank correlation analysis were used for comparisons between different organs and cell lines. An arbitrary classification system designating relative expression levels >1 as “high”, levels between 0.1 and 1 as “moderate”, and levels below 0.1 as “low” was applied to the data throughout the article.
Results
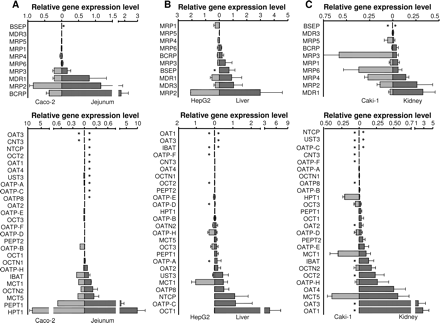
Transporter Expression in Human Jejunum and Colon. Twenty-six (69%) of the 36 transporter genes were expressed in the jejunum, and 21 (58%) were expressed in the colon (Table 1). HPT1 was identified as the most abundantly expressed transporter in the intestinal mucosa in this analysis. The well characterized dipeptide uptake carrier PepT1 and the ABC efflux transporters MRP2 and BCRP were highly expressed in jejunal tissue (rank order HPT1 > PepT1 > BCRP > MRP2 > MDR1; Fig. 1A). Rank correlation analysis comparing the relative transporter gene expression in jejunum and colon showed only a weak correlation (Spearman coefficient k′= 0.62 based on the 26 genes expressed in jejunum; figure not shown). Moreover, there were substantial differences in gene expression levels; on average, levels were 3- to 5-fold lower in colon than in jejunum. This was particularly significant for the highly expressed genes MRP2, BCRP, and PepT1.
Relative transporter gene expression levels of ABC (upper panel) and SLC (lower panel) transporters in human tissues isolated from jejunum mucosa (A), liver (B), and kidney (C). The transporters are ranked in increasing order according to gene expression levels in the respective organs plotted as dark bars on the right side of each graph. Each corresponding gene expression level in the commonly used cell lines is shown in the opposing direction on the graph (light gray bars to the left) for visualization of homologies and discrepancies in transporter expression profiles. The bars represent the mean relative expression levels; error bars indicate the standard deviation from three to six samples analyzed in duplicate. *, absence of gene expression.
Transporter Expression in Liver and Kidney. Thirty-four (94%) and 32 (89%) of the 36 transporter genes were expressed in liver and kidney tissues, respectively. Transporter gene expression was classified as high for 5 of the 34 expressed genes in the liver, ranked in the following order: OCT1 > MRP2 > OATP-C ≈ NTCP > BSEP. Genes expressed at a moderate level in the liver included MDR1 > MDR3 > OATP8 > MCT1 > MRP3 > UST3 > BCRP > OAT2 > OATP-A ≈ MRP6 > PepT1 > OCT3 (Table 1; Fig. 1). In the kidney samples, the highest expressed transporter genes were OAT1 and OAT3. Their expression was more than 5- and 3-fold higher than that of the next moderately expressed transporters. The following transporters were expressed in the kidney at a moderate level, ranked as MCT5 > OAT4 > MDR1 > MRP2 = OATP-H > OCT2 > OCTN2 > MRP4 > IBAT > MCT1 (Table 1; Fig. 1).
Specific Expression in One Organ (Organotypic Transporters). Semiquantitative expression mapping, with categorization into high, moderate, and low expression levels in all tissues, is shown in Table 1. This method of mapping enables depiction of expression patterns that are characteristic of each organ. HPT1, PepT1, and BCRP, the most highly expressed transporter genes in human jejunal mucosa, were confirmed as typical representatives for the upper intestinal tract. The highly expressed transporters NTCP and OATP-C, as well as the moderately expressed BSEP, were exclusively expressed in the liver. OCT1, the most highly expressed gene in the liver, was expressed at very low levels in the other organs and might therefore also be considered as liver-specific. In this study, the extent of OAT1 and OAT3 expression in the kidney was 5- and 3-fold higher, respectively, than that of the next most highly expressed transporter, OAT4. The values in Table 1 show that the patterns of transporter expression in the liver and kidney were complementary, since many of the liver-specific transporters (e.g., OATP-C, OCT1) were completely absent from the kidney, whereas the genes with predominant expression levels in the kidney (e.g., OAT1, OAT3) were absent from liver samples.
Ubiquitous Transporter Expression. In contrast to the organotypic transporter genes discussed above, which were mainly characterized by high expression levels in one or two tissues, we observed that some transporters were expressed at moderate levels in all three tissues. For instance, MDR1 and MCT1 were ubiquitously expressed at moderate levels (Table 1).
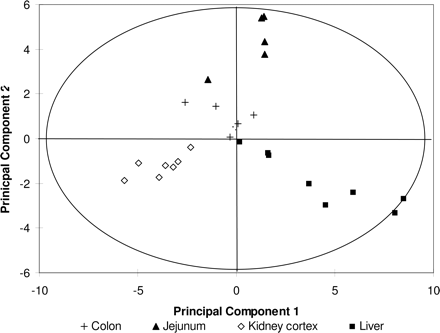
Principal Component Analysis. Global analysis of the tissue-specific expression patterns using principal component analysis (Fig. 2) revealed that the samples were clustered into four groups depending on the organ of origin, confirming the observations shown in Table 1.
Principal component analysis of all tissue transporter expression data. The graphic depiction of the first and second principal components shows that the liver, kidney, jejunum, and colon tissues are clustered into organ-specific groups.
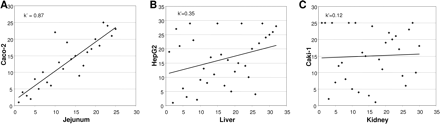
Comparison of mRNA Expression in Human Tissues and Representative Human-Derived Cell Lines.Figure 1 gives a detailed pair-wise comparison of transporter gene expression in jejunum, liver, and kidney and the corresponding organotypic cell lines. The five transporter genes expressed at the highest levels in jejunum matched those expressed at the highest levels in the Caco-2 cell line, although peak levels were lower in the cell line (Fig. 1A). The 13 genes that were absent from jejunal mucosa (indicated by an asterisk in Fig. 1) displayed low or no expression in the Caco-2 cells. The rank order correlation analysis of transporter genes expressed in human jejunum and Caco-2 cells indicated a good correlation between the systems (Spearman rank correlation coefficient k′= 0.87; Fig. 3A).
The most abundantly expressed genes in liver tissue were OCT1 and MRP2, followed by transporter genes involved in bile salt and phospholipid uptake and efflux (OATP-C, NTCP, MDR3, MDR1). Transporter expression in the liver cell line HepG2 did not match the tissue expression pattern (Fig. 1B). Thus, in the HepG2 cells, MRP2, MDR1, and MCT1 mRNAs were expressed at the highest levels, whereas OCT1 and OATP-C were expressed at low levels. The corresponding Spearman rank correlation was weak; k′= 0.35 (Fig. 3B).
The highest mRNA levels in Caki-1 cells were found for MRP3, MCT4, MRP1, MRP4, and MCT1. In contrast to genes expressed in kidney tissue, no mRNA for OATs and no OCT2 expression were detected in Caki-1 cells; instead, these cells expressed OCT3. The transporter expression profile in Caki-1 cells was narrower than that in kidney tissue; only 21 of the 32 transporters found in renal tissue were expressed, generally at lower levels, resulting in a weak Spearman rank correlation coefficient; k′= 0.12 (Fig. 3C).
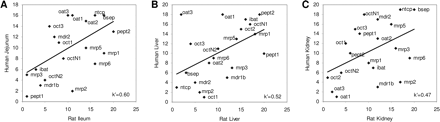
Species Differences in Expression Patterns.Comparison of human and rat transporter expression. Quantitative gene expression data from rat ileum, liver, and kidney have been published recently (Augustine et al., 2005). This analysis covered more than 30 transporter genes, 20 of which were orthologs to the genes in our analysis. Spearman rank correlations between the rat data for these 20 orthologs and our human data are shown in Fig. 4. The greatest overall similarity was observed for the intestinal tissues (Fig. 4A). The main differences were in MRP isoform expression; MRP2 is highly expressed in human jejunum, but mrp2 was low in rat ileum and high levels of the mrp3 isoform were seen instead. Expression of oat3 was second highest in rat liver, but it was completely absent from human liver samples. Expression of mrp/MRP in rats and humans was most diverse in the kidney. MRP2, ranking as fourth most highly expressed transporter in human kidney, was nearly absent from rat kidney. The most abundant transporter mRNAs in rat kidney were oct2 and oat3, followed at a lower rank by oat1.
Discussion
Our results and conclusions are based on mRNA expression data, which may not always correlate with the expression of encoded proteins. However, some examples of concordance between mRNA levels and protein expression can be found in the literature. In particular, MDR1 and PepT1 have been described in this respect. Taipalensuu et al. (2004) and Behrens et al. (2004) showed that mRNA expression data correlate well with the respective protein functionality. To fully align mRNA data with protein expression profiles, a protein expression analysis of all the included transport proteins would be necessary. However, quantification of integral membrane proteins remains a major technical challenge in proteomics, and, until this issue is solved, quantitative mRNA analysis is the most viable alternative. Consequently, we used mRNA profiles to estimate the expression patterns of the 36 transporter proteins investigated in this study.
In the following sections, discussion focuses on transporters expressed at moderate to high levels in the tissues, assuming that high mRNA levels are likely to be more relevant for transporter function. A number of transporter genes have been observed by others to be exclusively expressed in individual organs; the term “organ-specific transporters” has been applied by some authors, particularly for liver and kidney (Jung et al., 2001). Our study has put these observations into context, with an extensive transporter expression profile in human intestinal mucosa, liver, and kidney.
Intestine. The main characteristic of the intestinal transporter expression profile is the functional diversity of expressed transporters; both uptake (HPT1, PepT1, MCT1) and efflux (MRP2, MDR1, BCRP) transporters are expressed at relevant levels in the jejunum. Our data indicate that MRP2 and BCRP, which partially overlap in substrate specificity with MDR1 (Dietrich et al., 2003), are likely to be of similar importance. Because of their high expression levels, it is feasible that all three contribute to drug efflux in the intestinal mucosa, corroborating previous attempts to explain intestinal secretion or attenuation of absorption by implicating the involvement of multiple transport proteins (Englund et al., 2006; Seithel et al., 2006).
Liver. The high level of expression of OCT1 in liver tissue suggests that it is a potentially important component of drug transport in the liver. In confirmation of this, in vivo experiments in oct1(–/–) knockout mice (Wang et al., 2002) indicated that the hydrophilic antidiabetic drug metformin was cleared from the liver by this transporter. More lipophilic compounds, however, are more likely to be transported by passive membrane permeability than by OCT1-mediated uptake. Details of drug-drug interactions resulting from OCT1 inhibition are thus not currently clear (Jonker and Schinkel, 2004).
The high, exclusive expression of OATP-C and NTCP observed in liver tissue is in accordance with published data (Trauner and Boyer, 2003; Mikkaichi et al., 2004). Among the transporters expressed at moderate levels, we found UST3 to be exclusively expressed in liver tissue; no expression was detected in kidney or intestinal tissue. The clinical relevance of UST3 has not yet been investigated, although evidence of its expression was reported in a study by Sun et al. (2001).
Kidney. Our results showed that OAT1 and OAT3 are exclusively expressed in the kidney. OAT4 expression could also be considered to be kidney-specific, since it is 240-fold more highly expressed in the kidney than in the liver or jejunum. An OAT-dominated transporter profile for the human kidney has recently been sketched (Koepsell and Endou, 2004), but no quantitative transporter expression data have been presented. Thus, our data rank the expression of important renal drug transporters for the first time. However, these data should be used with caution in interpreting transporter function and roles in drug-drug interactions in the human kidney. It would, for instance, be interesting to complement the kidney data with region-specific expression data from renal tubuli, where active uptake and secretion processes take place.
Rank correlation analysis of relative gene expression levels in human tissue from jejunum (A), liver (B), and kidney (C) and the representative human-derived cell line. Spearman rank correlation coefficients (k′) are provided.
Rank correlation analysis of relative gene expression levels in rat and human tissues from upper intestine, human jejunum, and rat ileum (A); liver (B); and kidney (C). The transporter gene names on the figure refer to the respective rodent genes. Spearman rank correlation coefficients (k′) are provided.
Comparison of Organ Transporter Profiles. Comparison of liver and kidney data revealed complementary expression patterns. This comparison is physiologically sound, considering the different functions of these organs. The liver undertakes drug metabolism and biliary secretion, involving drug conjugates and bile salts; hence, high expression of OATP-C, NTCP, BSEP, and MRPs fits well. In the renal epithelium, drug transporters involved in active uptake from the blood into the epithelial cells and further secretion into urine (OAT1, 3, and 4) are highly expressed. A recently published comprehensive overview, comparing transporter expression in human tissues using gene-chip technology (Bleasby et al., 2006), described similar expression classification for the highly and moderately expressed genes. One large discrepancy is the finding for MRP2 expression in jejunal tissue, which we found to be high and which was described as low by Bleasby et al. (2006). A potential explanation might be that we restricted our analysis to the intestinal mucosal, whereas when underlying tissues of the intestinal wall are incorporated into the analysis, other cell types are introduced and a change in the expression profile might occur. A large undertaking in comparing mRNA expression profiles in human organs has been published by a Japanese group (Nishimura and Naito, 2005), in which pooled mRNA, as available from Clontech, from 23 different organs was considered. Their results provide a comprehensive overview comparing the expression of each transporter between different organs, and, particularly, the complementary expression of genes from the SLC22 family in liver and kidney is also reflected by their data.
Interplay between Uptake and Efflux Transporter Species. The high gene expression levels of MRP2 and OATP-C in liver tissue in our study provide further support for the importance of this transporter pair in vectorial transport in the liver (Cui et al., 2001; Ito et al., 2005). Drugs that utilize these transporters for biliary secretion include the angiotensin-converting enzyme inhibitor temocaprilat and the HMG CoA-reductase inhibitor pravastatin (Jedlitschky et al., 2006). The high expression of OCT1 in our study raises the question of whether it forms a transporter pair with an efflux transporter. Since OCT1 mediates the uptake of compounds that undergo extensive hepatic metabolism, the metabolites could become substrates for MRP efflux transporters, whereas the unchanged parent molecule could be secreted via MDR1, BCRP, or the recently presented MATE1 (Otsuka et al., 2005). The complex situation in hepatobiliary transport requires further investigation, which is ongoing in several laboratories (Shitara et al., 2005; Liu et al., 2006).
In the kidney, the high expression of renal OATs and moderate expression of MRP2 suggest the possibility of other transporter pairs cooperating in vectorial transport through renal epithelial cells. However, the situation is complex, as the OATs, found in the basolateral membrane, are thought to favor low molecular weight substrates, whereas MRP2 substrates are often bulky anionic conjugates. Furthermore, MRP2 expression levels were 5-fold lower than those of OAT1. OAT4, recently found in the luminal membrane (Ekaratanawong et al., 2004), could be a putative candidate from the basolateral OATs for forming a transporter pair. Further investigation of renal drug transporter expression and complementary functions is needed to map transport pathways in the kidney.
Some further consideration might be given to the coexpression of nuclear receptors being involved in regulation of transporter genes, since recent literature points to direct coupling of transporter expression levels to nuclear receptor activation (Zollner et al., 2005). Correlation of mRNA profiles of nuclear receptors and regulated transporters can help in studying the relevance of transporter induction in altered pharmacokinetics.
Ubiquitous Transporters. One additional observation from this study was the ubiquitous presence of MDR1, MCT1, MCT5, MRP1, MRP5, OCTN2, and OCTN1 in all investigated tissues, with maximal 5-fold inter-tissue variation in expression levels. This general expression pattern suggests that these transporter proteins might be involved in essential physiologic processes. Known ubiquitous genes code for components of the ribosome, structural proteins, and enzymes, being involved in common cellular functions or, as published for MRP1, in inflammatory responses, as well as protection from the toxic effect of oxidative stress (Bakos and Homolya, 2007). Although there is much speculation in the literature about the physiologic role of MDR1 (de Lange, 2004), concise information does exist on MCT1, 2, and 4 isoforms and their role in regulating the key processes of glycolysis and gluconeogenesis (Halestrap and Meredith, 2004).
It could be hypothesized from the high expression of MCT1 and MCT5 in all tissues that these transporters also play a role in drug transport. Indeed, the involvement of MCTs in membrane transport has been demonstrated for a small range of drugs (salicylic acid, valproic acid, nateglinide, atorvastatin) (Nagasawa et al., 2002; Okamura et al., 2002), and the MCT1 transporter was recently exploited to increase absorption of a gabapentin prodrug (Cundy et al., 2004). The MCT5 isoform has been studied less thoroughly than MCT1; in a recent publication, it was labeled an “orphan transporter” (Halestrap and Meredith, 2004). However, our finding that this transporter is ubiquitously expressed in the intestine, liver, and kidney suggests that its physiologic importance is equal to that of MCT1.
Comparison between Human Tissues and Organotypic Cell Lines. The transporter expression profiles of the commonly used organotypic cell systems Caco-2, HepG2, and Caki-1 confirmed that the relative expression of uptake and efflux transporters between Caco-2 cells and human jejunum are in good agreement (Taipalensuu et al., 2001; Englund et al., 2006). However, the expression levels of some transporters (e.g., PepT1) in Caco-2 cells were significantly lower than those in the jejunum, which should be taken into consideration when Caco-2 cells are used in transport studies focusing on these transporters. The high expression of HPT1 in Caco-2 cells, together with the findings of functional studies on peptide transport by HPT1, indicate a need for investigation of potential substrate overlap between these transporters (Landowski et al., 2003).
In contrast to transporter expression in Caco-2, expression in HepG2 and Caki-1 cells did not correlate with expression in native tissue from liver and kidney, respectively. The differences were both qualitative and quantitative. We conclude that HepG2 and Caki-1 should be used with caution in drug transport studies.
Comparison between Human and Rat Tissues. Our extensive characterization of transporter expression has allowed comparison with recently published data on as many as 20 orthologous rat transporters. The differences between rat and human transporter profiles are clear and obvious in all tissues. In human intestine, expression of MRP2 is high and that of MRP3 is low; in contrast, in rat ileum, the expression of mrp2 is low and that of mrp3 is high. It could be hypothesized that human MRP2 and rat mrp3 have comparable functions, which would provide a physiologic background for the described differences in substrate specificity of transporter orthologs (Ninomiya et al., 2005).
The most abundant transporter in human liver was OCT1; in contrast, the most highly expressed transporter protein in rat liver is oat3. Similarly, in human kidney tissue, OAT1 was the most commonly expressed transporter whereas, in rat kidney, oct2 and oat3 are most common. Understanding of the substrate specificities for each of these transporters is limited, and it remains to be shown whether the differences in expression reported here contribute to previously observed pharmacokinetic species differences.
In conclusion, our study provides the most complete quantitative analysis of drug transporter expression reported to date. This allowed us to perform detailed comparisons of transporters expressed within each organ, as well as comparisons between organs, between organs and organotypic cell lines, and between human organs and published rat organ data.
Classification of expression levels into high, moderate, and low allowed comparison of our data and previously published data on the relative importance of the transporters. The indication was that transporters that are highly expressed in human tissues are of significant importance for drug disposition. Based on our results, we tentatively conclude that the importance of each transporter for drug disposition can be roughly related to its expression levels, and that research in predictive pharmacokinetics should focus more on highly expressed drug transporters than on those expressed only weakly.
It is hoped that our comparisons of rat and human tissue will assist in improving the quality of predictions from animal studies to humans, help to explain the observed differences between in vivo models, and increase our understanding of the impact of active transport processes on pharmacokinetics and drug distribution.
Acknowledgments
Donation of human kidney material by Organ Recovery Systems (Des Plaines, IL) is gratefully acknowledged.
Footnotes
-
↵1 The nomenclature of transporter gene families SLC and ABC has been approved and unified over recent years by the HGNC (HUGO Gene Nomenclature Committee); however, since some readers may be more familiar with earlier transporter names such as MDR1, MRP2, or OCT1, the latter terms have been used throughout the text. Please refer to Table 1 for an overview of the accustomed and official consensus gene names.
-
This work was supported by grants from AstraZeneca (G.A.), Swedish research council (P.A.) and the Swedish Animal Welfare Agency (P.A.). Part of this work was presented at the BioParadigm 2005 Conference, 2005 Aug 14–18, St Gallen, Switzerland.
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.107.014902.
-
ABBREVIATIONS: PCR, polymerase chain reaction; RT-PCR, real-time PCR; ABC, ATP-binding cassette; SLC, solute carrier; HPT1, human peptide transporter 1.
- Received January 28, 2007.
- Accepted May 9, 2007.
- The American Society for Pharmacology and Experimental Therapeutics