Abstract
A major function of xenobiotic and endobiotic transporters is to move a wide range of organic substances across cell membranes. Sertoli cells play an important role in protecting developing germ cells by forming a physiological barrier, limiting exposure to potentially toxic substrates, or conversely, facilitating uptake of xenobiotics within the testis. The aim of this study was to quantitatively determine the constitutive expression of various transporters in isolated Sertoli cells from adult Sprague-Dawley rats. The following mRNA levels were measured in isolated Sertoli cells by the branched DNA signal amplification method, multidrug resistance (Mdr) protein 1a, 1b, and 2; multiple drug resistance protein (Mrp) 1, 2, 3, 4, 5, 6, 7, and 8; sodium taurocholate cotransporting polypeptide; bile salt excretory protein; ileal bile acid transporter; AbcG5 and AbcG8; organic anion transporting polypeptide (Oatp) 1, 2, 3, 4, 5, 9, and 12; prostaglandin transporter (Pgt); testis-specific transporter (Tst) 1 and Tst2; organic anion transporter (Oat) 1, 2, 3, and K; organic cation transporter (Oct) 1, 2, 3, N1, and N2; divalent metal transporter (Dmt) 1, Menke's, and Wilson's; zinc transporter (Znt) 1; equilibrative nucleoside transporter (Ent) 1 and 2; concentrative nucleoside transporter (Cnt) 1 and 2; and peptide transporter (Pept) 1 and 2. Levels were also determined in whole testis, liver, kidney, and ileum to provide a reference for determining relative expression levels. Mrp8, Tst1 and 2, and Ent1 and 2 were expressed in Sertoli cells at higher levels than in liver, kidney, or ileum, whereas Mrp1, 5, and 7, Mdr2, Oatp3, Oat2, OctN2, Dmt1, Menke's, Wilson's, and Znt1 were all significantly expressed in Sertoli cells, but Sertoli cell expression was not the tissue of highest expression. The remaining transporters were expressed at low levels in isolated Sertoli cells. Additionally, expression levels of Mrp1, Mrp7, Mrp8, Tst1, Tst2, OctN2, Wilson's, Znt1, Ent1, and Ent2 were greater in isolated Sertoli cells than in whole testis. Constitutive expression of transporters in Sertoli cells may provide an insight into the range of xenobiotics that can potentially be transported by Sertoli cells and thereby provide a mechanistic under standing of blood-testis barrier function.
Absorption, distribution, metabolism, and excretion play a major role in determining the target-organ toxicity of a xenobiotic. For any xenobiotic to have an effect, it must reach the target organ at sufficient concentrations. Transporters play a vital role in the absorption, distribution, and excretion processes that largely determine the pharmacodynamics of xenobiotics. Several previous studies have reported the existence of specific proteins that transport a variety of xenobiotics and endobiotics such as drugs, metals, flavonoids, nucleotides and nucleosides, di- and tripeptides, and bile acids (Saito et al., 1995; Miyamoto et al., 1996; Sekine et al., 1997; Yao et al., 1997; McMahon and Cousins, 1998). There are several transporter families, including multiple drug resistance (Mdr) proteins, multidrug resistance proteins (Mrps), bile acid and sterolin transporters [sodium taurocholate cotransporting polypeptide (Ntcp), bile salt excretory protein (Bsep), ileal bile acid transporter (Ibat), AbcG5, and AbcG8], organic anion transporting polypeptides (Oatps), organic anion transporters (Oats), organic cation transporters (Octs), metal transporters [divalent metal transporter (Dmt), Znt, Menke's, and Wilson's], and nucleoside and peptide transporters [equilibrative nucleoside transporter (Ent), concentrative nucleoside transporter (Cnt), and peptide transporter (Pept)]. Each transporter of a particular family may have overlapping as well as unique substrate specificity. Additionally, each transporter is expressed in a tissue-specific and developmental stage-specific manner that may share a coordinate temporal regulation with that of drug-metabolizing enzymes (Cherrington et al., 2002; Guo et al., 2002).
A physical barrier separating the adluminal compartment of the seminiferous tubules from the rest of the body is created by the formation of tight junctions near the base of Sertoli cells (Russell, 1977). Developing germ cells are protected in this microenvironment from xenobiotic exposure and immunological influences. To reach the adluminal space, xenobiotics must pass through Sertoli cells by either passive diffusion or facilitated transport. Selective movement of compounds across Sertoli cells, therefore, comprises the physiological or functional aspect of the blood-testis barrier. Because of its important role in regulating the exchange of materials between blood and adluminal compartment and thereby protecting the developing germ cells from various toxic insults, Sertoli cells are a toxicologically important part of the male reproductive system.
The constitutive expression levels of xenobiotic transporters in Sertoli cells may provide great insight into the range of xenobiotics that potentially move in and out of the male reproductive system. Additionally, a detailed knowledge of the expression of these transporters in Sertoli cells may help predict whether other xenobiotics may penetrate the blood-testis barrier. Therefore, the present study was undertaken in an effort to determine the constitutive expression levels of several xenobiotic transporter mRNAs in rat Sertoli cells in relation to whole testis, liver, kidney, and ileum. We have used QuantiGene signal amplification technology to specifically and quantitatively monitor the mRNA levels of 45 rat xenobiotic transporters.
Materials and Methods
Sertoli Cell Isolation. Sertoli cell isolations were performed as described previously (Anway et al., 2003) with minor modifications. Adult 90-day-old male Sprague-Dawley rats (Charles River, Wilmington, MA) were maintained on a 12-h alternating light/dark cycle and housed in 35 to 70% humidity and 68-73°F temperature-controlled rooms with access to Purina Rodent Chow 5001 (Farmer's Exchange, Framingham, MA) and water ad libitum. Animals were acclimated to housing conditions for at least 1 week before use. Animals were killed by carbon dioxide asphyxiation in accordance with the guidelines of Brown University's Institutional Animal Care and Use Committee in compliance with National Institute of Health guidelines.
Testes were removed, detunicated, and placed in 40 ml of 1× Hanks' (20-031-CV; Mediatech, Herndon, VA; calcium/magnesium free, pH 7.4, adjusted with 7.5% sodium bicarbonate), washed two times, and allowed to settle. Seminiferous tubules were dispersed (not fragmented) in a collagenase solution [25 ml, 0.5 mg/ml collagenase (C2674; Sigma-Aldrich, St. Louis, MO) in 1× Hanks', pH 7.4, 34°C, 10-15 min, shaking at 80 oscillations/min] and allowed to settle. Seminiferous tubules were washed three times in 40 ml of 1× Hanks' followed by incubation in a trypsin solution [25 ml, 0.5 mg/ml trypsin (T5266; Sigma-Aldrich) in 1× Hanks', pH 7.4, 5-10 min, 37°C, without shaking]. After two washes in 40 ml of 1× Hanks', tubules were washed a third time in a solution containing trypsin inhibitor [20 ml, 0.3 mg/ml trypsin (T6522; Sigma-Aldrich) in 1× Hanks', pH 7.4] and allowed to settle (Karl and Griswold, 1990).
To separate Sertoli cells from germ cells, tubules were incubated in an enzyme mixture solution [25 ml, 0.1% collagenase (C2674; Sigma-Aldrich), 0.2% hyaluronidase (H6254; Sigma-Aldrich), 0.04% DNase I (D5024; Sigma-Aldrich), and 0.03% trypsin inhibitor (T6522; Sigma-Aldrich) in 1× Hanks', pH 7.4, 34°C, 40 min, shaking at 80 oscillations/min]. The preparation was centrifuged (500 rpm, 4 min, GPR Tabletop centrifuge; Beckman Coulter, Palo Alto, CA) to pellet Sertoli cells, and washed three times in 40 ml of 1× Hanks' (Karzai and Wright, 1992).
Pelleted cells were then subjected to hypotonic shock in 10 ml (total volume) of 1× Hanks', to which 25 ml of a 1:10 dilution of 1× Hanks' in deionized water was added. Tubes were gently inverted three times and centrifuged at 500 rpm for 4 min, and the supernatant decanted. The resulting Sertoli cell pellet was resuspended by gently pipetting up and down in a total volume of 25 ml of 1× Hanks'. Sertoli cells were filtered through a 53-μm nylon mesh (Small Parts Inc., Miami Lake, FL), and washed three times with 40 ml of F-12/Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA). After pelleting, Sertoli cells were resuspended in 10 ml (total volume) of F-12/Dulbecco's modified Eagle's medium. A 1-ml aliquot was used for purity analyses and the rest (9 ml) was immediately snap frozen in liquid nitrogen for RNA isolations (Anway et al., 2003).
For purity analysis, cells were immersion fixed in a 10% neutral-buffered formalin solution for 10 min followed by dehydration in a graded series of ethanols, pelleted, embedded in glycol methacrylate (Technovit 7100; Heraeus Kulzer GmBH, Wehrheim, Germany), sectioned (2 μm), and stained with 1% toluidine blue/1% sodium borate. Sertoli cells were identified by nuclear morphology (Griswold et al., 1988). Isolations averaged between 75 and 85% Sertoli cells per animal.
Total RNA Isolation. Total RNA was isolated using RNAzol B reagent (Tel-Test Inc., Friendswood, TX) as per the manufacturer's protocol. Each RNA pellet was resuspended in 0.2 ml of 10 mM Tris-HCl buffer, pH 8.0. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm. RNA integrity and quality were analyzed by formaldehydeagarose gel electrophoresis with ethidium bromide staining. The quality of RNA samples was judged by the integrity and relative ratio of 28S and 18S rRNA bands.
Development of Specific Oligonucleotide Probe Sets. The following probe sets were used: Mdr1a and 1b (Brady et al., 2002); Mdr2 (Leazer and Klaassen, 2003); Mrp1, 2, and 3 (Cherrington et al., 2002); Mrp4, 5, and 6 (Leazer and Klaassen, 2003); Oatp1, 2, 3, 4, and 5 (Li et al., 2002); Oat-K, Oatp9 and 12, and Pgt (Leazer and Klaassen, 2003); Oct1, 2, 3, N1, and N2 (Slitt et al., 2002); Oat1, 2, and 3 (Buist et al., 2002); Dmt1 (Park et al., 2002); Wilson's, Menke's, and Znt1 (Leazer and Klaassen, 2003); Ntcp and Bsep (Leazer and Klaassen, 2003); Ibat (Choudhuri et al., 2003); Cnt1 and 2 as well as Ent1 and 2 (Leazer and Klaassen, 2003); Pept1 and 2 (Leazer and Klaassen, 2003); and AbcG5 and 8 (Leazer and Klaassen, 2003). The remaining sequences [Mrp7 (Abcc10), Mrp8, (Abcc12), Tst1 (homologous to mouse Slco6b1), and Tst2 (homologous to mouse Slco6c1)] were obtained from GenBank, and target sequences were analyzed by ProbeDesigner software version 1.0 (Genospectra, Fremont, CA). All oligonucleotide probes were designed with a Tm of approximately 63°C, enabling hybridization conditions to be held constant at 53°C for each oligonucleotide probe set (Table 1). Every probe developed through the ProbeDesigner software was BLAST-searched against the nucleotide database to ensure minimal or no cross-reactivity with other known rat sequences and expressed sequence tags.
List of oligonucleotide probes generated for analysis of mRNA expression
Branched DNA Signal Amplification Assay. Reagents required for RNA analysis (i.e., lysis buffer, amplifier/label probe buffer, and substrate solution) were supplied in the QuantiGene Discover kit (Genospectra). Expression levels were analyzed as described by Hartley and Klaassen (2000). Briefly, specific oligonucleotide probe sets were diluted in lysis buffer. Total RNA (1 μg/μl; 10 μl) was added to each well of a 96-well plate containing capture hybridization buffer [0.05 M HEPES sodium salt, 0.05 M HEPES free acid, 0.037M lithium lauryl sulfate, 0.5% (v/v) Micr-O-protect, 8 mM EDTA, and 0.3% (w/v) nucleic acid blocking agent] and 50 μl of diluted probe set (50, 100, and 200 fmol/μl for capture, blocker, and label probes, respectively). Total RNA was allowed to hybridize to each probe set containing all probes for a given transcript (blocker probes, capture probes, and label probes) overnight at 53°C. Subsequent hybridization and posthybridization wash steps were carried out according to the manufacturer's direction, and luminescence was measured with the Quantiplex 320 branched DNA luminometer (Bayer Diagnostics) interfaced with Quantiplex Data Management Software Version 5.02 (Bayer Corp.-Diagnostics Div. (Tarrytown, NY) for analysis of luminescence from 96-well plates.
Statistical Analysis. All values are expressed as relative luminescence units per 10 μg of total RNA with mean ± S.E. (n = 5). Differences in expression between isolated Sertoli cells and whole testis was determined using analysis of variance followed by Duncan's multiple range test. The level of significance was set at p < 0.05.
Results
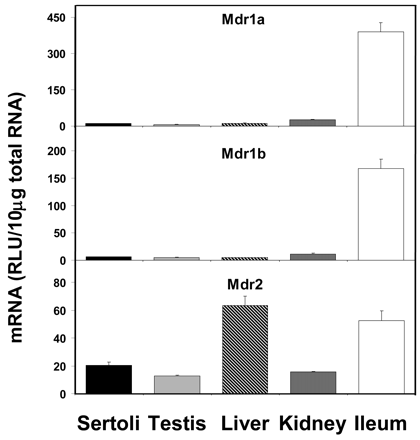
The constitutive expression levels of various transporter mRNAs in isolated Sertoli cells have been compared with that in whole testis, liver, kidney, and ileum. Figure 1 shows that the mRNA expression of Mdr1a and 1b was very low in Sertoli cells, whereas Mdr2 expression was moderate. Mdr1a and Mdr1b expressions were highest in ileum, and Mdr2 expression was highest in liver. Mdr1a and Mdr1b expressions in Sertoli cells were about 2.6 and 3.7%, respectively, of that in ileum, whereas Mdr2 expression in Sertoli cells was about 33% of that in liver.
Expression of Mdr mRNA levels in isolated Sertoli cells, whole testis, liver, kidney, and ileum (n = 5).
Expression of Mrp8 was almost exclusive to the testis, with levels in Sertoli cells almost double (188%) those found in whole testis (Fig. 2). Mrp5 was also expressed at high levels in Sertoli cells, where levels were roughly equal to other tissues examined. Mrp5 expression in Sertoli cells was 85, 95, 86, and 170% of that found in whole testis, liver, kidney, and ileum, respectively. Expression of mRNAs for Mrp1, 4, 6, and 7 were detected in Sertoli cells, although Sertoli cells were not the tissue of highest expression.
Expression of Mrp mRNA levels in isolated Sertoli cells, whole testis, liver, kidney, and ileum (n = 5).
Quantitatively, the expression of Mrp1, Mrp4, Mrp6, and Mrp7 in Sertoli cells was 190, 150, 116, and 188% of that in whole testis; 388, 430, 7, and 187% of that in liver; 41, 16, 25, and 58% of that in kidney; and ∼1100, 156, 35, and 39% of that found in ileum, respectively. In contrast, Mrp2 and Mrp3 mRNA expression in Sertoli cells was extremely low. Mrp2 expression was highest in liver, whereas Mrp3 expression was highest in ileum. Quantitatively, the expression of Mrp2 in Sertoli cells was about 2% of that in liver, and the expression of Mrp3 was less than 1% of that in ileum.
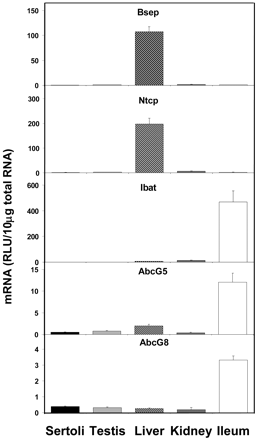
Figure 3 shows the expression of mRNA levels for the bile acid and sterolin transporters. Transporters involved in bile acid and sterolin transport were found to have almost no mRNA expression in Sertoli cells. Bile salt excretory protein (Bsep), Ntcp mRNA expression was less than 1% of that found in liver, whereas Ibat expression in Sertoli cells was also less than 1% compared with that in ileum. The expression of AbcG5 and AbcG8 mRNAs was about 4 and 11% of that in ileum for AbcG5 and AbcG8, respectively.
Expression of bile acid and sterolin transporter mRNA levels in isolated Sertoli cells, whole testis, liver, kidney, and ileum (n = 5).
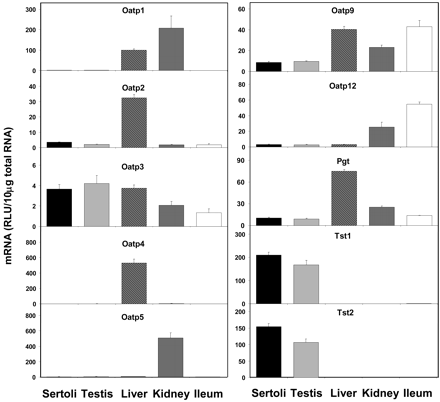
Expression of the Oatp mRNAs is shown in Fig. 4. Tst1 and Tst2 mRNA was found to be almost exclusively expressed in Sertoli cells and whole testis where expression was quantitatively more than 200-fold greater in Sertoli cells than in ileum (tissue with the next highest expression). Expression of Tst1 and Tst2 was 125 and 145% higher in Sertoli cells than in whole testis, respectively. Constitutive expression of Oatp3 mRNA in Sertoli cells was high in relation to other tissues where expression was 87, 97, 176, and 275% of that found in whole testis, liver, kidney, and ileum, respectively. Oatp2, Oatp9, Oatp12, and Pgt expression levels were measurable in Sertoli cells, although Sertoli cells were not the tissue of highest expression. Quantitatively, the expression of Oatp2, Oatp9, Oatp12, and Pgt in Sertoli cells was 168, 91, 107, and 94% of that in whole testis; 11, 22, 94, and 14% of that in liver, 208, 37, 12, and 41% of that in kidney; and 197, 20, 6, and 76% of that found in ileum, respectively. In contrast, Oatp1, Oatp4, and Oatp5 mRNA expression in Sertoli cells was virtually nonexistent. Oatp1 and Oatp5 expression was highest in kidney, whereas Oatp4 expression was highest in liver. The expression of Oatp1, Oatp4, and Oatp5 in Sertoli cells was less than 1% of that found in their respective tissues of highest expression.
Expression of Oatp mRNA levels in isolated Sertoli cells, whole testis, liver, kidney, and ileum (n = 5).
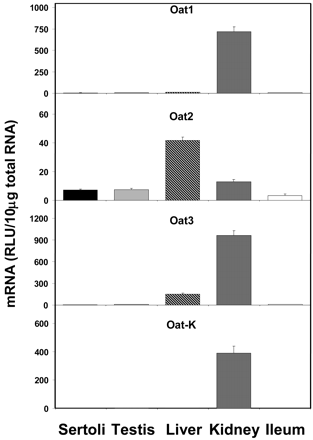
Figure 5 illustrates the expression of Oat mRNAs. Oat2 was expressed at moderate levels in Sertoli cells, although Sertoli cell expression was not as high as that found in liver. The expression of Oat2 in Sertoli cells was 98, 17, 56, and 219% of that found in whole testis, liver, kidney, and ileum, respectively. Conversely, Sertoli cell levels of Oat1, Oat3, and Oat-K are nearly nonexistent as evidenced by relative luminescence unit values less than 1% of those found in kidney (the tissue of highest expression for these transporters).
Expression of Oat mRNA levels in isolated Sertoli cells, whole testis, liver, kidney, and ileum (n = 5).
Expression of Oct3 mRNA was higher in Sertoli cells than in any other tissue examined (Fig. 6). Oct3 expression in Sertoli cells was 152, 159, 177, and 204% of that found in whole testis, liver, kidney, and ileum, respectively. OctN2 mRNA was also expressed highly in Sertoli cells, although kidney levels were higher than Sertoli cells. OctN2 expression in Sertoli cells was 168, ∼3700, 48, and 212% of that found in whole testis, liver, kidney, and ileum, respectively. The expression of Oct1, Oct2, and OctN1 in Sertoli cells was less than 2% of that found in kidney, the organ with the highest expression.
Expression of Oct mRNA levels in isolated Sertoli cells, whole testis, liver, kidney, and ileum (n = 5).
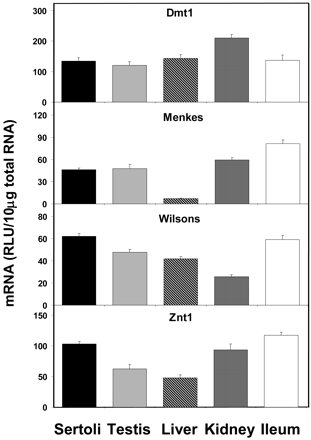
All of the metal transporters examined showed high expression levels in Sertoli cells (Fig. 7). Dmt1 expression was roughly equal in all tissues examined where Sertoli cell expression was 111, 94, 85, and 98% of that found in whole testis, liver, kidney, and ileum, respectively. The expression of Menke's transporter mRNA in Sertoli cells was roughly equal to levels in whole testis (98%), kidney (78%), and ileum (57%), but it was expressed at much higher levels than in liver (626%). The expression of Wilson's transporter mRNA was slightly higher in Sertoli cells than in whole testis, liver, and kidney (130, 148, and 243%, respectively), but similar to levels in ileum (105%). The expression of Znt1 mRNA was higher in Sertoli cells than in whole testis and liver (165 and 216%, respectively), but roughly similar to kidney and ileum (110 and 88%, respectively).
Expression of metal transporter mRNA levels in isolated Sertoli cells, whole testis, liver, kidney, and ileum (n = 5).
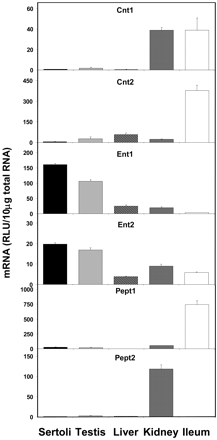
Figure 8 shows the expression of nucleoside and peptide transporters. Expression of both Cnt1 and Cnt2 mRNAs in Sertoli cells is extremely low when compared with the tissues of highest expression. Cnt1 is expressed equally in kidney and ileum where its expression is highest, with Sertoli cell levels at 2% in comparison. Cnt2 expression in Sertoli cells is also approximately 2% of that found in ileum, the tissue of highest expression. In contrast, expression of Ent1 and Ent2 was higher in Sertoli cells than in any other tissue examined. Constitutive expression of Ent1 in Sertoli cells was 151, 636, 806, and approximately 4400% of that found in whole testis, liver, kidney, and ileum, respectively. Similarly, Ent2 expression in Sertoli cells was 117, 508, 218, and 341% of that found in whole testis, liver, kidney, and ileum, respectively. The mRNA expression of Pept1 and Pept2 was very low in Sertoli cells. Pept1 expression was highest in ileum, whereas Pept2 expression was almost exclusively found in kidney. Sertoli cell expression of Pept1 was approximately 4% of that found in ileum, and Pept2 expression in Sertoli cells was 1% of that in kidney.
Expression of nucleoside and peptide transporter mRNA levels in isolated Sertoli cells, whole testis, liver, kidney, and ileum (n = 5).
Discussion
Sertoli cells perform numerous structural and supportive functions for the proper development of germ cells. An important toxicological function performed by Sertoli cells is the formation of the functional barrier that separates developing germ cells and the adluminal compartment from the rest of the body. Tight junctions form near the base of Sertoli cells, thereby sealing off the adluminal compartment as a distinct microenvironment. This barrier function, termed the blood-testis barrier, is likely to resemble the blood-brain barrier, which has been studied in greater detail. The blood-brain barrier, which is also formed by tight junctions in the brain capillary endothelial cells, protects the central nervous system from toxic injury. The specificity of the blood-brain barrier, where certain compounds are excluded but others are allowed to enter the central nervous system, is largely determined by the substrate specificity of P-glycoprotein and other transporters present (Schinkel et al., 1994; Hagenbuch et al., 2002; Potschka et al., 2003; Cisternino et al., 2004; Tohyama et al., 2004).
The current study was designed to quantitatively determine the constitutive expression of 45 transporter genes in Sertoli cells of adult Sprague-Dawley rats and to compare this expression with that found in whole testis, liver, kidney, and ileum. The overall results indicate a complex pattern of expression, where several transporters are highly expressed, whereas others are expressed at lower levels or are absent in Sertoli cells. This differential expression likely plays a major role in determining the selective distribution of xenobiotics in the male reproductive system. The observation that Mrp1, Mrp7, Mrp8, Tst1, Tst2, OctN2, Wilson's, Znt1, Ent1, and Ent2 are all expressed at higher levels in isolated Sertoli cells than in whole testis indicates that transport is an important function of Sertoli cells. Although several transporters may be localized to other cell types in testis, transporter expression in Sertoli cells underlies the integral role of transport in the blood-testis barrier.
One of the most interesting sites of metal toxicity is in the testes. Developing germ cells are particularly sensitive to toxic insult, and cadmium, arsenic, lead, and other metal-induced testicular injury has been reported in various species such as rat, mouse, rabbit, and pigeon. A most intriguing observation is the significant strain differences in mice regarding the sensitivity to cadmium-induced testicular toxicity. Several strains of mice, including 129/SvJ, are highly sensitive, whereas other strains, including A/J and C57BL/6J, are refractory to cadmium-induced toxicity (Liu et al., 2001). In the resistant A/J strain, the concentration of cadmium in the testes was shown to be diminished more than 10-fold compared with the sensitive strain (King et al., 1999). The mechanism of this altered accumulation of cadmium in the resistant strain was shown to be a facilitated transport that could be inhibited by zinc (King et al., 2000). In the current study, we find significant expression levels of several metal transporters including Dmt1 and Znt1 that may play an important role in the susceptibility to metals. Dmt1 has been shown to transport an unusually wide range of divalent metals including cadmium, whereas Znt1 has been implicated in the cellular resistance to both zinc and cadmium (Gunshin et al., 1997; Takiguchi et al., 2001). Together, these data suggest that Dmt1 and perhaps Znt1 play an important role in the transport of toxic metals across the blood-testis barrier.
The blood-testis barrier decreases the delivery of several cytotoxic agents to the testis in cancer patients. Up to 13% of relapses after complete remission in patients with acute lymphoblastic leukemia are testicular in origin (Miniero et al., 1995). Efflux transporters such as P-glycoprotein and Mrp1 have been shown to play an important role in limiting the disposition of certain compounds to the testis. Substrates for P-glycoprotein, including chemotherapeutics, were found to accumulate in the testis at higher concentrations in Mdr1a/b-/-double knockout mice than in wild-type mice (Schinkel et al., 1994). Mrp1 has recently been shown to be expressed at the basal side of Sertoli cells and to contribute to the blood-testis barrier (Wijnholds et al., 1998). In the current study, along with Mrp1, we found moderate expression of Mrp5 and Mrp7, but extremely high levels of Mrp8 in Sertoli cells. These transporters may also play a role in limiting the delivery of cytotoxic agents across the blood-testis barrier.
The blood-testis barrier also has a major impact during the therapy for human immunodeficiency virus infection. HIV-1 proviral DNA has been observed in the nuclei of germ cells at all stages of differentiation in HIV-seropositive men, but not in other epithelial cells of the male reproductive system, including prostate, epididymis, and seminal vesicles (Nuovo et al., 1994). It has also been suggested that germ cells may be directly infected by cell-free virus and that the testis represents a site of early viral localization (Muciaccia et al., 1998). The importance of this selective early-stage infection is underscored by the fact that developing germ cells are protected from the immune system by the blood-testis barrier and may serve as a primary source of venereal spread of the virus. Several nucleoside analog reverse-transcriptase inhibitors, including lamivudine, nevirapine, stavudine, and zidovudine, penetrate into the male genital tract and are effective at decreasing HIV type 1 concentrations in seminal fluid (Gulick et al., 1997; Taylor et al., 2000). However, the protease inhibitors ritonavir, and saquinavir do not cross the blood-testis barrier and reach concentrations in seminal fluid that are only 2 to 4% of that found in plasma (Taylor et al., 1999). These levels do not achieve the 95% inhibitory concentration, which has implications for the antiviral effect of these drugs in the male genital tract. It is not known to what extent the blood-testis barrier is responsible for the decreased concentrations of these protease inhibitors in seminal fluid, or whether other processes such as prostate secretions are involved. It is clear, however, that the male genital tract can be considered a dangerous reservoir for HIV type 1, particularly in respect to transmission of the virus (Eron et al., 1998).
The selective facilitating transport of nucleoside analogs as well as the specific exclusion of protease inhibitors by the blood-testis barrier may be the result of the differential expression of xenobiotic transporters in Sertoli cells. The current study shows the significant expression of both Ent1 and Ent2 in Sertoli cells. It has been demonstrated that these transporters are important in the uptake of the nucleoside analogs 2′3′-dideoxycytidine, 3′-azido-3′-deoxythymidine, and 2′3′-dideoxyinosine (Mangravite et al., 2003). The high expression levels of Ent1 and Ent2 in Sertoli cells may be the mechanism by which nucleoside analog therapies gain access to the male genital tract. Mrp4, Mrp5, and Mrp8 have also been shown to transport several nucleoside analog-based antiviral drugs (for review, see Borst et al., 2004) but may function to limit the exposure of nucleoside based drugs to the male genital tract. The current study finds moderate levels of Mrps 1, 5, and 7 as well as extremely high levels of Mrp8 in Sertoli cells. Conversely, Oat1 has also been shown to mediate the uptake of cidofovir and adefovir (Cihlar et al., 1999), but we find no expression of Oat1 in Sertoli cells.
In conclusion, the present study has determined the constitutive expression of 45 different transporters in Sertoli cells and compared that expression with that in whole testis, liver, kidney, and ileum. This study will serve as a useful reference of the constitutive transporter expression that functions as the blood-testis barrier in adult rats. Among the transporters studied, Mrp8, Tst1 and 2, and Ent1 and 2 were expressed in isolated Sertoli cells at higher levels than in liver, kidney or ileum, whereas Mrp1, 5 and 7, Mdr2, Oatp3, Oat2, OctN2, Dmt1, Menke's, Wilson's, and Znt1 were all significantly expressed in isolated Sertoli cells, but Sertoli cell expression was not the tissue of highest expression. The remaining transporters were expressed at low levels in isolated Sertoli cells. Additionally, expression levels of Mrp1, Mrp7, Mrp8, Tst1, Tst2, OctN2, Wilson's, Znt1, Ent1, and Ent2 were greater in isolated Sertoli cells than in whole testis. Thus, the constitutive expression levels of these transporters in isolated Sertoli cells may provide insight into the range of xenobiotics that can potentially pass into the male genital tract and thereby provide a mechanistic understanding of the blood-testis barrier.
Footnotes
-
doi:10.1124/dmd.104.001024.
-
ABBREVIATIONS: Mdr, multidrug resistance; Mrp, multiple drug resistance protein; Ntcp, sodium taurocholate cotransporting polypeptide; Bsep, bile salt excretory pump; Ibat, ileal bile acid transporter; Oatp, organic anion transporting polypeptide; Oat, organic anion transporter; Oct, organic cation transporter; Ent, equilibrative nucleoside transporter; Cnt, concentrative nucleoside transporter; Pept, peptide transporter; Dmt1, divalent metal transporter; Tst, testis-specific transporter; Pgt, prostaglandin transporter; Znt1, Zinc transporter; HIV, human immunodeficiency virus.
- Received June 16, 2004.
- Accepted October 15, 2004.
- The American Society for Pharmacology and Experimental Therapeutics