Abstract
P-glycoprotein is considered to be a major factor impeding effective drug therapy for many diseases of the central nervous system (CNS). Thus, efforts are being made to gain a better understanding of P-glycoprotein's role in drug distribution to brain parenchyma and cerebrospinal fluid (CSF). The goal of this study was to validate and introduce a novel P-glycoprotein–deficient (ABCB1-1Δ) canine model for studying P-glycoprotein–mediated effects of drug distribution to brain tissue and CSF. CSF concentrations of drug are often used to correlate efficacy of CNS drug therapy as a surrogate for determining drug concentration in brain tissue. A secondary goal of this study was to investigate the validity of using CSF concentrations of P-glycoprotein substrates to predict brain tissue concentrations. Loperamide, an opioid that is excluded from the brain by P-glycoprotein, was used to confirm a P-glycoprotein–null phenotype in the dog model. ABCB1-1Δ dogs experienced CNS depression following loperamide administration, whereas ABCB1 wild-type dogs experienced no CNS depression. In summary, we have validated a novel P-glycoprotein–deficient canine model and have used the model to investigate transport of the P-glycoprotein substrate 99mTc-sestamibi at the blood-brain barrier and blood-CSF barrier.
The effectiveness of many pharmacological agents, including human immunodeficiency virus 1 protease inhibitors, antineoplastic agents, and antiepileptic drugs, is limited by their ability to cross the blood-brain barrier (BBB) (Löscher and Potschka, 2002; McGee et al., 2006; Peak and Abrey, 2006). In addition to factors such as the drug's molecular weight, hydrophobicity, degree of ionization, and plasma protein and tissue binding (Golden and Pollack, 2003), whether the drug is a substrate for P-glycoprotein greatly influences its ability to cross the BBB. P-glycoprotein is a membrane transporter belonging to the ATP binding cassette superfamily encoded by the ABCB1 gene (formerly known as MDR1) (Lin and Yamazaki, 2003). P-glycoprotein is expressed on the lumenal surface of brain capillary endothelial cells, where it is believed to contribute to the BBB (Sugawara et al., 1990; Bendayan et al., 2002), and on the apical surface of choroid plexus epithelial cells, where it is believed to contribute to the blood–cerebrospinal fluid barrier (CSF) barrier (Rao et al., 1999).
Delineation of P-glycoprotein's role in the BBB and blood-CSF barrier is necessary to improve treatment strategies for patients with disorders of the central nervous system (CNS) such as AIDS dementia, brain tumors, and epilepsy. However, a quantitative, mechanistic understanding of the role of P-glycoprotein in the BBB and blood-CSF barrier is hindered by current models [cell culture and abcb1ab(–/–) knockout murine models]. When studying drug penetration of the BBB, particularly for P-glycoprotein substrate drugs, many investigators have resorted to using CSF drug concentrations as a surrogate for brain parenchymal concentrations in both rodent and human studies (Christensen et al., 2001; Antinori et al., 2005; Capparelli et al., 2005). The results of the study presented here, in which a spontaneous canine P-glycoprotein knockout model is used, question the validity of using CSF drug concentrations to predict the concentrations of P-glycoprotein substrate drugs in brain parenchyma.
We recently identified a functional polymorphism of the ABCB1 gene in collies (Mealey et al., 2001) and other herding breed dogs (Neff et al., 2004). Affected dogs (ABCB1-1Δ) harbor a 4-base pair deletion mutation at the 5′ end of the canine ABCB1 open reading frame. The frame shift generates several premature stop codons occurring within the first 10% of the coding region, resulting in premature termination of P-glycoprotein synthesis. Required elements for P-glycoprotein's drug-efflux function (ATP binding sites, substrate binding sites, phosphorylation sites, and multiple membrane-spanning motifs) (Yoshimura et al., 1989; Skach, 1998) are absent, resulting in a P-glycoprotein–null phenotype. We showed that the P-glycoprotein substrate loperamide exerts profound CNS effects in dogs with the ABCB1-1Δ mutation but exerts essentially no CNS effects in ABCB1 wild-type dogs. A radiolabeled P-glycoprotein substrate (99mTc-sestamibi) was excluded from the brain in ABCB1 wild-type dogs but penetrated the BBB in ABCB1-1Δ dogs. However, CSF concentrations of 99mTc-sestamibi did not differ in ABCB1 wild-type compared with ABCB1-1Δ dogs. This report describes a novel model that can be used to investigate the complexities of P-glycoprotein drug transport at the BBB and blood-CSF barrier.
Materials and Methods
Animals. Experiments involving animals were approved by the Washington State University animal care and use committee. Six collie dogs were used in these studies [three homozygous for ABCB1-1Δ (two female and one male) and three ABCB1 wild-type (two female and one male)]. The dogs ranged from 1.5 to 8 years. For individual dogs, a minimum 2-week washout period was allowed between experiments. Dogs were housed separately in runs and maintained on a 12-h light/dark cycle with free access to food and water. To prevent repeated venipuncture and/or repeated i.v. catheter placement for blood sample collections, venous access ports (Norfolk Veterinary Products, Skokie, IL) were surgically placed in one jugular vein of each dog at least 1 month before initial experiments.
Magnetic Resonance Imaging. Dogs were anesthetized with isoflurane in oxygen for magnetic resonance imaging (MRI) and positioned in sternal recumbency. MRI was performed with a 1.0-Tesla MRI system (Philips Gyroscan, Philips Medical Systems, Best, The Netherlands). Imaging sequences obtained included proton density, T2, fluid-attenuated inversion recovery, T1 precontrast, and T1 postcontrast in all three planes. Two board-certified veterinary radiologists, blinded as to the ABCB1 genotype of each animal, interpreted MRI images. Dogs considered to have abnormal brain images based on MRI results were to be excluded from the study and replaced with a different dog of the same genotype. Specifically, the investigators were looking for conditions that would alter the BBB (inflammatory, infectious, or neoplastic disease).
Neurological Examinations. Neurological examinations were performed before and 6 h after p.o. administration of loperamide (0.2 mg/kg) by veterinary neurologists blinded as to the ABCB1 genotype of each dog. Neurological parameters assessed included mental status, posture, gait, cranial nerves, and spinal reflexes.
Measurement of Bispectral Index. Bispectral index system (BIS) was measured before and 1 h after i.v. loperamide (0.08 mg/kg) administration. BIS uses electroencephalographic impulses to assess and monitor hypnosis or level of sedation. BIS generates a numerical value (derived from a complex mathematical algorithm) providing a quantitative means to compare CNS depression (Myles et al., 2004). The primary lead was placed on the midline approximately one third of the distance from a line connecting the zygomatic processes of the frontal bone and the most caudal portion of the external frontal crest that was palpable. A secondary lead was placed 2 cm lateral and 1 cm caudal to the primary lead over the right temple. A ground lead was placed rostral to the tragus of the right ear. A modified ECG cable was connected to the BIS cable distal to the analog-to-digital converter. Three 29-gauge platinum needle electrodes were connected to the modified cable and placed subdermally in the locations described. The BIS was measured using a BIS monitor and software. The BIS was recorded every 5 s for 5 min, and data were stored on a computer. The BIS was reported as a unitless whole number between 0 and 100. Filters for elimination of electrical noise were set as follows: the low cutoff was set at 2 Hz; the 50/60-Hz filter was set to 60 Hz; and the high cutoff was set at 70 Hz. At startup, the monitor required a skin-electrode impedance of <7.5 kΩ; thereafter, it provided for continuous impedance checking with impedance of <2kΩ at 16 Hz. High-frequency activity (70–110 Hz) was identified as electromyographic activity measured in decibels with respect to 0.0001 μV2 and was graphed in real time with the BIS. The monitor had automatic artifact detection and displayed a signal quality index as a function of good epochs and suppressed epochs in the previous 120 epochs (61.5 s) that were used for calculation of the BIS. The percentage of epochs in the past 63 s in which the electroencephalogram signal was suppressed was expressed as the suppression ratio. Burst suppression was identified as an isoelectric analog electroencephalogram for at least 1 s and detected by the monitor as indicated by an increase in the suppression ratio (i.e., suppression ratio >1). The BIS was recorded when the suppression ratio = 0.
CSF 99mTc-Sestamibi Activity.99mTc-sestamibi was obtained from a local commercial nuclear medicine supplier (Syncore, Spokane, WA). All the procedures involving use of 99mTc-sestamibi were approved by the Washington State University Environmental Health and Safety and Radiation Safety Offices. Dogs were anesthetized with desflurane in oxygen. Following sterile preparation of the collection site (cisterna magna), a 1.5-inch spinal needle with a stylet was inserted into the subarachnoid space. CSF was collected from the cisterna magna rather than the lumbar region because of the proximity of the cisterna magna to the ventricles (where CSF is produced). Approximately 0.5 ml of CSF was obtained at time 0 (before radioisotope administration) and 30, 60, 90, and 120 min after radioisotope administration. The flow rate of CSF in dogs is approximately 0.05 ml/min in beagles (Artru, 1991) but was approximately twice that in collies; therefore, it took 5 min to obtain a 0.5-ml sample. Blood (3 ml) samples were collected at the same time intervals. The stylet was replaced between sample collection periods. 99mTc-sestamibi (10 mCi/dog) was administered as a bolus injection in the lateral saphenous vein. Blood was collected from a cephalic or contralateral saphenous vein. After sampling was complete, meloxicam (0.15 mg/kg) was administered i.v. as an analgesic. Dogs were then allowed to recover from anesthesia. 99mTc-sestamibi activity in CSF and blood samples was counted using a well counter attached to a multichannel analyzer (Nucleus, Oak Ridge, TN). Region of interest was placed with a 20% window centered over the 99mTc photopeak of 140 KeV. Total activity within the defined photopeak for each sample was determined for 2-min acquisitions. 99mTc-sestamibi activity in blood and CSF was determined 4 h after injection in each subject. Background activity was determined by sampling before the administration of 99mTc-sestamibi and subtracted from all the subsequent samples.
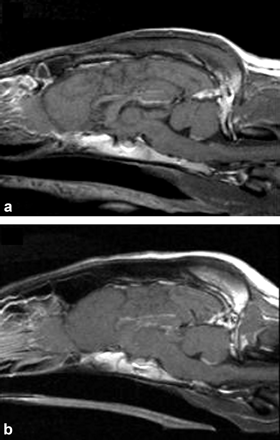
Sagittal T1-weighted post-gadolinium administration MRI images display normal brain anatomy, tissue signal characteristics, and contrast enhancement of an ABCB1 wild-type dog (A) and an ABCB1-1Δ dog (B).
Scintigraphic Studies. Anesthetized dogs were imaged using a large field of view gamma camera with a high resolution collimator (Starcam, General Electric Medical Systems, Milwaukee, WI). Images were obtained before and at 15-min intervals for 2 h after injection of 99mTc-sestamibi. The automated image analysis software program on the scintigraphy computer (Camstar, General Electric Medical Systems) was used to quantify brain uptake of 99mTc-sestamibi using ratios of the mean count density of regions of interest drawn of brain and nonbrain (neck muscle) tissue.
Statistics. To control for repeated measures on the same individuals over time, the data were analyzed using repeated measures analysis of variance as implemented by the statistical software NCSS 2004 (http://www.ncss.com/ncss.html). The conventional value of α = 0.05 was used as the threshold for statistical significance.
Results
MRI. Comparisons were made between two groups of dogs, one with normal P-glycoprotein function (ABCB1 wild-type) and one presumed to have a P-glycoprotein-null phenotype (ABCB1-1Δ), for each of the experiments performed. The latter group consists of dogs with a functional polymorphism of the ABCB1 gene that we identified (Mealey et al., 2001). We first wanted to establish that the two groups of dogs did not differ with respect to microvascular integrity or other anatomic abnormalities that would compromise the blood-CSF barrier or BBB. To accomplish this, we performed MRI of the brain, using both T1- and T2-weighted images. T1-weighted sequences best show anatomy (i.e., ventricular size differences or congenital malformations), whereas T2-weighted sequences best show pathological conditions (i.e., inflammation, neoplastic processes). Images were obtained before and after i.v. injection of gadolinium diethylenetriaminepentaacetic acid. Gadolinium diethylenetriaminepentaacetic acid does not normally cross the BBB, so it is particularly useful for assessing microvascular integrity. Two veterinary radiologists, blinded as to the ABCB1 genotype of each animal, independently interpreted MRI images of all the ABCB1 wild-type (Fig. 1A) and ABCB1-1Δ (Fig. 1B) dogs. There were no abnormal contrast enhancements, and macroscopic lesions of the brain were not detected.
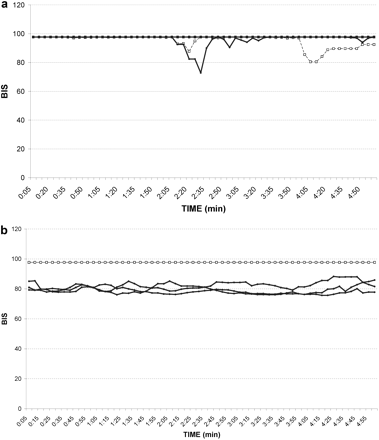
BIS values obtained before (A) and 1 h after (B) i.v. administration of loperamide showing significant (P = 0.0018) CNS depression in ABCB1-1Δ (solid line, •) compared with ABCB1 wild-type (broken line, □) dogs. BIS was assessed every 5 s for 5 min.
Neurological Effects of Loperamide Treatment on ABCB1-1Δ and ABCB1 Wild-Type Dogs. Loperamide was then used to compare the phenotype of ABCB1-1Δ and ABCB1 wild-type dogs to confirm the P-glycoprotein–null status of this potential canine model compared with the abcb1a(–/–) knockout mouse model. Loperamide is an opioid antidiarrheal agent that does not cause typical opioid-induced neurological signs in patients because, as a substrate of P-glycoprotein, it does not penetrate the BBB (Schinkel et al., 1996). It has been used as a probe to detect absent or altered P-glycoprotein function because pharmacological effects of opioids on the CNS lend themselves to objective measurement (Skarke et al., 2003). Before loperamide administration, all the dogs were assessed to be neurologically normal. Six hours after p.o. loperamide administration, all the ABCB1-1Δ dogs developed CNS depression and neurological deficits, whereas ABCB1 wild-type dogs remained neurologically normal (Supplemental Data Videos 1–5). Results of neurological examinations before and after loperamide administration are shown in Tables 1 and 2, respectively.
Results of neurological examination before loperamide (0.2 mg/kg p.o.) administration to ABCB1-1Δ and ABCB1 wild-type collies
Mental Status: alert, depressed, demented, disoriented, stuporous, and comatose. Posture: normal, head tilt (L or R), head turn (L or R), falling, tremor, tetany, decerebrate, decerebellate, Schiff-Sherrington, and other. Gait: ataxia (truncal, all, or pelvic limbs), paretic (tetra-, para-, hemi-, or mono), plegic (tetra-, para-, hemi-, or mono), lame (LT, RT, LP, RP), short-strided/stiff (T, P, all), and unable to stand (T, P, all). Postural reactions, cranial nerves, spinal reflexes: 0 = absent; 1 = reduced; 2 = normal; 3 = exaggerated.
Cranial nerves examined include II and VII (menace response, pupil size, direct and indirect pupillary light response); III, IV, VI (resting strabismus); III, IV, VI, VIII (positional strabismus); VIII (spontaneous, positional, or changing nystagmus); VIII, III, IV, VI (oculovestibular); V (facial sensation, mastication, ocular sensation); VII (facial symmetry); V, VII (palpebral); IX, X (swallowing); XI (trapezius muscle); XII (tongue).
Results of neurological examination 6 h after loperamide administration (0.2 mg/kg p.o.) to ABCB1-1Δ and ABCB1 wild-type collies
Mental status: alert, depressed, demented, disoriented, stuporous, and comatose. Posture: normal, head tilt (L or R), head turn (L or R), falling, tremor, tetany, decerebrate, decerebellate, Schiff-Sherrington, and other. Gait: ataxia (truncal, all, or pelvic limbs), paretic (tetra-, para-, hemi-, or mono), plegic (tetra-, para-, hemi-, or mono), lame (LT, RT, LP, RP), short-strided/stiff (T, P, all), and unable to stand (T, P, all). Postural reactions, cranial nerves, spinal reflexes: 0 = absent; 1 = reduced; 2 = normal; 3 = exaggerated.
Cranial nerves examined include II and VII (menace response, pupil size, direct and indirect pupillary light response); III, IV, VI (resting strabismus); III, IV, VI, VIII (positional strabismus); VIII (spontaneous, positional, or changing nystagmus); VIII, III, IV, VI (oculovestibular); V (facial sensation, mastication, ocular sensation); VII (facial symmetry); V, VII (palpebral); IX, X (swallowing); XI (trapezius muscle); and XII (tongue).
To more objectively measure the pharmacological effects of loperamide in the CNS, we used the BIS, which uses electroencephalographic impulses to assess and monitor hypnosis. BIS generates a numerical value (derived from a complex mathematical algorithm) providing a quantitative means to compare loperamide-induced CNS depression in ABCB1-1Δ and ABCB1 wild-type dogs (Myles et al., 2004). BIS values in an awake, alert subject range from 95 to 100, whereas the desired BIS for most anesthetized subjects at a surgical plane is 40 to 60. BIS was measured in dogs before and 1 h after loperamide administration (0.08 mg/kg i.v.). Mean BIS values in ABCB1 wild-type and ABCB1-1Δ dogs before loperamide administration were 96.5 and 97.2%, respectively (Fig. 2A). After loperamide administration, there was no change in mean BIS value in ABCB1 wild-type dogs (97.7%), but the BIS value in ABCB1-1Δ dogs was significantly decreased (80.0%; P = 0.0018), indicating that loperamide exerted CNS-depressant effects in dogs deficient for P-glycoprotein (Fig. 2B). Collectively, these data show that ABCB1-1Δ dogs are phenotypically similar to abcb1a(–/–) knockout mice with regard to loperamide and therefore may be useful as a model for studying the role of P-glycoprotein in the BBB.
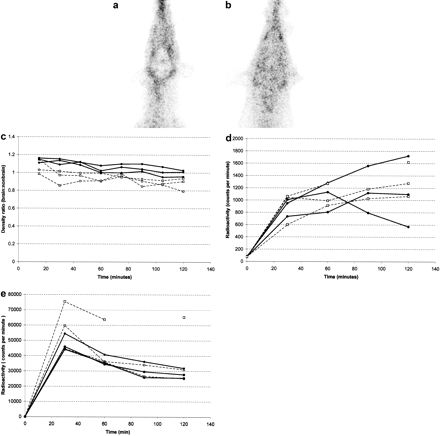
99mTc-Sestamibi Activity in Blood, Brain, and CSF. Although ABCB1-1Δ dogs are phenotypically similar to abcb1a(–/–) mice, their size offers several advantages over rodent models for studying P-glycoprotein's role in restricting access of substrate drugs to the CSF and brain parenchyma. For example, procurement of multiple CSF samples from the same animal over a time course can be accomplished in canine, but not rodent, models. Additionally, detailed imaging studies of the brain are often tedious and difficult to interpret in rodent models. In contrast, noninvasive imaging techniques such as MRI, nuclear scintigraphy, and others are routinely performed in dogs and could be easily used to assess drug penetration to restricted sites such as the CNS. We exploited both of these techniques in the P-glycoprotein–deficient canine model to compare CSF and brain concentrations of a P-glycoprotein substrate in ABCB1-1Δ and ABCB1 wild-type dogs. P-glycoprotein is thought to be involved in both the BBB and the blood-CSF barrier, but data regarding its comparative function at these separate locations are scarce. We decided to use this canine model to assess brain and CSF concentrations of 99mTc-sestamibi, a radiolabeled P-glycoprotein substrate (Dyszlewski et al., 2002), in ABCB1 wild-type and ABCB1-1Δ dogs. Serial nuclear scintigraphic imaging was used to assess uptake of 99mTc-sestamibi in brain tissue, whereas direct measurement of radioactivity in serially collected blood and CSF samples was used to assess 99mTc-sestamibi activity in blood and CSF. ABCB1-1Δ dogs had significantly greater 99mTc-sestamibi accumulation in the brain than did ABCB1 wild-type dogs (P = 0.027) (Fig. 3, A–C), indicating that P-glycoprotein at the BBB in dogs limits brain accumulation of P-glycoprotein substrates. Activity of 99mTc-sestamibi in blood samples did not differ between ABCB1 wild-type and ABCB1-1Δ dogs (Fig. 3D), indicating that differences in brain uptake 99mTc-sestamibi resulted from differences in P-glycoprotein function at the BBB. Interestingly, levels of 99mTc-sestamibi in CSF did not differ between groups (Fig. 3E), suggesting that P-glycoprotein does not influence entry of the substrate sestamibi into the CSF.
99mTc-sestamibi nuclear scintigraphic neuroimaging and direct blood and CSF 99mTc-sestamibi radioactivity measurements in ABCB1-1Δ and ABCB1 wild-type dogs indicate that P-glycoprotein functions at the BBB but not the blood-CSF barrier. A and B, representative 99mTc-sestamibi nuclear scintigraphic neuroimages showing diminished activity in the brain compared with surrounding tissue of an ABCB1 wild-type dog (A) but similar activity in the brain compared with surrounding tissue of an ABCB1-1Δ dog (B). 99mTc-sestamibi brain/nonbrain tissue ratios versus time curves in ABCB1-1Δ (bold lines, •) and ABCB1 wild-type (dashed lines, □) dogs showing a significant difference (P = 0.027) based on genotype (C). 99mTc-sestamibi activity in CSF (D) and blood (E) does not differ between groups.
Discussion
There are two significant outcomes of the study reported here. The first is the validation of ABCB1-1Δ dogs as a P-glycoprotein-null animal model. With regard to loperamide distribution to the brain, ABCB1-1Δ dogs are comparable with abcb1a knockout mice (Schinkel et al., 1996). These mice exhibit signs typical of opiate intoxication (immobility, circling) at doses of loperamide that do not affect wild-type mice. ABCB1-1Δ dogs exhibited multiple neurological abnormalities of posture, gait, postural reactions, cranial nerve reflexes, and spinal reflexes at doses of loperamide that did not affect wild-type dogs. Ivermectin is another drug that does not penetrate the BBB in animals with normal P-glycoprotein function. Similar to abcb1 knockout mice, ABCB1-1Δ dogs are viable and fertile and appear phenotypically normal, but they are exquisitely sensitive to the neurotoxic effects of the antiparasitic drug ivermectin (Mealey et al., 2001). The apparent difference in brain penetration of loperamide in ABCB1-1Δ dogs as compared with wild-type dogs is not a result of either macroscopic anatomic defects or microvascular permeability abnormalities because MRI findings, including contrast studies, identified no defects or abnormalities in any of the dogs studied.
The second significant outcome is the finding that P-glycoprotein does not appear to contribute equally to the BBB and blood-CSF barrier in this canine model using the P-glycoprotein substrate 99mTc-sestamibi. The latter result is notable in that it challenges the assumptions of some researchers that P-glycoprotein contributes to both the blood-CSF barrier and BBB and raises questions about the practice of using CSF drug concentrations to predict efficacy of P-glycoprotein substrate drugs intended for treating CNS diseases such as AIDS. Furthermore, our results differ from recently reported results in abcb1a(–/–) and abcb1ab(–/–)(–/–) mice in which CSF concentrations of some P-glycoprotein substrates were greater in knockout mice as compared with wild-type mice (Doran et al., 2005). It is likely that species differences account for the disparate findings in dogs and mice. Whereas humans and dogs have a single gene that codes for P-glycoprotein (ABCB1), the rodent ortholog consists of two genes, abcb1a and abcb1b (Dean, 2005). The greater concordance between canine and human ABCB1 genes represents yet another potential advantage of the ABCB1-1Δ canine model over murine models for studying the role of P-glycoprotein in the blood-CSF barrier and BBB. However, substrate specificity between canine and human P-glycoprotein has not been fully investigated.
Effective delivery of CNS-active pharmaceutical agents to the brain represents an important therapeutic problem for many brain disorders. In particular, P-glycoprotein–mediated efflux of a variety of drugs is considered a major hindrance to effective treatment for CNS diseases, including AIDS dementia complex, epilepsy, psychosis, and others. For example, all the currently available human immunodeficiency virus 1 protease inhibitors are substrates for P-glycoprotein and therefore are actively extruded from the CNS by P-glycoprotein, rendering them ineffective for suppressing viral replication there (Thuerauf and Fromm, 2006). Furthermore, recent evidence suggests that P-glycoprotein–mediated efflux of anticonvulsant drugs from epileptic foci is a major cause of refractory epilepsy (Tishler et al., 1995; Kwan and Brodie, 2005). A quantitative, mechanistic understanding of the role of P-glycoprotein–mediated efflux transport on the pharmacokinetics and pharmacodynamics of substrate drugs acting in the CNS is needed to improve drug delivery to the brain but is hindered by current models [cell culture and abcb1a(–/–) knockout murine models]. Although immortalized cell lines provide a readily accessible in vitro model, their ability to predict a fully representative in vivo situation is limited. For example, a greater degree of up-regulation and down-regulation of membrane transporters occurs compared with what has been observed in vivo (Barrand et al., 1995; Regina et al., 1998). Other factors that do not adequately reflect the in vivo situation include electrical resistance and absence of the closely associated astrocyte foot processes and pericytes that provide signals and/or soluble factors required for normal expression and function of transport proteins (Pardridge, 1999; Feng, 2002).
Although the abcb1a(–/–) knockout mouse model was extremely valuable in determining that P-glycoprotein plays an important role in restricting access of substrate drugs across the BBB (Schinkel et al., 1994, 1995; Sills et al., 2002), size limitations restrict the use of many important research applications in this murine model. The naturally occurring canine model presented here overcomes the disadvantages of current models as a means of dissecting the role of P-glycoprotein in CSF and brain penetration of substrate drugs. Classification of candidate drugs as substrates or inhibitors of P-glycoprotein is of crucial importance in CNS drug development. This canine model has immediate relevance in the CNS drug development process by providing in vivo CNS drug distribution data after the putative drug has passed in vitro tests.
Footnotes
-
This work was supported by Pfizer, Inc., and by National Institutes of Health Grant MH074956.
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.107.018978.
-
ABBREVIATIONS: BBB, blood-brain barrier; CSF, cerebrospinal fluid; CNS, central nervous system; MRI, magnetic resonance imaging; BIS, bispectral index system.
-
↵
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material. - Received September 25, 2007.
- Accepted March 7, 2008.
- The American Society for Pharmacology and Experimental Therapeutics