Abstract
Carboxylesterase (CES) 1 and CES2 are two major hepatic hydrolases responsible for the metabolism of numerous endogenous and exogenous compounds. In this study, age- and sex-dependent expression and activity of CES1 and CES2 were investigated using both animal models and individual human liver s9 samples. The expression and activity of mouse CES1 (mCES1) and mCES2 in the liver were markedly lower in newborns relative to adults and increased gradually with age, approximating levels of adult animals by age 2 to 4 weeks. Likewise, the average human CES1 (hCES1) expression in the subjects <1 year of age was significantly lower than that of pooled samples. In particular, hCES1 expression in the 13-day and 1-month-old subjects was just 20.3 and 11.1%, respectively, of the pooled sample values. In addition, the subjects <1 year of age exhibited a trend suggestive of low hCES2 expression, but this difference failed to reach statistical significance because of large interindividual variability. The expression and activity of mCES1 and mCES2 were not significantly altered after the animals were treated with human growth hormone, indicating growth hormone may not be associated with the low level of CES expression during early developmental stages. No significant differences of the expression and activity of mCES1 and mCES2 were observed between sexually mature male and female mice. In conclusion, the expression and activity of CES1 and CES2 are age-related but independent of growth hormone level. Sex seems to be an unlikely factor contributing to the regulation of CES1 and CES2.
Carboxylesterase (CES) 1 and CES2 are two major hydrolytic enzymes responsible for the metabolism of numerous carboxylic acid esters, carbamates, thioesters, and amide agents. A variety of widely prescribed therapeutic drugs such as methylphenidate (MPH), oseltamivir, and irinotecan are metabolized by CES1 and/or CES2, resulting in the formation of hydrolytic metabolites (Imai et al., 2006; Hosokawa et al., 2008). A number of endogenous substrates, such as triacylglycerols and fatty acyl-CoA esters, are recognized. In addition, a variety of environmental toxins and specific chemical warfare agents have been identified as substrates of CES1 and CES2 (Satoh et al., 2002). Distinct differences between CES1 and CES2 have been identified relative to localization, substrate specificity, immunological properties, and gene regulation (Satoh and Hosokawa, 1998; Imai et al., 2006).
Significant differences in drug metabolism and disposition are known to exist between very young children, adolescents, and adults as a result of delayed maturation of drug-metabolizing enzymes (DMEs) (Kearns et al., 2003). The vast majority of what is presently understood regarding developmental aspects of DMEs in humans has been generated in studies of the cytochrome P450 (P450) enzymes, with very little data available on hydrolases by comparison (Hines, 2008). The enzymatic activity of many P450s changes significantly during the early stages of development. For example, the activity of the major isoenzymes CYP1A2, CYP2C9, CYP2E1, CYP2D6, and CYP3A4 increases gradually after birth (Vieira et al., 1996; Sonnier and Cresteil, 1998; Stevens et al., 2003, 2008; Koukouritaki et al., 2004). Conversely, the level of CYP3A7, the predominant P450 isoform in fetal liver, decreases nearly 5-fold within the first year after birth (Stevens et al., 2003). In addition to the pediatric population, elderly individuals may also display a significantly altered ability to metabolize a variety of medications relative to younger healthy adults (Benedetti et al., 2007). Besides age, sex is increasingly appreciated as another important factor affecting the expression and/or activity of DMEs in humans and in experimental animal models (Mugford and Kedderis, 1998; Scandlyn et al., 2008). For example, in humans, the expression of CYP3A4, the major P450 in the liver, was determined to be 2-fold higher in females compared with males (Wolbold et al., 2003). Thus, subject age and sex are among the major determinants known to influence the expression and function of DMEs. Accordingly, a better understanding of age- and sex-related factors that may also influence hydrolytic drug metabolism is warranted. Gained insights offer the potential for improvements in the safety and efficacy of pharmacotherapy.
Compared with the available biomedical literature devoted to the P450 enzymes, studies addressing the influence of developmental age and sex on the activity of CES1 and CES2 are extremely limited. It has recently been documented that blood concentrations of MPH, a selective substrate of CES1, are significantly higher in preschool children than in school-aged children (Wigal et al., 2007) and also higher in children in general when compared with adults following oral dosing of MPH (Wargin et al., 1983). Given that CES1-mediated hydrolysis is the major metabolic route of MPH, we hypothesize that human CES (hCES) 1 function and/or expression may be premature during the earlier developmental stages of life. In addition, sex differences in both pharmacokinetics (PK) and pharmacodynamics (PD) of MPH have also been described in our recent clinical study (Patrick et al., 2007). This study revealed that the mean area under the plasma concentration versus time curves of d-MPH in women was significantly lower than in men after oral dosing with 0.3 mg/kg dl-MPH. However, despite low exposure to d-MPH, women reported a significantly greater stimulant effect than men when questioned, “Do you feel any drug effect?” The underlying mechanism(s) of the differing PK and PD response to MPH between sexes is uncertain and an area of ongoing study. We have further hypothesized that the lower d-MPH plasma concentrations observed in women are at least partially related to a higher catalytic activity of hCES1 in females.
In the present study, we investigated age- and sex-related expression and activity of CES1 and CES2 using both a mouse model and human liver s9 fractions from 32 individual donors ranging from age 13 days to 85 years. Furthermore, we initially explored one avenue of developmental expression and activity of CES1 and CES2 by determining the association of human growth hormone (HGH) with CES1 and CES2 expression and activity.
Materials and Methods
Materials and Animals. Individual and pooled human liver s9 fractions were purchased from CellzDirect, Inc. (Durham, NC) and XenoTech, LLC (Lenexa, KS). The ages of individual donors ranged from 13 days to 85 years. The known biological characteristics of the human liver donors are summarized in Table 1. CES1 and CES2 antibodies were the products from Abcam Inc. (Cambridge, MA). The antibodies react with the antigens derived from both humans and mice. Racemic MPH was purchased from Sigma-Aldrich (St. Louis, MO). MPH hydrolytic metabolite ritalinic acid was a gift from Dr. Kennerly Patrick (Medical University of South Carolina, Charleston, SC). Irinotecan and its metabolite SN38 were obtained from Toronto Research Chemicals Inc. (North York, ON, Canada). HGH [Nutropin (somatropin, rDNA origin, for injection) lyophilized powder] was from Genentech (South San Francisco, CA). All the other agents were of high analytical grade and obtained commercially. FVB mice were obtained from Charles River Laboratories, Inc. (Wilmington, MA) and housed in a colony room with temperature maintained at 22 ± 1°C on a 12-h light/dark cycle with lights on at 6:00 AM and off at 6:00 PM. The breeding colony was established to produce the animals with various ages for the study of developmental expression and activity of CES1 and CES2. All the protocols were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Biological descriptors of human donor samples
Western Blot Studies. Western blot studies were conducted to explore the potential relationship(s) between CES1 and CES2 expression with age and/or sex. Male FVB mice between the age of 1 and 4 days, as well as 1, 2, 4, and 9 weeks, were used to study the developmental expression of mouse CES (mCES) 1 and mCES2 in the liver. After anesthesia with isoflurane, animals were sacrificed by decapitation, and liver tissues were harvested. Liver tissues were rinsed in the assay buffer (Dulbecco's phosphate-buffered saline containing 20 mM HEPES, pH 7.4) and then homogenized using a Polytron homogenizer (Kinematica, Inc., Lucerne, Switzerland). The homogenate then underwent centrifugation at 9000g for 30 min at 4°C, and the supernatant was collected as the s9 fraction. The protein concentrations of prepared mouse liver s9 samples and purchased individual and pooled human liver s9 fractions were measured using a Pierce (Rockford, IL) BCA assay kit. The expression of CES1 and CES2 in both mouse and human liver s9 fraction samples was determined using the Western blot assay described previously (Zhu et al., 2009). Antiactin and glyceraldehyde-3-phosphate dehydrogenase were included in the study as the loading controls for mouse and human samples, respectively. The relative expression levels of CES1 and CES2 were estimated by analyzing the blots with ImageJ software (National Institutes of Health, Bethesda, MD) and calculating the ratio of CES1 and CES2 to actin or glyceraldehyde-3-phosphate dehydrogenase. In addition, to determine the potential difference of mCES1 and mCES2 expression between sexes, the liver s9 fractions were prepared from 9-week-old male and female FVB mice and subjected to Western blot analysis using the method described above.
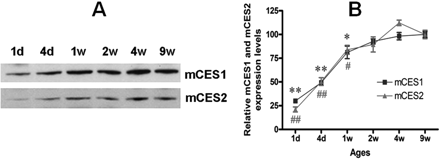
Developmental expression of mCES1 and mCES2 in the mouse liver. A, representative blots of mCES1 and mCES2 expression in the liver tissues of mice from different age groups. B, the expression levels of hepatic mCES1 and mCES2 in different age groups relative to that of 9-week-old mice. **, p < 0.01 and *, p < 0.05 versus mCES1 of 9-week-old mice; ##, p < 0.01 and #, p < 0.05 versus mCES2 of 9-week-old mice (n = 4).
Enzymatic Activity Studies. The enzymatic activity of CES1 and CES2 in both human and mouse liver s9 fractions was measured using established substrates MPH (CES1) and irinotecan (CES2) (Sanghani et al., 2004; Sun et al., 2004). The hydrolysis of MPH and irinotecan was carried out in 1.5-ml Eppendorf tubes at a total volume of 100 μl. Before incubations, substrate solutions were freshly prepared in the assay buffer (Dulbecco's phosphate buffered saline containing 20 mM HEPES, pH 7.4). Reactions were initiated by mixing 50 μl of the respective substrates with 50 μl of liver s9 samples. The final MPH and irinotecan concentrations were 1 mM and 200 μM, respectively, and the protein concentration of s9 samples was 5 mg/ml. After incubation at 37°C for 1 h, the MPH hydrolysis reaction study was terminated by adding 500 μl of ice-cold methanol. The irinotecan hydrolysis reaction was terminated by adding 500 μl of methanol containing 5 mM hydrochloric acid after incubation at 37°C for 10 min. Precipitated protein was removed by centrifugation at 16,000g for 5 min at 4°C. Concentrations of MPH metabolite ritalinic acid were determined using a previously published high-performance liquid chromatography method (Zhu et al., 2008). SN38, the hydrolytic product of irinotecan, was quantified using a validated high-performance liquid chromatography/fluorescence assay (Rivory and Robert, 1994).
Expression of hCES1 and hCES2 in the individual liver s9 samples (A) and the analysis of relative expression levels of hCES1 (B) and hCES2 (C) among different age groups. **, p < 0.01 versus pooled samples.
Effect of HGH on the Expression and Activity of mCES1 in Vivo. Both animals and humans exhibit substantially higher concentration of growth hormone during the early developmental period. In the present study, a mouse model was used to investigate the potential association between HGH and mCES1 expression and activity. Nine-week-old male FVB mice were randomly divided into four groups (n = 4): HGH short-term treatment group (5 mg/kg single-dose i.p. injection), HGH long-term treatment group (5 mg/kg/day i.p. injection for 9 days), and two control groups injected with an equal volume of water once and for 9 days. HGH was reconstituted in sterile water, producing a concentration of 0.5 mg/ml. The injection volume was 10 μl/g b.wt. Animals were dosed with HGH or water on a single occasion or once daily for 9 days. Twenty-four hours after single dose or 1 h after the last dose of 9-day administration of HGH, the mice were sacrificed by decapitation after anesthesia with isoflurane. The liver s9 fractions were prepared using the method described previously. The expression and activity of mCES1 were determined by Western blot analysis and MPH hydrolysis assay, respectively (Zhu et al., 2008, 2009).
Data Analysis. All the values were presented as mean ± S.D. Comparisons of the expression and activity of CES1 and CES2 within different age groups were performed by one-way analysis of variance followed by Dunnett's post-test. The differences in the expression and activity of CES1 and CES2 between male and female mice, as well as between the mice treated with HGH and controls, were analyzed by the unpaired Student's t test. All the statistical analyses were performed using Prism software (GraphPad Software Inc., La Jolla, CA). A p value of less than 0.05 was considered statistically significant.
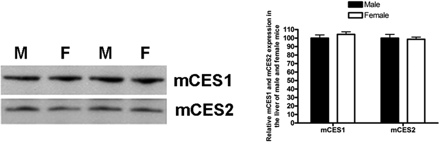
Expression levels of mCES1 and mCES2 in the livers did not differ between male and female mice.
Results
Age- and Sex-Related CES1 and CES2 Expression. The expression of both mCES1 and mCES2 in the liver of 1-day-old mice was only ∼25% of that of adult animals (9 weeks). The expression levels increased with animal age and achieved the levels of fully mature animals by 2 weeks of age (Fig. 1). Consistent with observations in mice, the expression of hCES1 was generally low in very young human subjects when compared with the pooled samples (Fig. 2). Particularly low expression of hCES1 was detected in the samples from the 13-day and 1-month-old subjects with the relative expression level just 20.3 and 11.1% of the pooled samples, respectively. The average expression levels of hCES1 in the age groups of <1 year (n = 7), 1 to 4 years (n = 5), 6 to 9 years (n = 5), 12 to 18 years (n = 4), and 75 to 85 years (n = 8) were 60.0 ± 34.9% (p < 0.01), 78.1 ± 14.0%, 81.2 ± 21.0%, 95.5 ± 8.2%, and 101.2 ± 28.9%, respectively. The hCES1 expression displayed a significant trend of increase with ages (p = 0.0026). With regard to hCES2, the subjects with the ages <1 year exhibited a tendency of low hCES2 expression but did not reach statistic significance when compared with the pooled samples. The expression levels of hCES2 for ages <12 months, 1 to 4 years, 6 to 9 years, 12 to 18 years, and 75 to 85 years were determined to be 83.8 ± 14.7, 106.0 ± 26.6, 98.2 ± 13.8, 105.8 ± 7.7, and 109.4 ± 20.2%, respectively, relative to the pooled samples. It is noted that both hCES1 and hCES2 expression in elderly subjects (>75 years) are comparable with that of the pooled samples. It is noteworthy that significant interindividual variability in expression levels of both hCES1 and hCES2 was observed in subjects even within similar age ranges. No significant differences in the expression of mCES1 and mCES2 were observed between adult male and female mice (Fig. 3).
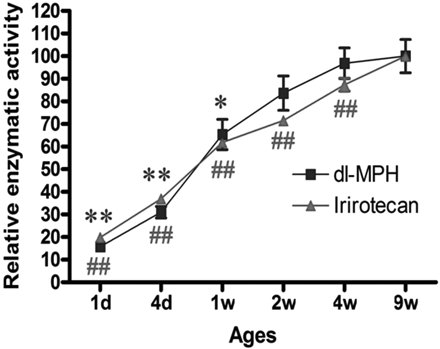
Relative enzymatic activity (hydrolysis) of MPH and irinotecan in the livers of mice across different age ranges (n = 4). **, p < 0.01 and *, p < 0.05 versus mCES1 of 9-week-old mice; ##, p < 0.01 versus mCES2 of 9-week-old mice.
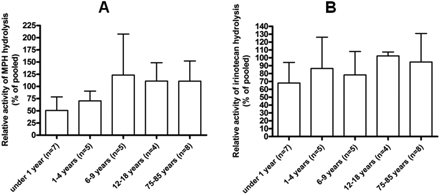
Enzymatic Activity of CES1 and CES2. Consistent with the expression levels described above, the enzymatic activity of mCES1 and mCES2 determined by MPH and irinotecan hydrolysis, respectively, was significantly lower in the young animals (Fig. 4). The catalytic activity of mCES1 and mCES2 in the liver s9 samples prepared from 1-day-old mice was approximately 25% of mature animals. The activity of mCES1 and mCES2 reached mature levels after 1 and 4 weeks, respectively. With regard to human samples, in vitro MPH hydrolysis studies showed that the catalytic activity of the liver hCES1 in the 13-day and 1-month-old subjects was 9.9 and 12.5%, respectively, relative to the pooled. The hCES1 activity in the age groups of <1 year, 1 to 4 years, 6 to 9 years, 12 to 18 years, and 75 to 85 years was 50.8 ± 27.8, 70.4 ± 20.0, 123.2 ± 84.3, 111.0 ± 50.0, and 110.9 ± 41.2, respectively, when compared with the pooled sample values (Fig. 5A). A significant trend (p = 0.0092) was observed in the increase of hCES1 activity through different age groups. The relative activity of hCES2 as determined by irinotecan hydrolysis was 68.0 ± 26.1, 84.1 ± 39.6, 91.1 ± 29.7, 102.3 ± 4.9, and 94.6 ± 36.2% in the liver s9 samples of the subjects with the ages of <1 year, 1 to 4 years, 6 to 9 years, 12 to 18 years, and 75 to 85 years, respectively (Fig. 5B).
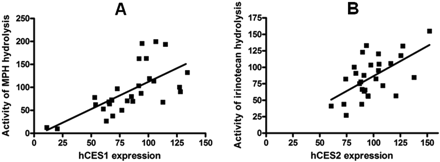
The hydrolysis of MPH and irinotecan significantly correlated with the levels of expression of hCES1 (p < 0.0001; r = 0.6694) and hCES2 (p = 0.0008; r = 0.5892), respectively (Fig. 6). Incubation studies did not reveal any significant differences in catalytic activity of mCES1 and mCES2 between male and female mice (data not shown).
HGH Does Not Regulate CES1 Expression and Function in the Liver. The expression and activity of mCES1 in the mouse liver was measured after short-term (1 day) and long-term (9 days) treatment with 5 mg/kg/day HGH. No significant difference of mCES1 expression was found between the animals treated with HGH and water. The liver s9 samples prepared from HGH-treated mice also showed similar catalytic activity to control animals with regard to catalyzing MPH hydrolysis. The data indicated that significantly low expression and activity of CES1 in very young mice and humans may not be related to the levels of growth hormone. In addition, it was clear that HGH administration did exert significant pharmacologic effects via dosing by the intraperitoneal route as evidenced by recorded body weight gain measurements. Mice treated with HGH gained 5.2 ± 0.2 g after the exposure to HGH for 9 days, whereas that of controls was only 2.0 ± 0.4 g (p < 0.01).
Activity of human liver s9 samples representing different age groups on catalyzing MPH (A) and irinotecan (B) hydrolysis.
Discussion
CES1 and CES2 expressed in the liver play critical roles in the deactivation or activation of numerous therapeutic agents. In the present study, we investigated the developmental aspects of CES1 and CES2 expression and activity in both mice and humans. The animal study showed that the expression and activity of CES1 and CES2 were markedly low in newborn mice and then increased with age. The expression and activity reached levels equaling that of fully mature 9-week-old animals between 2 and 4 weeks after birth. In agreement with these observations in mice, extremely low levels of CES1 expression and activity were observed in two liver s9 samples derived from both a 13-day-old and 1-month-old human donor. The expression and activity of hCES1 in the age group <1 year were significantly lower than those of pooled samples. Our study also suggested a trend of hCES1 expression with different-aged groups. Similar to hCES1, hCES2 expression and activity were determined to be low in the age group <1 year, but this finding did not reach statistical significance compared with the pooled samples because of large interindividual variability. It is noted that the expression and activity of both hCES1 and hCES2 did not differ between elderly subjects (75–85 years) and pooled samples. Therefore, the data suggested that the expression and activity of CES1 and CES2 in both mice and humans are age-related with significantly low levels in very early developmental stages.
Varied CES1 and CES2 catalytic activity among different age groups could have significant influence on both PK and PD of the respective substrates of CES1 and CES2. Because of less than mature CES1 and CES2 function, younger subjects may be more likely to experience higher systemic exposure to the drugs than adults, resulting in undesirable pharmacological effects and/or toxicity. Oseltamivir, an antiviral prodrug, requires hydrolysis via CES1 to release its intended active metabolite, oseltamivir carboxylate (Shi et al., 2006; Zhu and Markowitz, 2009). Animal studies showed that the unweaned juvenile rats within the age range of 4 to 21 days did not hydrolyze oseltamivir efficiently during at least one toxicity assessment (http://www.fda.gov/cder/foi/nda/2000/21-246_Tamiflu_Pharmr.pdf). The systemic exposure to the prodrug after a single dose of oseltamivir was reportedly 10-fold higher than that in adult rats. The high systemic exposure to the prodrug could likewise be the cause of a 75% mortality rate observed in juvenile rats dosed with 1000 mg/kg/day oseltamivir, although this is a matter of speculation at present. Furthermore, an in vitro incubation study using s9 fractions of the marmoset liver indicated that the activation of oseltamivir was 5- to 10-fold slower in juveniles (6 weeks old and younger) of this species, as well as relative to adults (aged 3 months and older) (http://www.fda.gov/cder/foi/nda/2000/21-246_Tamiflu_Pharmr.pdf).
The correlation of the levels of hCES1 (A) and hCES2 (B) expression with the activity of MPH and irinotecan hydrolysis. The hydrolysis of MPH and irinotecan significantly correlated with the expression levels of hCES1 (p < 0.0001; r = 0.6694) and hCES2 (p = 0.0008; r = 0.5892), respectively.
During the process of preparing this report, an independent study led by Dr. Yan reported the patterns of hCES1 and hCES2 expression and activity in the liver tissues of three age groups: fetuses (82–224 gestation days), children (<10 years), and adults (Yang et al., 2009). The reverse transcription-quantitative polymerase chain reaction and Western blot analysis showed that the expression of hCES1 and hCES2 in adults was significantly higher than children, and the expression in children was significantly higher than fetuses, in both mRNA and protein levels. Consistent with the expression, highest catalytic activity of hCES1 and hCES2 was determined in adults, whereas the least activity was found within the fetus. Thus, these findings are in good agreement with our observations that the function of both hCES1 and hCES2 is age-related, and young children exhibit significant lower expression and activity relative to adults.
The mechanisms responsible for ontogenic expression of DMEs remain unclear. However, developmental profiles of DME expression and activity closely reflect that of major shifts in hormonal levels associated with growth and sexual maturation (Leeder and Kearns, 1997). Growth and/or sex hormones can potentially serve as biochemical regulators of DME expression through interaction with nuclear hormone receptors such as the pregnane X receptor and/or the constitutive androstane receptor (Kennedy, 2008). Hosokawa and Satoh (1988) reported that the expression of carboxylesterase isoenzymes RL1 and RH1 in the liver of hypophysectomized rats was significantly decreased after the treatment of HGH. In the present study, we investigated the effects of HGH on mCES1 expression and activity in normal mice. No significant differences of the expression and activity of mCES1 in the liver were observed between control animals and the mice administered HGH at a dose of 5 mg/kg/day for 1 and 9 days. Thus, the impaired expression of hCES1 and hCES2 during early developmental stages was unlikely to be related to high growth hormone levels in youths.
Sex-related differences in the activity of DMEs have been documented in humans previously. For example, CYP2E1 and CYP1A2 activity are higher in males than in females, whereas CYP3A4 is more abundant in females than males (Scandlyn et al., 2008). It has been reported that the mean area under the plasma concentration versus time curve of d-MPH was significantly higher in males than females after oral dosing of 0.3 mg/kg dl-MPH, a selective CES1 substrate (Patrick et al., 2007), which raised the question whether CES1 activity differs between sexes. Accordingly, we investigated the expression and activity of mCES1 and mCES2 in sexually mature male and female mice. The results showed that both expression and activity in males were comparable with that in females. In addition, the study found that the randomly selected female mice exhibited very consistent mCES1 and mCES2 expression and activity, indicating the expression of the enzymes in females may not be related to estrous cycle.
Consistent with other reports, remarkable interindividual variability of the expression and activity of hCES1 and hCES2 was observed within similar age ranges (Jewell et al., 2007; Takahashi et al., 2008; Yang et al., 2009). This variability may reflect the collected influence of multiple factors that may modulate CES expression and activity. First, genetic polymorphisms are relatively common within CES1 and CES2 genes, although few have been rigorously studied in terms of their metabolic consequences. Nevertheless, genetic polymorphisms have the potential to lead to significant alteration of both expression and activity of these enzymes. It was recently reported that hCES1 catalytic activity was dramatically impaired in the two natural hCES1 variants G143E and D260fs (Zhu et al., 2008). In addition, mutations located in the promoter regions could result in significant changes of the transcription efficiency of CES1 and CES2 genes (Geshi et al., 2005; Bellott et al., 2008). Second, because most of the liver donors were under medical care, the large interindividual variability of hCES1 and hCES2 could be partially the result of various therapeutic agents the liver samples were exposed to before donation. Finally, pathophysiological conditions such as inflammation are often associated with dramatic alterations of DME expression, as well as PK and PD of many therapeutic drugs (Morgan et al., 2008; Morgan, 2009). In particular, the expression of both hCES1 and hCES2 in primary hepatocytes and HepG2 cells was remarkably suppressed after the treatment of proinflammatory cytokine interleukin 6 (Yang et al., 2007).
In conclusion, the expression of CES1 and CES2 is age-dependent. The enzymatic function appears to be immature in young mice and children within early developmental stages. Both expression and activity remain unchanged in elderly subjects within the ages of 75 to 85 years when compared with pooled samples. In addition, our preliminary studies suggest that lower levels of CES1 and CES2 observed in youth do not seem to be directly related to growth hormone levels. The animal study revealed similar expression levels of mCES1 and mCES2 between sexes. However, potential sex-related differences in humans cannot be excluded because of the possibility of species-specific regulation of CES1 and CES2 expression between humans and rodents, as well as potentially different responses to exogenously administered HGH.
Footnotes
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.109.028209.
-
ABBREVIATIONS: CES, carboxylesterase; MPH, methylphenidate; DMEs, drug-metabolizing enzymes; P450, cytochrome P450; hCES, human carboxylesterase; PK, pharmacokinetics; PD, pharmacodynamics; HGH, human growth hormone; mCES, mouse carboxylesterase.
- Accepted May 27, 2009.
- Received April 22, 2009.
- The American Society for Pharmacology and Experimental Therapeutics