Abstract
The absorption characteristics of temocapril were investigated using Caco-2 cells, and the esterases expressed in Caco-2 cells were identified. Temocapril was almost completely hydrolyzed to temocaprilat during transport across Caco-2 cells. Hydrolysis experiments of temocapril in Caco-2 cell 9000g supernatant (S9) and brush-border membrane vesicles showed that temocapril was mainly hydrolyzed within the cells after uptake, after which the temocaprilat formed was transported to both the apical and basolateral surfaces. In native polyacrylamide gel electrophoresis by detection of hydrolase activity for 1-naphthylbutyrate, Caco-2 cell S9 showed a band with high esterase activity and another band with extremely low activity. The proteins in the major and minor bands were identified as carboxylesterase-1 (hCE-1) and carboxylesterase-2 (hCE-2). The abundant expression of hCE-1 in Caco-2 cells was supported by reverse transcription-polymerase chain reaction. In the normal human small intestine, hCE-2 is abundantly present, although the human liver expresses much higher levels of hCE-1 and lower levels of hCE-2. The expression pattern of carboxylesterases in Caco-2 cells is completely different from that in human small intestine but very similar to that in human liver. Since the substrate specificity of hCE-1 differs from that of hCE-2, it is suggested that the prediction of human intestinal absorption using Caco-2 cell monolayers should be performed carefully in the case of ester- and amide-containing drugs such as prodrugs.
Recently, reports in the literature have presented an increasingly large body of evidence that the degradation of a variety of drugs in the intestinal epithelium may limit their oral absorption. The effect of P450 isoforms on intestinal absorption is well documented; it has been shown that CYP3A4 in intestinal mucosal cells is responsible for decreased oral absorption (Paine et al., 1997; Lin et al., 1999; Zhang et al., 1999). Similarly, the absorption of ester-containing drugs is limited by esterase (Okudaira et al., 2000; Ruiz-Balaguer et al., 2002), and more specifically, carboxylesterases (CESs; E.C. 3.1.1.1).
Various CESs are present in a variety of organs and tissues of mammalian species (Satoh and Hosokawa, 1998). CESs catalyze the hydrolysis of a variety of ester-containing and amide-containing endogenous compounds to their respective free acids such as acyl-CoA and acylcarnitine. CESs also play important roles in the inactivation of a variety of structurally diverse drugs and the activation of prodrugs. The mammalian CESs comprise a multigene family, and the isozymes are classified into four main CES groups and several subgroups. The major human liver isozymes hCE-1 (GI:119576) and hCE-2 (GI: 37622885) belong to classes CES1 and CES2, respectively. The CES expressed in human small intestine is mainly hCE-2 (Satoh et al., 2002). Therefore, the hydrolysis activity of hCE-2 affects the absorption of ester-containing and amide-containing drugs such as prodrugs that are known to enhance the membrane permeability and transepithelial transport of hydrophilic drugs by increasing the lipophilicity of their parent compounds (Taylor, 1996).
Caco-2 cells, derived from a colon adenocarcinoma, spontaneously differentiate under defined culture conditions to exhibit the structural and functional characteristics of mature human enterocytes. Therefore, Caco-2 cells have been widely accepted as a most useful in vitro model for rapid screening of intestinal drug absorption (Artursson et al., 2001). The fraction of a dose absorbed in vivo correlates significantly with the permeability of passively transported compounds across a Caco-2 cell monolayer (Lennernäs, 1998). Caco-2 cells express several drug-metabolizing enzymes and transporters that are present in the human enterocyte. However, the Caco-2 cell has thus far fallen short as an ideal model for predicting oral availability of first-pass metabolized drugs in the intestine because of its failure to express substantial amounts of metabolic enzymes such as cytochrome P450 isozymes (Prueksaritanont et al., 1996; Nakamura et al., 2002). In addition, the Caco-2 cell line shows markedly lower esterase activity than the human small intestine (Yoshigae et al., 1998). The CES isoform in the Caco-2 cell line has not been identified, although hCE-2 is present in the human intestine. Therefore, it is doubtful whether the Caco-2 cell line is suitable for predicting the oral absorption of prodrugs.
In the present study, the hydrolyzing capacity of Caco-2 cells was evaluated using two substrates, temocapril and p-nitrophenylacetate (PNPA). Temocapril is a good and a poor substrate for hCE-1 and hCE-2, respectively (Takai et al., 1997). PNPA is a good substrate for several CES isozymes and other hydrolases. Temocapril was nearly completely hydrolyzed during transport from apical to basolateral through Caco-2 cell monolayer. The hydrolysis of temocapril was attributed to the cytoplasm of Caco-2 cells rather than brush-border membrane vesicles (BBMVs). It was demonstrated that the culture period of Caco-2 cell hardly affected the hydrolysis of temocapril and PNPA. Furthermore, the major CES isoform expressed in Caco-2 cells was determined to be hCE-1 by native polyacrylamide gel electrophoresis (PAGE) and the reverse transcription-polymerase chain reaction (RT-PCR), although hCE-2 was abundantly present in the human intestine. Finally, the difficulty in accurately predicting the intestinal absorption of prodrugs using the Caco-2 cell line was assessed.
Materials and Methods
Materials. Caco-2 cells were purchased from American Type Culture Collection (Manassas, VA). Human liver and intestine microsomes were obtained from BD Gentest (Woburn, MA) and Tissue Transformation Technologies (Edison, NJ). Dulbecco's modified Eagle's medium, 0.25% trypsin-EDTA, Dulbecco's phosphate-buffered saline (PBS), and Hanks' balanced salt solution (HBSS) were purchased from Sigma (St. Louis, MO). Nonessential amino acids, l-glutamine, and penicillin-streptomycin were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum was purchased from Cansera International Inc. (Rexdale, ON, Canada). Temocapril and temocaprilat were provided by Sankyo Co., Ltd. (Tokyo, Japan). p-Nitrophenol and PNPA were purchased from Nacalai Tesque (Kyoto, Japan). Human liver total RNA (lot 2041228) and intestine total RNA (lot 2060019) were purchased from BD Biosciences Clontech (Palo Alto, CA) and colon total RNA (lot 0620271) was purchased from Stratagene (La Jolla, CA). All other chemicals and reagents were of analytical grade.
Cell Culture. Caco-2 cells were grown in 75-cm2 culture flasks in culture medium consisting of Dulbecco's modified Eagle's medium with 10% heat-inactivated fetal bovine serum, 0.1 mM nonessential amino acids, 2 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin in an atmosphere of 95% air and 5% CO2 at 37°C. Before reaching confluence, the cells were trypsinized with 0.25% trypsin and 0.53 mM EDTA and plated at a density of 8.0 × 104 cells/cm2 in culture medium on a polycarbonate membrane (Transwell, 3-μm pore size, 24.5 mm in diameter; Costar, Cambridge, MA). Culture medium was replaced [1.5 ml on the apical side (AP) and 2.6 ml on the basolateral (BL) side] every other day for the first week and daily thereafter. After seeding, the cells were cultured for 21 to 28 days to allow them to fully differentiate into confluent enterocyte-like monolayers. Transepithelial electrical resistance was measured using a Millicell ERS ohmmeter (Millipore Corporation, Billerica, MA). All cell monolayers in these studies showed transepithelial electrical resistance values ranging from 800 to 1100 ohm·cm2. The Caco-2 cells used in this study were between passages 27 and 59.
Preparation of Caco-2 Cell 9000g Supernatant (S9). Caco-2 cells cultured for 7 days in 75-cm2 culture flasks and cells cultured for 21 days on Transwell were washed with ice-cold PBS and then removed with a cell scraper. The cells were suspended in SET buffer (0.29 M sucrose, 1 mM EDTA, and 50 mM Tris) and then sonicated with a Sonifier cell disruptor (Branson, Danbury, CT). The sonicated cells were then homogenized in a Potter-Elvehjem glass homogenizer with a Teflon pestle under ice-cold conditions. After centrifugation of the cell homogenate at 9000g for 20 min at 4°C, the supernatant (S9) was obtained. Protein content was determined by the method described by Bradford (1976) with bovine serum albumin used as the standard.
Preparation of BBMVs. BBMVs were isolated using the divalent cation precipitation method described by Kessler et al. (1978) with minor modifications. Caco-2 cells cultured in 75-cm2 flasks were washed with ice-cold PBS and then removed with a cell scraper. The cells were homogenized in buffer A (2 mM Tris and 50 mM mannitol, pH 7.1) with a Waring Blender for 5 min at maximum speed. An aliquot of the homogenate was saved for protein and marker enzyme assays. The entire procedure was carried out on ice or at 4°C.
A 1/100 volume of 1 M CaCl2 was added to the homogenate (final concentration 10 mM), which was then stirred and allowed to stand for 15 min on ice. The homogenate was centrifuged at 3000g for 15 min at 4°C. The supernatant was centrifuged at 27,000g for 30 min at 4°C. The pellet was resuspended in 0.5 ml of buffer B (250 mM mannitol and 10 mM Tris-HCl, pH 7.4) using a syringe with a 25-gauge needle (20 strokes) and homogenized in a Potter-Elvehjem glass homogenizer with a Teflon pestle. The suspension was centrifuged at 27,000g for 30 min at 4°C. The pellet was resuspended in 0.3 ml of buffer B using a syringe with a 25-gauge needle (20 strokes). The purity of the obtained BBMVs was estimated by using the specific activity of alkaline phosphatase as a marker enzyme for the apical brush-border membrane. The enrichment factor relative to that in the crude homogenate was approximately 11.
Hydrolysis Experiments. Hydrolysis of PNPA and temocapril was performed in human liver microsomes, intestine microsomes, and S9 and BBMVs of Caco-2 cells. The biological samples were diluted with 50 mM HEPES, pH 7.4, buffer at an appropriate protein concentration and preincubated for 5 min at 37°C. For the hydrolysis of PNPA, the reaction was initiated by the addition of PNPA (final concentration: 25–500 μM), and the p-nitrophenol formed was spectrophotometrically determined at 405 nm (V-530; Jasco, Tokyo, Japan).
For the hydrolysis of temocapril, the reaction was initiated by the addition of temocapril dissolved in dimethyl sulfoxide (final concentration 50–500 μM) after preincubation of the biological samples at 37°C for 5 min. At an appropriate time, 200 μl of ice-cold acetonitrile was added into the reaction sample (200 μl) to terminate the reaction. After centrifugation of the reaction mixture at 1600g for 10 min, the supernatant (100 μl) was added to 2% H3PO4 (100 μl) and analyzed by HPLC. Degradation of temocapril was not observed by this quantitative procedure. The final concentration of dimethyl sulfoxide was less than 1%, which has no effect on hydrolase activity.
Transport Experiments. Caco-2 cell monolayers were gently washed with HBSS, followed by preincubation of both the apical and basolateral sides for 30 min with HBSS. After preincubation, temocapril or temocaprilat solution (100 μM in HBSS) was added to the apical compartment (1.5 ml) of the cell monolayer and HBSS was added to the basolateral compartment (2.6 ml). The cell monolayers were incubated at 37°C. Samples (190 μl on the receiver side and 10 μl on the donor side) were withdrawn at various times up to 120 min. The volume removed from the receiver side was always replaced with fresh HBSS. Samples were stabilized by the addition of 10 μl of 20% H3PO4 and analyzed by HPLC.
Apparent permeability coefficients (Papp) were calculated using the following equation: Papp = dQ/dt/A/C0 (square centimeters per second), where dQ/dt is the rate of appearance of drugs in the basolateral compartment (steady-state flux, micromoles per second), A is the surface area of the monolayer (i.e., 4.71 cm2), and C0 is the initial concentration (micromolar) in the donor compartment.
HPLC Analysis. The HPLC system was comprised of a Jasco 880-PU pump, a Jasco 875-UV detector, a Jasco autosampler, a Jasco column oven, and a Shimadzu Chromatopack C-R7A (Shimadzu, Kyoto, Japan). An aliquot of the sample was injected onto a Mightysil RP-18 column (5 μm, 4.6 i.d. × 250 mm; Cica Merk Co., Tokyo, Japan) and eluted at a flow rate of 0.8 ml/min with 0.2% H3PO4/acetonitrile [3:7 (v/v), solvent A] and 0.2% H3PO4 (solvent B) according to the following gradient schedule: 45% solvent A for the first 10 min, a linear gradient from 45 to 100% solvent A over the next 10 min, and 100% solvent A for 5 min. The temperature of the column was maintained at 40°C. The elution times of temocapril and temocaprilat were 17.6 and 6.4 min, respectively. UV detection was performed at 258 nm, and the detection limit for both temocapril and temocaprilat was 100 nM.
RT-PCR. Caco-2 cell pellets were prepared 7 days after seeding at 3.0 × 105 cells in 15 ml in 75-cm2 flasks. Total RNA was extracted from the Caco-2 cells using ISOGEN (Nippon Gene, Toyama, Japan). The RNA concentration and purity were determined using a spectrophotometer. Five micrograms of total RNA was reverse-transcribed using 25 pmol of oligo(dT) primer, 1 mM dNTP, and RNase H free ReverTra Ace (Toyobo, Osaka, Japan) with one cycle of the reverse transcription reaction (42°C for 50 min). After digestion of the remaining RNA with RNase H, the reverse transcription samples were subjected to subsequent PCR reaction. PCR was performed with TaKaRa Ex Taq (Takara Bio Inc., Otsu, Japan). The PCR conditions and the sequences of the forward and reverse primers were as follows.
For hCE-1, PCR conditions were 25 cycles at 95°C for 15 s, 54°C for 30 s, and 72°C for 45 s; forward primer: 5′-TCCCCTTGTTTGCATTGCTA (nucleotides 1155–1174); and reverse primer: 5′-AAAGAGGTTGGTCCAGAAAG (nucleotides 1650–1631).
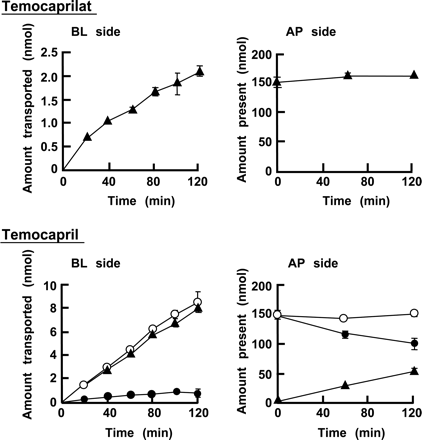
Apical (AP)-to-basolateral (BL) transport of temocaprilat and temocapril through a Caco-2 cell monolayer. Temocaprilat and temocapril were applied to the apical side at concentration of 100 μM. Values are mean ± S.D. (n = 3). ○, total; •, temocapril; ▴, temocaprilat.
For hCE-2, PCR conditions were 25 cycles at 95°C for 15 s, 56°C for 30 s, and 72°C for 45 s; forward primer: 5′-AGCCTGTCCCTAGCATTGTT (nucleotides 998-1017); and reverse primer: 5′-GCCCCCAAAGAAACTTCTGA (nucleotides 1413–1394).
For glyceraldehyde-3-phosphate dehydrogenase, PCR conditions were 25 cycles at 95°C for 15 s, 58°C for 30 s, and 72°C for 45 s; forward primer: 5′-ACCACAGTCCATGCCATCAC (nucleotides 526–545); and reverse primer: 5′-TCCACCACCCTGTTGCTGTA (nucleotides 977–958).
Amplified PCR products were separated on 1.5% agarose gel and stained with ethidium bromide.
Polyacrylamide Gel Electrophoresis. PAGE was performed as described by Mentlein et al. (1980). Polyacrylamide gels [7.5% (w/w)] containing 1% (w/v) Nonidet P-40 for solubilization of proteins were used for the separation of native enzymes. After electrophoresis of the microsomes and S9 samples (5–15 μg of protein), the gels were stained for esterase activity with 1-naphtylbutyrate through coupling to liberated 1-naphthol with Fast Red TR-salt.
Results
Transport of Temocapril and Temocaprilat through a Caco-2 Cell Monolayer. Apical-to-basolateral transport of temocaprilat and temocapril through the Caco-2 cell monolayer is shown in Fig. 1. The Papp values from apical-to-basolateral were 5.68 ± 0.81 × 10-7 and 27.7 ± 1.03 × 10-7 cm/s for temocaprilat and temocapril, respectively. When temocapril was applied to the apical side, the Papp of temocapril was calculated from the total concentrations of temocapril and temocaprilat on the receiver side. The Papp of temocapril was about 5-fold greater than that of temocaprilat. This higher Papp of temocapril is supported by the partition coefficients between n-octanol and pH 7.4 phosphate buffer; the log P values are -0.1 and -2.5 for temocapril and temocaprilat, respectively (Shionoiri et al., 2001).
When temocapril was applied to the apical side, interestingly, the transported compound in the basolateral compartment was mainly temocaprilat rather than temocapril. This finding suggests that temocapril was hydrolyzed to temocaprilat during transport across the Caco-2 cell. Furthermore, temocaprilat was observed not only in the basolateral compartment but also on the apical side. The amounts of temocapril and temocaprilat after a 2-h transport experiment are shown in Table 1. The donor (apical) side contained 50 nmol of hydrolyzed temocaprilat, which was much greater than the amount transported (7.94 nmol) to the receiver (basolateral) compartment. These findings suggest that the presence of temocaprilat in the apical and basolateral compartments was due to the hydrolysis of temocapril by an enzyme present in the cellular membrane and/or cytoplasm of Caco-2 cells, because temocapril was scarcely hydrolyzed in pH 7.4 HBSS (percentage of hydrolysis of temocapril in pH 7.4 HBSS for 2 h is less than 0.1%).
Amounts of temocapril and temocaprilat in each compartment after apical-to-basolateral transport of temocapril for 2 h through a Caco-2 monolayer After 2 h of apical-to-basolateral transport experiment of temocapril, the amounts of temocapril and temoaprilat in apical and basolateral compartments were determined. Values are mean ± S.D. (n = 3).
Hydrolase Activity in Caco-2 Cells. To estimate the distribution of hydrolase activity in Caco-2 cells, the hydrolysis of temocapril and PNPA was measured in Caco-2 cell S9 and BBMVs. Table 2 lists the hydrolytic parameters for PNPA and temocapril in Caco-2 cell S9 and BBMVs. Since the hydrolysis in Caco-2 cell BBMVs was extremely low, its enzymatic parameters could not be determined. Temocapril, a good substrate for hCE-1, was hydrolyzed by BBMVs in the range of 1 to 2% of its hydrolysis in S9. However, PNPA, a good substrate for several CES isozymes and other hydrolases, was hydrolyzed in BBMVs. These data suggest that the hydrolysis of temocapril during transport across the Caco-2 cell monolayer is associated with an enzyme present in S9 rather than BBMVs.
Hydrolysis of temocapril and PNPA in Caco-2 cell BBMVs and S9 The hydrolysis rate (ν) of temocapril and PNPA was measured at substrate concentrations of 250 and 500 μM, respectively.
The Km values of PNPA and temocapril in S9 of Caco-2 cells cultured in a flask for 7 days were nearly same as those for passage numbers 28 to 59 and were also similar to those for Caco-2 cell S9 cultured on Transwell for 21 days. Moreover, each Vmax value for temocapril and PNPA was nearly same among all S9 samples except for Vmax of PNPA in cells cultured for 21 days. These findings indicate that the hydrolase species expressed in Caco-2 cells are only slightly affected by the culture conditions used in this study, although the expression level of hydrolases contributed to PNPA hydrolysis is affected.
Furthermore, the hydrolytic parameters of PNPA and temocapril in Caco-2 cell S9 were compared those in the human liver and small intestine microsomes. The Km value of PNPA in Caco-2 cell S9 was about 2-fold higher than that in the human liver and small intestine microsomes. The Vmax was more than 10-fold lower in Caco-2 cell S9 than in human tissue. In contrast, the Km value of temocapril was almost the same in human liver microsomes and Caco-2 cell S9, although the Km value in small intestine microsomes could not be measured due to the extremely low hydrolase activity. The Vmax value of temocapril in Caco-2 cell S9 was much lower than that in human liver due to the low expression of esterase in Caco-2 cells.
Expression of Carboxylesterase in Caco-2 Cells and Human Tissues. RT-PCR and esterase activity staining after nondenaturing PAGE were performed to demonstrate the expression of CES mRNA and its protein, respectively. As shown in Fig. 2, PCR using hCE-1 gene-specific primers showed that hCE-1 mRNA expression was present in the liver and Caco-2 cells and slightly present in the small intestine and colon under conditions of nearly equal expression of glyceraldehyde-3-phosphate dehydrogenase mRNA in all samples. Expression of hCE-2 mRNA was detected in the small intestine and colon in addition to the liver. However, expression of hCE-2 mRNA was hardly detected in Caco-2 cells.
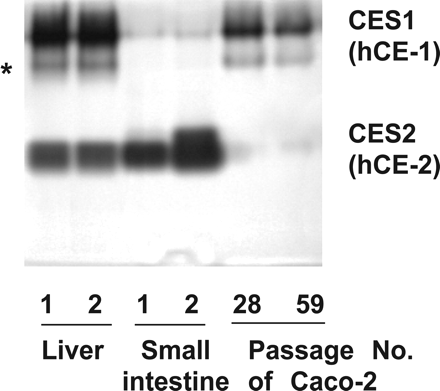
In accordance with the results of RT-PCR, human liver microsomes showed two electrophoretically distinct esterases with activity toward 1-naphthylbutyrate (Fig. 3). The upper and lower bands revealed the presence of hCE-1 protein and hCE-2 protein, respectively. An accessory band was observed at a migration point faster than that of hCE-1 protein, which is thought to be a monomeric form of hCE-1 (Satoh et al., 2002). Small intestine microsomes showed mainly expression of hCE-2 protein, whereas S9 of Caco-2 cells at passage numbers 28 and 59 showed mainly expression of hCE-1 protein. Caco-2 cells cultured in two other laboratories also showed high expression of hCE-1 and quite low expression of hCE-2 (data not shown). The human samples obtained from two different companies showed the same expression pattern of CES proteins in both the liver and small intestine. Interestingly, the pattern of esterase activity in Caco-2 cell S9, an in vitro model of human enterocytes, was similar to that in liver microsomes rather than that in the small intestine.
Discussion
The present study has demonstrated that Caco-2 cells express hydrolyzing enzymes that are responsible for the almost complete hydrolysis of temocapril during transport, despite the remarkably low level of hydrolysis of temocapril in human small intestine microsomes. The hydrolyzed product of temocapril, temocaprilat, was observed not only in the basolateral compartment but also in the apical compartment. The presence of a large amount of temocaprilat in the apical compartment suggests that hydrolysis of temocapril occurs at the surface of the apical membrane. However, the amount of temocaprilat transported to the basolateral side was found to be 4-fold greater in the experiment in which temocapril was applied to the apical compartment, compared with when temocaprilat itself was applied in an initial amount of 150 nmol (Fig. 1). In addition, the hydrolase activity of Caco-2 BBMVs was markedly lower than that of S9, which comprises most of the cellular volume. In particular, the contribution of the hydrolase activity of S9 to the overall hydrolysis of temocapril in Caco-2 cells was much greater than cells BBMVs, in contrast to the findings for PNPA (Table 2). Therefore, it seems that temocapril is abundantly hydrolyzed to temocaprilat in the cytoplasm after its uptake into Caco-2 cells, followed by the efflux of temocaprilat to the apical and basolateral sides. Temocaprilat, a hydrolyzed product, is theoretically present at the highest concentration within the cell compared with the apical and basolateral compartments. Therefore, the temocaprilat can diffuse into both the apical and basolateral sides at the same concentration. However, the concentration of temocaprilat in the apical compartment (33.3 μM) was found to be 10-fold higher than that in the basolateral compartment (3.05 μM). It has been reported that temocaprilat is a good substrate of multidrug resistance protein 2, which is located on the apical membrane of Caco-2 cells (Ishizuka et al., 1997; Taipalensuu et al., 2001; Kusuhara and Sugiyama, 2002). Therefore, the temocaprilat generated from temocapril within Caco-2 cells may be preferentially transported by multidrug resistance protein 2 into the apical compartment rather than the basolateral compartment. Further experiments are necessary to clarify the detail mechanism of efflux transport of temocaprilat in Caco-2 cell monolayers.
RT-PCR analysis of hCE-1 and hCE-2 mRNA in human tissue and Caco-2 cells. The product of RT-PCR was electrophoresed in 1.5% agarose gel and stained with ethidium bromide. In all reactions, a single band of precise length deduced from the primers was detected. It was confirmed by further amplification that all PCR products in this figure were not saturated (data not shown).
Polyacrylamide gel electrophoresis of liver and small intestine microsomes (5 μg of protein) and Caco-2 cell S9 (15 μg of protein), followed by staining for esterase activity using 1-naphthylbutyrate. 1, microsomes from GENTEST. 2, microsomes from TTT. *, monomeric form of hCE-1.
To demonstrate the presence of hydrolyzing enzymes in Caco-2 cell S9, native PAGE has been performed with the detection of hydrolase activity using 1-naphthylbutyrate as the substrate. Interestingly, native PAGE of Caco-2 cell S9 showed a major enzyme that was identified as hCE-1. The abundant expression of hCE-1 rather than hCE-2 in Caco-2 cells was supported by the results of RT-PCR (Fig. 2). In general, the human small intestine and colon express abundant quantities of hCE-2 (Khanna et al., 2000; Satoh et al., 2002). Schwer et al. (1997) have reported that the higher expression in the small intestine is mainly attributable to high expression in the jejunum, compared with moderate expression in the duodenum and low expression in the ileum.
The findings of the present study indicate high temocapril hydrolase activity in human liver microsomes and extremely low activity in small intestine microsomes. These data are in agreement with reports that temocapril can be a good substrate for hCE-1 (Takai et al., 1997; Mori et al., 1999). Furthermore, Caco-2 cell S9 exhibits high temocapril hydrolase activity, with the same Km value as human liver microsomes. It is thought that the almost complete hydrolysis of temocapril during transport across a Caco-2 cell monolayer is due to the expression of hCE-1. If it is assumed that temocapril is not metabolized to temocaprilat in Caco-2 cells like in human small intestine, a greater permeability of temocapril across the Caco-2 cell monolayer may be obtained and rapid human intestinal absorption can be predicted. Therefore, prediction of the human intestinal absorption of ester- and amide-containing drugs based on the transport results obtained using a Caco-2 cell monolayer should be performed with care.
Although hCE-1 and hCE-2 are both members of the 60-kDa serine esterase superfamily, they differ substantially. The sequence homology between these two enzymes is only 48% (Pindel et al., 1997). hCE-1 is an Mr 180,000 trimer with an isoelectric point of 5.8 (Pindel et al., 1997), whereas hCE-2 is a monomer with an isoelectric point of 4.9 (Brzezinski et al., 1994). Substantial differences in substrate specificity also exist between the two isoforms. For example, hCE-1 hydrolyzes the methyl ester of cocaine, whereas hCE-2 hydrolyzes the benzoyl ester (Brzezinski et al., 1994; Pindel et al., 1997). In addition, hCE-1, but not hCE-2, hydrolyzes the ethyl ester of meperidine and delapril. In contrast, hCE-2 hydrolyzes aspirin and procaine, whereas hCE-1 does not (Takai et al., 1997). The activation of irinotecan to 7-ethyl-10-hydroxycamptothecin (SN38) is catalyzed by hCE-2 with greater affinity and higher velocity compared with hCE-1 (Humerickhouse et al., 2000). The differences in the substrate specificity of hCE-1 and hCE-2 may lead to erroneous results when predicting intestinal absorption based on the transport data obtained using a Caco-2 cell monolayer.
It is difficult to understand why Caco-2 cells express hCE-1 in a manner similar to the human liver, given the fact that these cells are derived from a colon adenocarcinoma. Sanghani et al. (2003) studied the expression of hCE-1 and hCE-2 by Northern blotting and RT-PCR in the normal colon and colon tumors. The expression of hCE-2 was much higher than that of hCE-1, and both proteins were similarly expressed in paired tumors and normal colon tissue. Xie et al. (2002) also reported higher expression of hCE-2 than hCE-1 in the normal colon and colon tumors, with greater expression of both proteins in adjacent normal colon tissue than in adenocarcinoma. It is clear that hCE-2 is an abundant protein in the colon tissue, although the expression level differs between the normal colon and colon tumors. Furthermore, Pavillard et al. (2002) demonstrated the expression of CES in colon cell lines by RT-PCR. The expression of hCE-1 is higher in HT-29 cells than in LoVo cells, whereas the expression of hCE-2 is very similar in both cell lines and always much higher than the expression of hCE-1. The expression pattern of CES in Caco-2 cells is very different from that in colon tissue and other colon cell lines.
In summary, Caco-2 cells show high levels of hydrolase activity that can nearly completely hydrolyze temocapril during transport. The major hydrolyzing enzyme is hCE-1, which is abundantly expressed in the human liver, in contrast to the abundant expression of hCE-2 in the human small intestine and colon. Since the substrate specificity of hCE-1 differs from that of hCE-2, it is suggested that the prediction of human intestinal absorption based on the results obtained using a Caco-2 cell monolayer should be performed with care for ester- and amide-containing drugs such as prodrugs.
Footnotes
-
This work was supported in part by Grant-in-Aid for Scientific Research 16590085 from the Japan Society for the Promotion of Science (to T.I.) and a Research Grant for Young Scientists from the KAO Foundation for Arts and Sciences (to M.H.).
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.105.004226.
-
ABBREVIATIONS: CES, carboxylesterase; PNPA, p-nitrophenylacetate; hCE-1, human carboxylesterase-1; hCE-2, human carboxylesterase-2; BBMV, brush-border membrane vesicle; PAGE, polyacrylamide gel electrophoresis; RT-PCR, reverse transcription-polymerase chain reaction; AP, apical; BL, basolateral; PBS, phosphate-buffered saline; HBSS, Hanks' balanced salt solution; HPLC, high-performance liquid chromatography.
- Received February 11, 2005.
- Accepted May 18, 2005.
- The American Society for Pharmacology and Experimental Therapeutics