Abstract
Over the last decade HepaRG cells have emerged as a promising alternative to primary human hepatocytes (PHH) and have been featured in over 300 research publications. Most of these reports employed freshly differentiated HepaRG cells that require time-consuming culture (∼28 days) for full differentiation. Recently, a cryopreserved, predifferentiated format of HepaRG cells (termed here “cryo-HepaRG”) has emerged as a new model that improves global availability and experimental flexibility; however, it is largely unknown whether HepaRG cells in this format fully retain their hepatic characteristics. Therefore, we systematically investigated the hepatocyte functionality of cryo-HepaRG cultures in context with the range of interindividual variation observed with PHH in both sandwich-culture and suspension formats. These evaluations uncovered a novel adaptation period for the cryo-HepaRG format and demonstrated the impact of extracellular matrix on cryo-HepaRG functionality. Pharmacologically important drug-metabolizing alleles were genotyped in HepaRG cells and poor metabolizer alleles for CYP2D6, CYP2C9, and CYP3A5 were identified and consistent with higher frequency alleles found in individuals of Caucasian decent. We observed liver enzyme inducibility with aryl hydrocarbon receptor, constitutive androstane receptor (CAR), and pregnane X receptor activators comparable to that of sandwich-cultured PHH. Finally, we show for the first time that cryo-HepaRG supports proper CAR cytosolic sequestration and translocation to hepatocyte nuclei in response to phenobarbital treatment. Taken together, these data reveal important considerations for the use of this cell model and demonstrate that cryo-HepaRG are suitable for metabolism and toxicology screening.
Introduction
The liver is a major organ involved in the detoxification of both endobiotic and xenobiotic chemicals. Primary human hepatocytes (PHH) are a well accepted in vitro liver model for prediction of drug metabolism and toxicity, owing to their proper maintenance of metabolism, transport, and receptor signaling pathways. However, the pronounced interindividual variability and high cost of PHH has led to the emergence of alternative cell models, such as the hepatoma-derived HepG2 and the immortalized Fa2N-4 for screening purposes. To date, these immortalized models have been associated with insufficient hepatocyte differentiation and low metabolic functionality (Hariparsad et al., 2008; Donato et al., 2010).
In recent years, freshly differentiated HepaRG cells have emerged as a promising alternative to PHH for in vitro drug-drug interaction and toxicology studies. To reach phenotypic maturity, HepaRG cells grow to confluence and differentiate over 4 weeks (from progenitor cells) into cocultures of hepatocyte-like and cholangiocyte-like cells (Gripon et al., 2002). Since this model was discovered, many studies have shown that freshly differentiated HepaRG cultures exhibit cellular interactions, drug metabolism/transport, and drug induction responsiveness comparable to PHH cultures (Grime et al., 2010; McGill et al., 2011; Gerets et al., 2012; Le Vee et al., 2013; Szabo et al., 2013). A cryopreserved format of differentiated HepaRG cells (cryo-HepaRG) has recently become available, improving the global availability and experimental flexibility of this model. However, the impact of detachment, cryopreservation, and replating on HepaRG function has not been comprehensively evaluated. It is known that disruption of cellular interactions during liver isolations results in PHH dedifferentiation (Godoy et al., 2013). Therefore, it is important to understand the consequences of detachment/reattachment for cryo-HepaRG. To date, the effect of culture time on cryo-HepaRG metabolic competence (postreattachment to monolayers), liver enzyme induction, and uptake transport has not been characterized or compared with interindividual variation across large numbers of sandwich-cultured primary human hepatocytes (SC-PHH) and suspensions of PHH. Finally, no immortalized-liver-cell-line alternative to PHH has been found to properly model the constitutive androstane receptor (CAR) activation pathway by which CAR is sequestered in the cytosol of hepatocytes and translocates to the nucleus upon activation by phenobarbital, a hallmark feature of functional PHH.
In the current study, we evaluated cryo-HepaRG and found them to resemble freshly differentiated HepaRG after 7–10 days in culture. We observed bile canaliculi formation over time, a hallmark of hepatocyte polarization operative in PHH cultures, with morphologies (i.e., cords of hepatocyte-like cells) stabilizing after 7–10 days in culture. We monitored the temporal dynamics of metabolic competence in cultured cryo-HepaRG and observed an adaptation period with an initial loss of metabolic competence that was restored to suspension cryo-HepaRG levels after 7–10 days in culture. Metabolic activities, liver enzyme induction, and uptake/efflux transport in cryo-HepaRG were compared with numerous lots of SC-PHH and suspension PHH to provide a broader context for cryo-HepaRG functionality. Our results reveal the impact of extracellular matrix overlay on cryo-HepaRG functionality, provide genotyping analysis of pharmacologically important poor metabolizer alleles, and demonstrate that cryo-HepaRG can properly sequester CAR in the cytosol and translocate it to the nucleus upon phenobarbital treatment.
Materials and Methods
Materials.
Cryo-HepaRG, William’s E Media (WEM), 96-well collagen I-coated plates, GlutaMAX Supplement, HPRG770, HPRG720, and HPRG740 media supplements, Cryopreserved Hepatocyte Recovery Media, Geltrex Matrix, Carboxy Dichlorofluorescein Diacetate (CDFDA), and PHH were obtained from Life Technologies/Thermo Fisher Scientific (Carlsbad, CA). Serum-free hepatocyte culture supplement ITS+ was obtained from BD Biosciences (San Jose, CA). Phenacetin, acetaminophen, coumarin, 7-hydroxycoumarin, bupropion, hydroxybupropion, paclitaxel, 6α-hydroxypaclitaxel, diclofenac, 4′-hydroxydiclofenac, mephenytoin, 4′-hydroxymephenytoin, dextromethorphan, dextrorphan, testosterone, 6β-hydroxytestosterone, midazolam, 1-hydroxymidazolam, benzydamine, benzydamine N-oxide, 7-hydroxycoumarin glucuronide and sulfate, omeprazole, phenobarbital (PB), rifampicin (RIF), dimethyl sulfoxide (DMSO), aflatoxin B1, ketoconazole, benzonapthoflavone, hyperforin, 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl)oxime (CITCO), taurocholate, and estrone sulfate were obtained from Sigma-Aldrich (St. Louis, MO) or other commercial sources.
Culturing of Cryo-HepaRG.
Cryo-HepaRG were thawed and plated (1 × 105 cells/well) on 96-well flat-bottom collagen (I)-coated cell culture plates following the Life Technologies standard plating protocol. Briefly, supplemented culture media (WEM supplemented with HPRG770 and GlutaMAX) was warmed to 37°C (water bath). Media (9 ml/plate) was transferred to a 50-ml conical tube. Cryo-HepaRG were thawed at 37°C (water bath) until a small amount of ice crystal remained. Cryo-HepaRG were aseptically transferred to the 50-ml conical tube and centrifuged for 2 minutes at 357g at room temperature. Media was aspirated and cells were resuspended in 5 ml of HPRG770-supplemented media, and cells were counted and assessed for viability with Trypan Blue (0.05%) using a Countess (Life Technologies). Cell densities were adjusted to 1.25 × 106 cells/ml, and 80 μl was delivered to each well (prewetted with 45 μl of plating media) of cell-culture plates using a multichannel pipettor. Plated cells were subsequently allowed to settle in cell culture plates for ∼10 minutes in the biosafety cabinet and moved to a humidified incubator at 5% CO2 and 37°C. Supplemented media was changed, as needed, for each assay type >1 hour after plating, and culture media was renewed every 2–3 days, unless otherwise stated. All HepaRG and PHH cell culture images were captured using a Zeiss Axiovert inverted research microscope equipped with phase-contrast optics, a 3CCD camera, and imaging computer with image analysis software. Freshly differentiated HepaRG cells were grown and differentiated from progenitor cells as previously described (Gripon et al., 2002).
PHH.
Cryopreserved PHH were thawed and recovered using Cryopreserved Hepatocyte Recovery Media, then directly assayed in suspension form or plated using WEM (Hepatocyte Plating Supplement Pack, serum-containing; Life Technologies) at optimal density (e.g., 0.8 × 106 cells/ml) in 24-well culture plates. Cryopreserved and fresh hepatocytes were allowed to attach for 4–6 hours in a humidified incubator at 5% CO2 and 37°C, prior to overlay with 0.35 mg/ml Geltrex in serum-free WEM supplemented with ITS+, GlutaMAX, 1% Pen/Strep, 15 mM HEPES buffer, and 100 nM dexamethasone. Culture media was replaced daily. PHH reference data from lot characterizations across hundreds of donor preparations (cultured and suspension formats) represent approximately equal numbers of male and female donor preparations. For Hu8033, primary hepatocytes were derived from a female donor.
CDFDA Staining.
Cryo-HepaRG were thawed and plated (3 × 105 cells/well) onto 24-well culture plates using WEM supplemented with HPRG770 and GlutaMAX. Media was replaced the next day with WEM supplemented with HPRG720 and GlutaMAX supplement. Media was refreshed every 2 days thereafter. After 10 days in culture, media was aspirated from plates, and cell monolayers were washed with warm Hanks’ balanced salt solution (HBSS). HepaRG cultures were incubated for 15 minutes with cell culture media containing 5 μM CDFDA. After incubations, fluorescence photomicrographs were captured as described above with a fluorescent light source.
Drug-Metabolism Assays.
Cryo-HepaRG were cultured for 0 (suspension), 1, 2, 3, 4, 10, or 22 days (media was renewed every 2–3 days) in HPRG720 (metabolism media supplement) and GlutaMAX prior to conducting in situ incubations for metabolic activities, with the exception of induction assays (as described below). Primary human hepatocytes were cultured 48–72 hours in WEM (ITS+, GlutaMAX, 1% Pen/Strep, and 100 nM dexamethasone) prior to in situ metabolism assays with probe substrates. The following final substrate concentrations were used in cell culture media: 100 μM phenacetin (CYP1A2), 5 μM coumarin (CYP2A6), 500 μM bupropion (CYP2B6), 20 μM paclitaxel (CYP2C8), 25 μM diclofenac (CYP2C9), 250 μM S-mephenytoin (CYP2C19), 15 μM dextromethorphan (CYP2D6), 250 μM chlorzoxazone, 10 μM midazolam (CYP3A4/5), 200 μM testosterone (CYP3A4/5), 250 μM benzydamine (FMO), or 100 μM 7-hydroxycoumarin (sulfotransferase, UDP-glucuronosyltransferase). All in situ metabolism assays were conducted in single-probe format as previously described (Jackson et al., 2009). All cell suspension metabolism assays were performed in single probe format as previously described (Smith et al., 2012). All cryo-HepaRG metabolism assays for all substrates examined were conducted for 1 hour. PHH metabolism assay incubation periods were substrate-dependent (15 min phenacetin, 20 min bupropion, 45 min paclitaxel, 15 min diclofenac, 30 min (S)-mephenytoin, 15 min dextromethorphan, 15 min chlorzoxazone, 14 min testosterone, 10 min midazolam, 30 min 7ethoxycoumarin, 30 min benzydamine). Metabolism assay samples were collected and stored frozen at –80°C until they were processed for liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis. LC-MS/MS analysis of metabolism assay samples was conducted as previously described (Jackson et al., 2009; Smith et al., 2012) and included standard curves with at least six calibration standards along with 12 quality control samples (at three different concentrations) dispersed at the beginning, middle, and end of analytical runs to assess quantitative continuity throughout the run.
Induction Assays.
To evaluate cytochrome P450 (P450) inducibility, cryo-HepaRG were maintained in WEM supplemented with HPRG740 (no DMSO) and GlutaMAX supplement beginning on day 3 of culture (initially plated in HPRG770-supplemented media). Dosing with 50 μM omeprazole, 1 mM PB, or 10 μM RIF was initiated on day 3 of culture and renewed once daily over the ∼72-hour treatment period. Inducers were dissolved in 100% DMSO and diluted 1/1000 in cell culture media for a final DMSO concentration of 0.1%. The extent of induction was evaluated with in situ metabolism assays for CYP1A2, CYP2B6, and CYP3A4 enzymatic activity compared with vehicle control levels. Probe substrates phenacetin, bupropion, and testosterone were dissolved in 100% DMSO and applied to cell cultures (as described above) for 60 minutes. Assay media was subsequently transferred to round-bottom polypropylene plates (96-well) for LC-MS/MS analysis as described earlier. For gene expression assays, established (inventoried, validated) TaqMan assays for CYP1A2, CYP2B6, CYP3A4, and GAPDH were used. TaqMan universal PCR Master Mix and dNTPs were obtained from Applied Biosystems/Thermo Fisher Scientific (Foster City, CA) and used according to the manufacturer’s protocol. Complementary DNA (cDNA) was generated from isolated total RNA from cell cultures using an ABI PRISM 6100 Nucleic Acid Prep Station and ABI chemistry (ABI, Foster City, CA) following the manufacturer’s protocol for cell culture lysates. Total RNA was quantified using a NanoDrop spectrophotometer (NanoDrop, Wilmington, DE). A High-Capacity cDNA Archive Kit (Life Technologies) was used to synthesize cDNA from 200 ng of total RNA, using random hexamers, following the manufacturer’s protocol.
Cytotoxicity Assays.
For cytotoxicity assays, plating media was replaced on day 2 in culture with WEM supplemented with HPRG720 or HRG770 and GlutaMAX supplement. Media was refreshed every 2 days thereafter. HepaRG cultures were treated with aflatoxin B1 on day 7 in culture. Twenty-four hours after treatment, ATP content assays were performed. ATP levels were determined using CellTiter-GLO Luminescent Cell Viability Assay according to the manufacturer’s instructions (Promega Corporation, Madison, WI) using a FLUOstar Omega luminometer (BMG Labtech, Cary, NC). Dose-response curves were modeled with the Hill equation, as previously described (Beam and Motsinger-Reif, 2011).
Uptake Transport Assays.
Plated uptake assays were performed on day-4 of cryo-HepaRG culture. Cell-culture media was aspirated from culture plates, and cell monolayers were washed three times with warm HBSS plus HEPES buffer. Cells were preincubated with the third wash in a 37°C humidified incubator with 95% air/5% CO2 for 10 minutes. Buffer was subsequently replaced with dosing solution containing varying concentrations of 3H-radiolabeled taurocholate, estradiol-17β-glucuronide, or estrone sulfate prepared in HBSS plus HEPES buffer, and incubated for 30 minutes. After incubation, dosing solutions were aspirated and HepaRG or PHH cultures were washed three times with ice-cold buffer and placed at –80°C for a minimum of 15 minutes prior to cell lysis with 0.1% Triton X solution. Samples were analyzed using a MicroBeta TriLux scintillation counter (Perkin Elmer, Waltham, MA). Primary hepatocyte uptake transport assays were performed analogously to HepaRG cultures, with the exception that 10-minute incubations were performed, and cultures were maintained for 18–24 hours (postplating), prior to uptake assays.
CYP3A4 Immunohistochemistry.
CYP3A4 antibody was purchased from Abcam (Cambridge, MA). Treated cell cultures (24-well) were washed with phosphate-buffered saline (PBS), fixed with 500 μl of 2% paraformaldehyde solution with 0.1% saponin in PBS for 30 minutes at room temperature. Fixed cells were washed three times with PBS for 1 minute on a micro-orbital shaker (∼200 rpm). Subsequently, blocking was performed with 300 μl of Image-iT FX Signal Enhancer (Thermo Fisher Scientific) and incubated at room temperature overnight. Blocked cells were washed three times with 500 μl/well of warm PBS for 1 minute on an orbital shaker (∼200 rpm). Cells were further incubated with primary antibody at 1/200 dilution for 1 hour at 37°C and imaged with a Zeiss Axiovert inverted research microscope as described earlier.
Protein Quantification Assays.
Protein quantification assays were performed using Thermo Scientific Pierce BCA Protein Assay Kit following the manufacturer’s instructions, and absorbance was quantified using a FLUOstar Omega plate reader (BMG Labtech).
Genotyping Analysis.
Genomic DNA was isolated from HepaRG cells utilizing an ABI PRISM 6100 Nucleic Acid Prep Station and ABI chemistry following manufacturer’s instructions (Life Technologies). Genotyping of HepaRG genomic DNA was conducted using TaqMan Drug Metabolism Genotyping Assays on an ABI 7900 Real-Time PCR instrument following manufacturer’s instructions (Thermo Fisher Scientific).
Translocation of CAR in HepaRG Cells.
An adenovirus-expressing enhanced yellow fluorescent protein–tagged hCAR (Ad/EYFP-hCAR) construct was generated and functionally characterized, as previously reported (Li et al., 2009a). Cryo-HepaRG were thawed and seeded on 24-well BioCoat plates (Thermo Fisher) in WEM containing HepaRG Induction Media Supplements HPRG740 (serum-containing) and HPRG750 (serum-free). Twenty-four hours after plating, HepaRG cells were infected with Ad/EYFP-hCAR (5 μl virus per well) for 12 hours, followed by treatment with vehicle control (0.1% DMSO) or PB (1 mM) for an additional 8 hours. Treated cells were then subjected to confocal microscopy analysis with a Nikon C1-LU3 instrument attached to an inverted Nikon Eclipse TE2000 microscope (Tokyo, Japan). Localization of hCAR was quantitatively characterized as nuclear, cytosolic, and mixed (nuclear + cytosolic) expression by counting 100 Ad/EYFP-hCAR–expressing HepaRG cells from each treatment group.
Statistical Analyses.
Multiple t-test comparisons of mean enzymatic activities were determined in GraphPad Prism v.6.07 (GraphPad Software, La Jolla, CA) by analyzing each row individually without assumption of consistent standard deviations, correction for multiple comparisons using Holm-Sidak method, and an alpha of 0.05. Tukey’s pairwise comparisons of mean response data were performed after initial one-way analysis of variance using JMP 11.0.0 (SAS, NC) at an alpha of 0.05.
Results
Culture Model Evaluation
Hepatocyte morphology is known to be a useful indicator of monolayer integrity/differentiation status with in vitro liver models (Hamilton et al., 2001). Therefore, we assessed cryo-HepaRG culture morphologies compared with freshly differentiated HepaRG and SC-PHH (Fig. 1, A and C). In initial cell biology evaluations with cryo-HepaRG, we observed a rapid attachment (<10 minutes) to cell culture plates compared with the ∼4–6 hours generally required for PHH. After 1–2 hours in culture, cryo-HepaRG appeared rounded (Fig. 1D) and lacked characteristic cobblestone appearance and cord-like networks of hepatocyte-like cells observed with fully/freshly differentiated HepaRG (Fig. 1A). Cobblestone-like networks are common to epithelial cells in confluent monolayers that autoassemble, resembling structures found in vivo within the liver (Godoy et al., 2013). Their absence after only a few hours in culture indicates that cryo-HepaRG were undergoing a significant transition to their new culture environment. While cryo-HepaRG began to resemble fully differentiated HepaRG ∼24 hours postattachment (Fig. 1E), 7–10 days were required for full recovery of morphology (Fig. 1, F and B). Initial experiments to optimize cryo-HepaRG cell seeding densities using metabolic competence (Supplemental Fig. 1) and cell morphologies resulted in selection of ∼100,000 cells/well (96-well format). Seeding densities greater than ∼100,000 cells/well resulted in overcrowding of monolayers and forced HepaRG cells to intermittently rise out of plane. Analogously to PHH cultures (LeCluyse et al., 1994) and freshly differentiated HepaRG (Antherieu et al., 2010), cryo-HepaRG appeared to form bile canalicular structures after ∼3 days in culture). In general, these structures were more rounded between multiple cells compared with longer linear or y-like structures common to SC-PHH. Bile canalicular formation was further evaluated using CDFDA staining. CDFDA is converted to CDF in hepatocytes, and CDF is a substrate of bile efflux transporter multidrug resistance–associated protein 2 (MRP2) in liver (Hoffmaster et al., 2004). MRP2 and other apical efflux transporters are generally thought to be internalized upon disruption of cell-cell interactions during isolation of PHH. Under suitable culture conditions, hepatocytes repolarize in culture and canalicular transporters (i.e., MRP2) migrate back to the canalicular membrane over time (2–4 days). CDFDA staining of cryo-HepaRG cultures demonstrated clear localization of CDF between hepatocytes that perfectly overlie with canalicular domains in phase contrast images (Figs. 1, G–I). Approximately 3 days in culture were required to form bile canaliculi in cryo-HepaRG. Collectively these observations demonstrate that cryo-HepaRG repolarize over time in culture analogously to PHH isolated from human livers and do not immediately reform fully differentiated monolayers.
Morphologic evaluation, seeding density optimization, and bile canaliculi staining. Cell morphology photomicrographs with HepaRG cell cultures at various stages of culture. (A) Freshly differentiated HepaRG after ∼4 weeks in culture. (B) Cryo-HepaRG after 10 days in culture (postplating). (C) Sandwich cultures of cryopreserved PHH (3 days in culture). (D) Cryo-HepaRG in culture ∼6 hours postplating. (E) Cryo-HepaRG ∼24 hours in culture. (F) Cryo-HepaRG ∼7 days in culture. (G) Phase contrast bright-field image of cryo-HepaRG matched to CFDFA staining (H), and the merged image (I) highlighting MRP2 efflux function indicative of a polarized monolayer functional for biliary efflux.
Phase I and Phase II Drug-Metabolism Competence Characterization and Temporal Dynamics
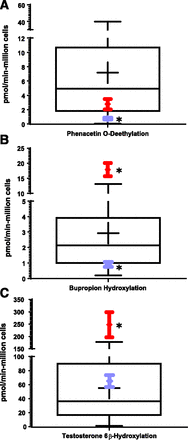
As a marker of hepatocyte differentiation, we evaluated the metabolic competence of cryo-HepaRG after 10 days in culture with a panel of 12 pharmacologically important drug-metabolizing enzymes (Table 1). Enzymatic activities [CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4/5, flavin monooxygenase (FMO), UDP-glucuronyltransferase (UGT), and sulfotransferase (SULT)] were determined in situ and compared with the ranges of interindividual variation observed in SC-PHH under comparable assay conditions. Following 10 days of culture, CYP1A2 activity (phenacetin O-deethylase) was observed at 2.78 ± 0.728 pmol/min-million cells in cryo-HepaRG compared with a mean activity of 7.18 ± 7.78 (near 25th percentile, Fig. 2A in red) compiled from a panel of 52 donor preparations of SC-PHH. Cryo-HepaRG CYP1A2 mean activity was statistically different from the SC-PHH mean activity, but the magnitude was less than 2-fold from the median CYP1A2 activity of the 52 different SC-PHH preparations. Notably, both plated cryo-HepaRG and SC-PHH fall profoundly short of in vivo-like levels at ∼6% of median CYP1A2 activity observed across 212 PHH suspension preparations evaluated (48.0 pmol/min-million cells), which of course, assumes PHH suspensions approximate in vivo levels.
Baseline drug-metabolizing enzyme activity at day-10 (metabolism media) with cryo-HepaRG versus sandwich-culture and suspension PHH
Cryo-HepaRG cells were cultured for 10 days postplating in HepaRG metabolism media–supplemented culture media in eight replicate wells. Primary human hepatocytes were directly assayed or cultured for 4–5 days in serum-free induction media with ITS+, 100 nM dexamethasone, 15 mM HEPES, 1% pen/strep. Drug-metabolism enzyme–specific activities were evaluated using in situ incubations with probe substrates in cryopreserved human hepatocyte culture. LC-MS/MS methods were used to quantify specific metabolite concentrations. Means were compared using unpaired t tests (i.e., Welch’s correction).
HepaRG metabolic competence contextualized to PHH sandwich cultures. Box and whisker plots show ranges of metabolic competence for CYP1A2 (A), CYP2B6 (B), and CYP3A4/5 (C) from PHH sandwich cultures (52 donor preparations) with violet diamonds representing cryo-HepaRG metabolic activity at day-4 and red diamonds representing day-10 cryo-HepaRG. Error bars show standard deviations of eight replicate wells from a single lot, and box and whiskers show the PHH distribution (52 donor preparations) including: minimum/maximum of the range, mean (crosshair), 25th and 75th percentiles, and median of the distribution. Data were generated using probe substrates phenacetin, bupropion, and testosterone for CYP1A2, CYP2B6, and CYP3A4/5, respectively. LC-MS/MS methods were used to quantify metabolite concentrations from in situ incubations of spent culture media to calculate specific activities. Statistical significance (multiple t tests, P < 0.05) between SC-PHH and cryo-HepaRG indicated with an asterisk (*).
CYP3A4/5 activity in cryo-HepaRG was evaluated utilizing two probe substrates (testosterone and midazolam) following 10 days in culture under metabolism media (day-10). Testosterone 6β-hydroxylase activity was 248 ± 50.9 pmol/min-million cells in cryo-HepaRG, whereas midazolam 1-hydroxylase activity was 28.4 ± 2.39 pmol/min-million cells. Interestingly, the testosterone 6β-hydroxylase activity in cryo-HepaRG was ∼6.8-fold higher than median and was significantly higher (P < 0.0001) than the mean, median, and top of the range observed with 52 donor preparations of SC-PHH (1.47–178 pmol/min million cells, mean of 55.2 ± 49.4). Although initially appearing super-physiologic, this level of CYP3A4/5 activity was lower than median testosterone 6β-hydroxylase activity observed across 195 suspensions of PHH (407 pmol/min million cells). Consistent with the elevated CYP3A4/5 basal activity observed across 52 cryo-HepaRG preparations, CYP2B6 activity levels also exceeded the range observed with SC-PHH (P < 0.05) at 17.9 pmol/min-million cells compared with 0.21–13.1 pmol/min-million cells. Although CYP3A4 and CYP2B6 exhibited disproportionately high levels of activity in cryo-HepaRG, it is important to note that these enzymes are also among the most inducible drug-metabolizing enzymes in human liver. Less inducible enzymes such as CYP2A6, CYP2C8, CYP2C9, CYP2C19, and CYP2D6 were not statistically different (P < 0.05) from the mean activities of 52 SC-PHH preparations evaluated (Table 1). Generally, all activities measured in situ were around 10% of the median activities observed with PHH suspensions. CYP2D6 activity in cryo-HepaRG was near the low end of the range compared with SC-PHH and is further discussed below in Genotyping of Important Human Absorption, Distribution, Metabolism, and Excretion Genes HepaRG. CYP2E1 activity was also evaluated in cryo-HepaRG cultures under these conditions (data not shown); however, the potent inhibitory effects of DMSO on CYP2E1 prohibited accurate evaluation of CYP2E1 metabolic competence. Enzymatic activity data for FMO, UGT, and SULT were also determined for cryo-HepaRG and reported in Table 1. While no FMO, UGT, or SULT data were available in SC-PHH under these assay conditions, cryo-HepaRG exhibited ∼10% FMO and ∼30% SULT activity levels compared with PHH suspensions, and UGT activity levels were comparable between cryo-HepaRG and PHH suspensions.
As demonstrated in Table 1, SC-PHH lose a substantial proportion of drug-metabolism competence over time in culture from their initial suspension format. We hypothesized that with cryo-HepaRG a similar phenomenon may occur that has not been reported previously and may impact metabolism-related research findings. Therefore, we compared CYP1A2, CYP2B6, and CYP3A4/5 enzymatic activities of day-4 cryo-HepaRG (Table 1) with day-10 cryo-HepaRG against the activities observed across 52 SC-PHH preparations (Fig. 2, A–C). Here we observed that day-4 CYP1A2 activity in cryo-HepaRG fell below the 25th percentile of the SC-PHH range compared with day-10 activity that rose ∼3-fold to levels near the 25th percentile of SC-PHH (Table 2, Fig. 2A). CYP2B6 and CYP3A4 showed similar trends, shifting from lower activities at day-4 (near the 25th percentile and average of the distributions, respectively) to levels above the SC-PHH range by day-10, with increases of 19.7-fold and 3.8-fold, respectively (Fig. 2, B and C). One possible explanation for the observed increases in P450 activity (normalized to cell numbers plated) over time in culture could be cellular proliferation occurring between day-4 and day-10. Although proliferation is improbable in contact-inhibited confluent monolayers (Gripon et al., 2002), we measured total protein content over time as a marker of cell number and observed no statistically significant changes in total protein content across the time points examined (Supplemental Fig. 1). Therefore, increased drug-metabolism competence over time along with morphologic observations appears to reflect an improved hepatocyte differentiation status rather than an increase in the number of HepaRG cells.
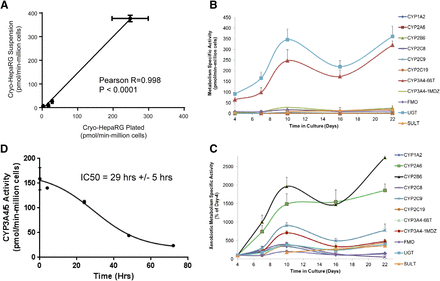
HepaRG metabolic competence summary: suspension HepaRG versus cultures (metabolism media)
To further characterize the temporal dynamics of metabolic competence with cryo-HepaRG cells, we generated specific activity data with suspensions of cryo-HepaRG in their initial form for comparison with cultured cryo-HepaRG at day-4 and day-10 (Table 2). In general, cryo-HepaRG suspensions and day-10 cultures of cryo-HepaRG (in HPRG720 metabolism media) produced comparable metabolic activities across nine metabolizing enzymes, consistent with cell morphology observations. A good correlation (Pearson correlation with R = 0.998) of enzymatic activities was observed between cryo-HepaRG suspensions and day-10 cultures of cryo-HepaRG (Fig. 3A). These data coupled with the day-4 enzymatic activity data suggest that cryo-HepaRG cells (under metabolism media) initially lose much of their metabolic competence but recover to suspension-like levels over ∼10 days. For the cytochromes P450 evaluated, day-10 metabolic competence was on average 8.6-fold higher than day-4, while FMO, UGT, and SULT activities were 2.0-, 3.8-, and 1.8-fold higher on day-10 relative to day-4, respectively. We extended this study out to 22 days with activity data shown in Fig. 3C that were normalized to respective day-4 activities in Fig. 3D. Metabolic competence appeared to achieve maximal levels at ∼10 days in culture across the metabolic pathways assessed and was largely maintained at comparable levels until day-22. Notably, day 16 enzymatic activities were lower than metabolic activities on day-10 and day-22 across the panel of enzymes. It is not clear whether this decrease was biologically important as monolayer morphology appeared unchanged after day-10 and time points on both sides of day 16 were generally comparable.
Metabolic competence (Phase I and Phase II) comparisons over time in culture. Cryo-HepaRG cultures were evaluated for metabolic competence at various times in culture over a range of specific activities with a panel of probe substrates reflective of various metabolic pathways (P450, FMO, UGT, and SULT). Cryo-HepaRG were thawed and plated using HPRG770-supplemented media then switched and maintained in HPRG720-supplemented media (metabolism media) after ∼24 hours in culture in sandwich cultures on collagen (Type I) coated plates. (A) Pearson correlation analysis relating observed specific activities from suspension cryo-HepaRG (preplating) compared with day-10 cultures of cryo-HepaRG over a range of Phase I and Phase II drug-metabolizing enzymes. (B, C) Evaluation of the temporal dynamics of cryo-HepaRG activity over time in culture. (D) Loss of CYP3A4/5 with cryo-HepaRG in culture during the initial days of culture, fit in GraphPad Prism 6.07 using nonlinear regression. Data represent mean and standard deviation of eight independent replicate wells (A–C) and nine independent replicate wells in (D).
As a result of the observed lower P450 activities with day-4 cryo-HepaRG, we further evaluated the loss of activity from suspensions through the first 96 hours in culture. As hypothesized, cryo-HepaRG cultures appear to undergo a loss of activity (∼10-fold for CYP3A4) similar to that observed with PHH (Smith et al., 2012) as they transition from suspension to cell culture monolayers after removal from the liver (Fig. 3B). The observed “half-life” of CYP3A4/5 testosterone 6β-hydroxylase activity was 34.1 hours ± 1.02 hours, which is consistent with the time frame observed with PHH (∼29 hours) (Smith et al., 2012). Minimum activity level was observed after ∼72 hours in culture, as later time points appeared to produce higher activities. In summary, physiologically relevant levels of drug-metabolism activities were observed in cryo-HepaRG cultures compared to SC-PHH. However, cryo-HepaRG metabolic competence did not appear to be a static, intrinsic property of cryo-HepaRG postdifferentiation/cryopreservation, as cryo-HepaRG metabolism levels exhibit marked temporal dynamics over the initial 10 days in culture, which is important to understand in metabolism-related research.
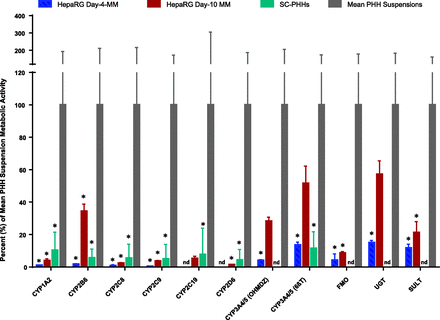
To more comprehensively contextualize cryo-HepaRG metabolic competence, we calculated cryo-HepaRG day-4, cryo-HepaRG day-10, and SC-PHH day-4 enzymatic activities as percentages of mean suspension PHH activities (Fig. 4). From these data, PHH suspension activities far exceeded (P < 0.05) those observed with SC-PHH for all enzymes investigated. This was also true for day-4 cryo-HepaRG. For day-10 cryo-HepaRG, mean activities were also lower and generally statistically significant (P < 0.05) compared with mean and median PHH suspension activities. However, owing to high interindividual variability observed in CYP3A4/5 (P = 0.058 for 1-hydroxymidazolam and P = 0.064 with 6βT), CYP2C19 (P = 0.19), and UGT (P = 0.15) activities, statistical significance was not reached at the P < 0.05 level. These data also show significant (P < 0.05) increases for all evaluated enzymatic activities from day-4 to day-10 with cryo-HepaRG cultures in metabolism media. Day-10 cryo-HepaRG were also statistically (P < 0.05) indistinguishable from SC-PHH across this panel of metabolic activities with the exception of the higher observed activities of CYP2B6 and CYP3A4/5 as described earlier. Taken together, these data demonstrate that day-10 cryo-HepaRG cultures were largely comparable to SC-PHH with elevated levels of CYP2B6 and CYP3A4/5, whereas day-4 cryo-HepaRG were generally lower in metabolic capacity than SC-PHH with the exception of CYP3A4/5 (6βT).
Panel of liver enzyme activities characterizing cryo-HepaRG versus PHH: Metabolic activities with cryo-HepaRG cultures and SC-PHH were contextualized to mean activities from ranges of interindividual variation observed with suspensions of PHH. Data represent mean and standard deviation percentages of PHH suspension activities (mean) reflective of liver-like levels of enzymatic activities. Statistical significance (multiple t tests, P < 0.05) between mean suspension PHH enzymatic activities versus other models indicated via asterisk (*).
Effects of Overlay with Extracellular Matrix on Metabolism and Cell Culture Morphologies
Overlay of PHH cultures with extracellular matrices (e.g., Geltrex, Matrigel) in “sandwich” configuration is standard practice with PHH to improve longevity, cuboidal three-dimensionality, and hepatocyte differentiation (LeCluyse et al., 1994). Therefore, we assessed the impact of Geltrex overlay on cultures of cryo-HepaRG cells in serum-free HPRG750-supplemented induction media (contains no DMSO). Figure 5, A and B, show cryo-HepaRG cell morphology photomicrographs with and without Geltrex overlay (0.35 mg/ml). Overlay of cryo-HepaRG cultures led to a marked change in cell morphologies, producing improved homogenous monolayers within a single focus plane. Overlay appeared to shift the cell populations toward more three-dimensional morphologies analogous to SC-PHH (i.e., smaller nuclear-to-cytosolic ratios), and qualitatively appeared to produce higher proportions of hepatocyte-like cells (fewer cholangiocyte-like cells). These characteristics are generally associated with improved hepatocyte functionalities; therefore, we further evaluated the effects of overlay on metabolic competence. CYP1A2, CYP2B6, and CYP3A4 were examined in situ, and the results are shown in Fig. 5C. Approximately 2- to 3-fold increases in basal metabolic activities were observed in the presence of Geltrex overlay consistent with cell morphology observations. To further evaluate the effects of Geltrex overlay on cryo-HepaRG cultures, immunohistochemistry was performed for CYP3A4, a marker of differentiated hepatocytes (Fig. 5, D–G). Immunohistochemistry for CYP3A4 was able to effectively stain hepatocyte-like HepaRG cells in standard cryo-HepaRG cultures (Fig. 5, D versus E). Upon overlay with Geltrex, the CYP3A4 staining became more prevalent across the culture with increased fluorescence coverage overall and larger areas of CYP3A4 staining (Fig. 5, F and G). Taken together, these data indicate that overlay of cryo-HepaRG improved their hepatocyte functionality under the conditions examined.
Cell morphologies and metabolic competence of cryo-HepaRG with Geltrex overlay. Cryo-HepaRG were cultured without (A) or with (B) Geltrex overlay (0.35 mg/ml) yielding a more coplanar culture in the presence of Geltrex overlay and more cuboidal cell morphologies consistent with a more three-dimensional configuration. (C) After 4 days in culture, metabolic competence for CYP1A2, CYP2B6, and CYP3A4/5 was assessed in cryo-HepaRG with and without Geltrex overlay in HPRG750-supplemented serum-free culture media. (D) Phase contrast image of HepaRG without Geltrex overlay matched to (E) immunohistochemistry for CYP3A4-immunoreactive protein without Geltrex overlay after 7 days in culture in HPRG720-supplemented “metabolism” media. (F) Phase contrast of image of HepaRG with Geltrex overlay matched to (G) immunohistochemistry for CYP3A4-immunoreactive protein with Geltrex overlay after 7 days in culture in HPRG720-supplemented “metabolism” media. Data represent mean of three independent replicate wells, and error bars represent the standard deviations of the respective mean responses. Asterisk (*) indicates P < 0.05 (Tukey’s Pairwise Comparison) relative to respective No Overlay control.
Genotyping of Important Human Absorption, Distribution, Metabolism, and Excretion Genes HepaRG
CYP2D6 and CYP2C9 enzymatic activities (Table 1) in cryo-HepaRG cultures yielded lower levels of metabolic activity (near the lower end of the ranges of interindividual variation) compared with SC-PHH. HepaRG cells are known to contain only a single copy of chromosome 22, which encodes for CYP2D6 and could contribute to the lower CYP2D6 activity (Gripon et al., 2002). However, low levels of CYP2D6 and CYP2C9 could arise from the presence of poor metabolizer alleles in cells originating from a female Caucasian donor. Therefore, we assessed common poor metabolizer alleles in a panel of drug-metabolizing enzymes (CYP2C9, CYP2C19, CYP2D6, CYP3A5) known to be important for interindividual variation in drug clearance (Ingelman-Sundberg et al., 2007). Genotyping analyses revealed the presence of multiple P450 single-nucleotide polymorphisms (summarized in Table 3). As suspected from enzymatic activity data, HepaRG cells contain poor metabolizer alleles for CYP2D6 (*2 and *9). These results suggest that HepaRG cells are deficient in CYP2D6 metabolism. The small observable dextromethorphan conversion to dextrorphan could be the product of other enzymes including CYP3A4/5 as previously reported (Yu and Haining, 2001). Our genotyping analysis also revealed poor metabolizer alleles for CYP2C9 (*2/*2) consistent with the observed low CYP2C9 activity. For CYP3A5, HepaRG cells contained two CYP3A5*3 alleles. These are known to be null alleles owing to expressed RNA instability and are present in the majority of individuals of Caucasian descent. Therefore, it is probable that CYP3A4/5 activity in HepaRG cells is predominantly attributable to CYP3A4 activity.
Genotyping summary for ADME genes in HepaRG
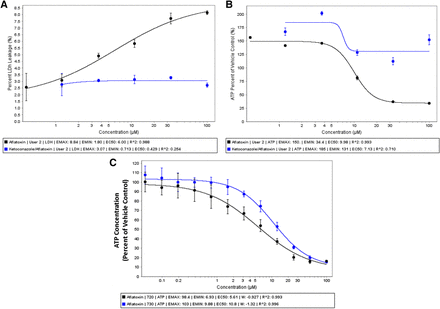
Metabolism-Dependent Cytotoxicity in Cryo-HepaRG Cultures
Aflatoxin B1, a well known metabolism-dependent hepatotoxicant, has been shown to undergo metabolic activation via CYP3A4 and CYP1A2 producing a highly reactive epoxide metabolite (Essigmann et al., 1977). The well documented mechanism of action of aflatoxin B1 makes it an attractive probe toxicant for evaluating the utility of cryo-HepaRG to support evaluation of metabolism-dependent toxicity. HepaRG cells were cultured in HPRG720 metabolism media for 7 days prior to dosing cultures with aflatoxin B1, ketoconazole (a reversible inhibitor of CYP3A metabolism in humans), or the combination of these two chemicals. Cryo-HepaRG cultures were treated for ∼24 hours prior to cell health/stress evaluations (Fig. 6). Both lactate dehydrogenase and ATP data confirm aflatoxin B1-related cytotoxicity at 5–10 μM half-maximal effective concentrations (EC50). Ketoconazole (20 μM) was able to attenuate the observed cytotoxicity (Fig. 6, A and B). Comparison of aflatoxin B1 cytotoxicity under HPRG720 versus HPRG730 (“Tox” media) media supplements, known to modulate P450 basal activities, produced a shift in aflatoxin B1 potency consistent a metabolically-activated toxicity (Fig. 6C). These data highlight the utility of the cryo-HepaRG culture model for assessing metabolically activated toxicity, supporting previous studies that showed the utility of P450 inhibitors for studying mechanisms of toxicity (Li, 2009b), and replicating previous studies that demonstrated freshly differentiated HepaRG are a sensitive model in which to study aflatoxin B1 cytotoxicity (Aninat et al., 2006).
Metabolism-dependent cytotoxicity in cryo-HepaRG. Cryo-HepaRG were cultured for 3 days in HPRG720-supplemented media prior to initiating cytotoxicity experiments. (A, B) Aflatoxin B1 was applied to cryo-HepaRG cultures in concentration-response for 24 hours and assayed for lactate dehydrogenase leakage (A) (CytoTox-One; Promega) and ATP content (B) (CellTiter-GLO; Promega) in the presence/absence of ketoconazole (20 μM). Data represent the mean and standard deviation of three independent replicates for each treatment group. (C) Concentration-response of aflatoxin B1 in cryo-HepaRG cultures (7 days in culture) using HPRG720 (metabolism media) and HPRG770-supplemented media after 24 hours of treatment with aflatoxin B1. Data represent mean of three independent replicate wells, and error bars represent the standard deviations of the respective mean responses.
Active Uptake Transport Characterization
An important and often rate-limiting step in drug clearance is hepatic uptake transport (Shitara et al., 2013). Active uptake transport function was evaluated in cultures of cryo-HepaRG using tritium-labeled taurocholate [a sodium/taurocholate cotransporting polypeptide (NTCP) substrate], estrone sulfate [an organic anion–transporting polypeptides 1B1 and 1B3 (OATP1B1/3) substrate], and estradiol-17β-glucuronide (OATP1B1/3). For these evaluations HepaRG cells were cultured for 10 days prior to initiating experiments. Active uptake with taurocholate was assessed at multiple concentrations at both 4°C and 37°C to estimate the respective passive and active uptake transport capacity of cultured cryo-HepaRG (Fig. 7A) with representative uptake transport data from a single SC-PHH preparation (Hu8083) shown in Fig. 7B. For cryo-HepaRG, active uptake transport at 37°C was readily distinguishable from uptake evaluated at 4°C, consistent with competence for active uptake via NTCP. The estimated Km for taurocholate uptake was 21.1 μM ± 3.08 μM for cryo-HepaRG compared with 6.68 ± 0.564 for Hu8083 SC-PHH fit to a Michaelis-Menten model. The extent of taurocholate uptake in cryo-HepaRG cultures appeared to be somewhat lower than in SC-PHH, but it is notable that the percentages of hepatocytes in these models are different. To further contextualize the extent of taurocholate uptake transport in cryo-HepaRG cultures, we plotted 1 μM taurocholate uptake transport rates versus the range of transport rates across 76 donor preparations of SC-PHH (37 female, 39 male) under the analogous assay conditions (Fig. 7C). Cryo-HepaRG uptake was comparable to SC-PHH, falling above the 25th percentile of the distribution (9.3 pmol/min per milligram) but lower than the median rate of SC-PHH taurocholate update (11.8 pmol/min per milligram). We further evaluated cryo-HepaRG uptake transport competence with estrone sulfate (ES) and estradiol-17β-glucuronide (E2-17βG), known substrates for OATP uptake transporters OATP1B1 and OATP1B3 (Fig. 7, D and E, Table 4). Cryo-HepaRG cultures produced some active uptake with estrone sulfate at both 0.5 and 40 μM concentrations (Fig. 7, D and E). Lower proportions of active uptake were observed with E2-17βG (∼2-fold over 4°C control, data not shown). Reference SC-PHH data were not available for ES uptake. However, median E2-17βG (1 μM) uptake (37°C) across 76 lots of SC-PHH preparations was 2.92 pmol/min per milligram compared with 0.357 ± 0.0181 pmol/min per milligram (0.5μM) under comparable culture conditions with cryo-HepaRG (Table 4). The presence of serum in cell culture media did appear to elevate uptake transport with both ES and E2-17βG. Geltrex overlay of cryo-HepaRG cultures did not appear to have a profound effect on uptake transport, but uptake did appear marginally higher in the presence of overlay. Collectively these data demonstrate that cryo-HepaRG cultures were competent for active uptake transport but have a somewhat reduced capacity compared with SC-PHH under the culture conditions examined.
Active uptake transport in cryo-HepaRG. Cryo-HepaRG were cultured for 10 days in HPRG720-, HPRG740-, and HPRG750-supplemented media and subsequently evaluated for active uptake transport with taurocholate and estrone sulfate. (A) Concentration-response curves for taurocholate uptake at 37°C and 4°C in cryo-HepaRG cultures. (B) Representative concentration-response curves for taurocholate uptake at 37°C and 4°C in SC-PHH (Hu8083). (C) Box and whisker plot of taurocholate uptake distributions (at 37°C) for 76 donor preparations of sandwich-cultured primary human hepatocytes (black) and day-10 cultures of cryo-HepaRG (red diamond). (D, E) Estrone sulfate uptake in cryo-HepaRG in culture for 10 days in metabolism media (HPRG720), serum-containing induction media (HPRG740), and serum-free induction media (HPRG750) media supplements (cryo-HepaRG were initially plated in HPRG770-plating/maintenance media and changed ∼24 hours postplating) and assayed for estrone sulfate at 0.5 and 40 μM concentrations. Data represent mean of three or four independent replicate wells, and error bars represent the standard deviations of the respective mean responses.
Uptake OATP1B1/1B3 transport with cryo-HepaRG and overlay effects
Functional Xenobiotic Signaling Pathways in Cryo-HepaRG.
Hepatic cell lines such as HepG2 and Fa2N-4 have been used in screening and mechanistic research to represent hepatic function in vitro. However, numerous reports have demonstrated the severely limited differentiation status for mature hepatocyte phenotypes in these cell lines, including xenobiotic metabolism and receptor signaling pathways (Donato et al., 2008; Hariparsad et al., 2008). Therefore, we evaluated cryo-HepaRG cultures with various selective activators of aryl hydrocarbon receptor (AhR), CAR, pregnane X receptor (PXR), and farnesoid X receptor (FXR), monitoring induction of downstream target genes (Fig. 8, A–D). After cells were cultured for 48 hours post-thaw, they were treated with inducers for 72 hours at three concentrations prior to in situ metabolism assays. Concentration-related increases in CYP1A2 enzymatic activity were observed with the AhR agonist omeprazole. These responses were compared to ranges of induction responses observed in cultures of PHH and generally showed comparable (and substantially less variable) induction well within the ranges of “normal” response (Fig. 9). Additionally, CYP1A2 mRNA was induced over three independent assay plates with an average of ∼350-fold over 0.2% DMSO control (Fig. 10A). Induction of CYP1A2 mRNA was larger (fold over control) compared with enzymatic activity responses, a phenomenon common to SC-PHH (unpublished observations). It is also notable that for induction assays, metabolism incubations with phenacetin required ∼1 hour to produce sufficient levels of the CYP1A2-specific metabolite acetaminophen. This is comparable to the incubation time frame (30–60 minutes) effective with SC-PHH that produces robust activity levels yet avoids substrate depletion. By comparison, ∼24 hours are commonly required to observe specific metabolite levels with dedifferentiated cell lines such as HepG2 (Granata et al., 2006).
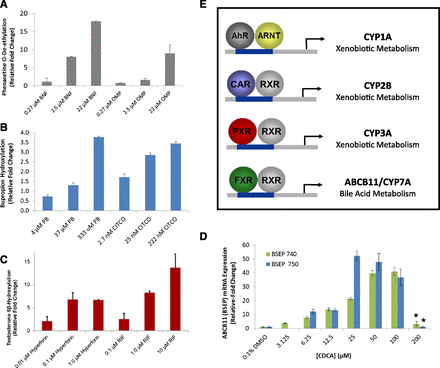
P450 enzyme induction as a marker of hepatocyte functionality with cryo-HepaRG. Cultures of cryo-HepaRG were maintained in serum-free induction media (HPRG750) for ∼24 hours prior to initiating induction experiments with prototypical receptor activators for AhR, CAR, PXR, and FXR. Receptor activators were applied at three concentrations to monitor concentration-responsiveness and were applied at “lower” concentrations less prone to crosstalk with other receptor pathways (e.g., CAR and PXR with PB). (A) AhR agonists β-naphthoflavone and omeprazole produced induction of CYP1A2 enzymatic activity compared with vehicle control metabolic activity indicating a functional AhR receptor pathway in cryo-HepaRG cell cultures. (B) CAR direct activator CITCO and indirect activator PB effectively induced CYP2B6 enzymatic activity at lower concentrations, suggesting a functional CAR pathway in cryo-HepaRG cell cultures. (C) PXR activators hyperforin and rifampicin (RIF) were effective in inducing CYP3A4/5 enzymatic activity consistent with a functional PXR receptor pathway in cryo-HepaRG cell cultures. (D) ABCB11 (bile salt export pump protein) gene expression was evaluated with FXR agonist CDCA in concentration-response in cryo-HepaRG cell cultures and showed a clear induction response in both HPRG750 (serum-free) and HPRG740-supplemented induction media. (E) Summary of relationships between sentinel gene targets and hepatic receptor pathways. *Cytotoxicity was observed at 200 μM concentrations of CDCA in cryo-HepaRG cultures. Data represent the mean of three independent replicates and error bars indicate standard deviations of the mean.
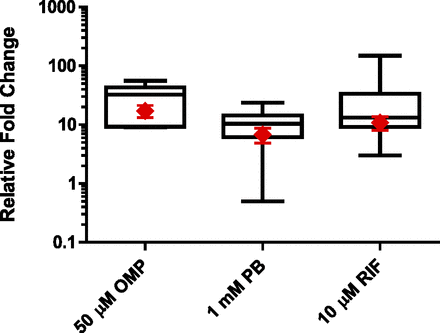
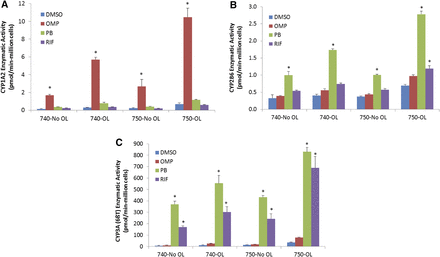
HepaRG and PHH induction response comparison (enzyme-specific activities). Three separate lots of cryo-HepaRG cultures (red diamonds) were contextualized to ranges of induction response observed with sandwich-culture PHH (52 lots for CYP2B6 and CYP3A4, 11 lots for CYP1A2) for induction-of-enzymatic-activity levels (box and whisker plots). P450-specific activities were evaluated after 72 hours of treatment with 50 μM omeprazole (OMP) for CYP1A2 (phenacetin O-deethylation activity), 1 mM PB for CYP2B6 (bupropion hydroxylase activity), or 10 μM RIF for CYP3A4/5 (midazolam 1-hydroxylation and testosterone 6β-hydroxylation activities). Fold over control values were calculated relative to respective vehicle controls. Statistical analysis of lot variation with cryo-HepaRG was performed using an analysis of variance followed by a Tukey’s pairwise comparison revealed no statistically significantly differences at the P < 0.05 level as shown in Table 5.
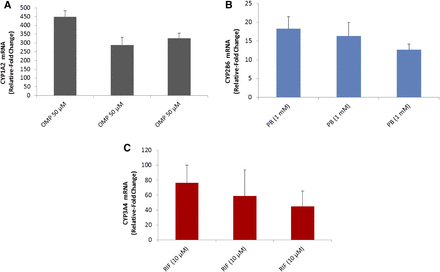
P450 mRNA induction of CYP1A2, CYP2B6, and CYP3A4/5 with cryo-HepaRG cultures. Cryo-HepaRG cultures were treated with omeprazole (OMP) at 50 μM, PB at 1 mM, and RIF (RIF) at 10 μM for 48 hours, and mRNA expression was quantified via quantitative reverse transcription-polymerase chain reaction (qRT-PCR) using gene-specific TaqMan assays. (A) CYP1A2 mRNA induction in three independent assay plates. (B) CYP2B6 mRNA induction in three independent assay plates. (C) CYP3A4 mRNA induction in three independent assay plates. Data represent mean responses of three independent replicate wells and error bars indicate standard deviations of mean responses.
Induction of CYP2B6 is known to be a marker of CAR and PXR activation, with both receptors activating response elements within the CYP2B6 upstream regulatory regions (Wang et al., 2003). Although there is crosstalk between these pathways, CYP2B6 induction is more responsive to CAR activators compared with PXR activators (Faucette et al., 2006). With SC-PHH, CYP2B6 induction is generally a sensitive marker of hepatocyte culture integrity, with lower quality lots failing to support CYP2B6 induction in response to PB (unpublished observations over hundreds of donor preparations of SC-PHH). In cryo-HepaRG cultures, CYP2B6 was clearly inducible in a concentration-related manner with both direct (CITCO) and indirect (PB) activators of CAR at sufficiently low concentrations for observing induction and minimizing the contributions of PXR to CYP2B6 induction (Fig. 8B). Induction of CYP2B6 in response to indirect activators (i.e., PB and phenytoin) has not been observed with any immortalized hepatic cell lines (i.e., HepG2, Huh-7, Fa2N-4) to date, which has been attributed to insufficient expression and cell-signaling functionality to sequester CAR within the cytosol or translocate it to the nucleus (Sueyoshi et al., 2008; Templeton et al., 2011). CYP2B6 induction in cryo-HepaRG cultures (three independent lots) was further contextualized against the range of responses observed in SC-PHH and showed comparable (and substantially less variable) CYP2B6 induction in response to PB (Fig. 9). CYP2B6 mRNA induction with PB over three independent assay plates produced ∼15-fold induction responses that were larger but consistent with enzymatic activity induction (Fig. 10B). Consistent with the CYP1A2 induction evaluations, 1-hour bupropion substrate incubations were effective in cryo-HepaRG, further supporting their metabolic competence akin to SC-PHH and distinguishing them from dedifferentiated cell lines that are generally known to have undetectable enzymatic activity levels.
Two agonists of PXR, RIF and hyperforin, were used to evaluate the competence of cryo-HepaRG cultures (Fig. 8C). According to these data, cryo-HepaRG cultures were clearly responsive to PXR agonists as has been reported previously with freshly differentiated HepaRG cultures (Andersson et al., 2012). Analogously to the CYP2B6 induction evaluations with cryo-HepaRG, lower concentrations of inducers (hyperforin and RIF) were used to activate PXR at the same time minimizing the potential for crosstalk with the CAR pathway. Induction of CYP3A4/5 metabolic activity compared favorably with the range of responses observed with SC-PHH (Fig. 9). The inter-lot variation with cryo-HepaRG (three independent lots show no statistically significant differences, Table 5) was profoundly lower than the variability observed across 52 SC-PHH preparations. CYP3A4 mRNA was also effectively induced with 10 μM RIF over three independent assay plates. The ∼60-fold mRNA induction produced was larger than the fold changes observed at the enzymatic activity level (Fig. 10C).
Inter-lot reproducibility
The farnesoid X receptor is an important regulator of bile homeostasis, and plays an important role in liver function (Teodoro et al., 2011). We assessed FXR functionality in cryo-HepaRG cultures with the agonist chenodeoxycholic acid (CDCA) and measured induction of ABCB11 mRNA expression (Fig. 8D). ABCB11 is a FXR target gene encoding for the bile salt export pump, which is heavily involved in bile acid homeostasis and plays an important role in biliary efflux of bile acids that can be quantitatively modeled in vitro with SC-PHH (Plass et al., 2002),(Liu et al., 1999). With CDCA treatment we observed a concentration-related induction of ABCB11 in cultures of cryo-HepaRG consistent with reports in SC-PHH (Yu et al., 2002). These data suggest that HepaRG cells support the FXR signaling pathway.
We further explored the effects of cell culture media supplements and Geltrex overlay with cryo-HepaRG on liver enzyme inducibility (Fig. 11). Here we compared CYP1A2, CYP2B6, and CYP3A4/5 enzymatic induction responses with both the HPRG740 (serum-containing) and HPRG750 (serum-free)-supplemented media in the presence and absence of Geltrex overlay. Cultures were treated for 72 hours with prototypical AhR, CAR, and PXR activators. In general, comparable fold induction responses were observed with either HPRG740- or HPRG750-supplemented media using prototypical inducers in the presence or absence of Geltrex overlay. However, with overlay, serum-free HPRG750-supplemented media supported more robust induction responses for CYP1A2 and CYP2B6, whereas the more balanced CYP3A induction by RIF and PB was consistent with induction responses of SC-PHH in serum-free induction media. Analogously to overlay experiments with HPRG720-supplemented metabolism media, the use of Geltrex overlay appeared to elevate basal levels of metabolism. In addition, the use of Geltrex overlay in general gave higher induction responses for CYP1A2, CYP2B6, and CYP3A4/5 with either HPRG740- or HPRG750-supplemented media. These data indicate that overlaying cultures of cryopreserved (and potentially freshly differentiated) HepaRG cells results in higher baseline metabolic capacities and comparable fold induction responses. The use of serum-containing or serum-free media supplements alone did not result in a substantial change in induction responsiveness with these prototypical inducers in cryo-HepaRG cultures.
Impact of media and overlay (Geltrex) on induction with cultures of cryo-HepaRG. In these experiments the effects of Geltrex overlay in cryo-HepaRG cultures on induction responses were evaluated by assaying enzymatic activities in response to specific receptor activators for AhR, CAR, and PXR and by monitoring CYP1A2 (A), CYP2B6 (B), and CYP3A4/5 (C) enzymatic activities after 72 hours of exposure. Data represent the mean of three independent wells with error bars showing the standard deviations of mean responses. Asterisk (*) indicates P < 0.05 (Tukey’s pairwise comparison) relative to respective DMSO control groups for each culture media/overlay combination.
CAR Translocation in HepaRG Cells.
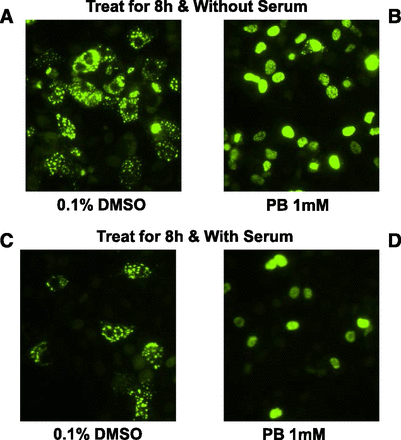
To date, no immortalized hepatic cell line has been shown to effectively support the CAR signal transduction pathway, which is characterized by appreciable levels of CAR expression, cytoplasmic retention of expressed CAR, and nuclear translocation of CAR in response to activators such as PB. Previous reports with overexpressed CAR–green fluorescent protein fusion protein (transfected via expression vectors) in HepG2 cells demonstrated that the CAR fusion protein spontaneously accumulates in cell nuclei, resulting in constitutive activity that renders CAR nonresponsive to its activators like PB, which rely on cytosolic sequestration and nuclear translocation as an activation mechanism (Kobayashi et al., 2003). Proper CAR sequestration and translocation are hallmark features of hepatocyte functionality that has only been shown in vivo or with cultures of PHH to date. To evaluate the function of the CAR cytoplasmic retention mechanism within cryo-HepaRG, cells were thawed, plated, and infected with adenovirus-expressing EYFP-hCAR. Cells were cultured in serum-containing and serum-free media conditions treated with vehicle or with 1 mM PB. Fluorescent images were captured ∼8 hours after treatment to examine the cellular localization of EYFP-hCAR within HepaRG cells (Fig. 12). Fluorescent images and cell counts demonstrated that EYFP-hCAR was predominantly localized to the cytoplasm of HepaRG cells (70% cytosolic, 15% nuclear, and 15% mixed) without activation. In contrast, over 90% of EYFP-hCAR accumulated in the nuclei of PB-treated HepaRG cells. For the first time in a hepatic cell line, these data demonstrate the existence of a functioning CAR cytoplasmic retention mechanism, as well as the ability of CAR to translocate to the nucleus in response to activation by PB in cryo-HepaRG.
CAR translocation in cryo-HepaRG cultures in response to phenobarbital. Cryo-HepaRG cultures were plated in HPRG770-supplemented media and allowed to equilibrate overnight for ∼24 hours prior to changing media to HPRG750 (A and B) or HPRG740 (C and D) induction media. Cryo-HepaRG were transduced with a green fluorescent protein–tagged CAR using an adenoviral delivery system and treated with indirect CAR activator PB or vehicle control (0.1% DMSO). CAR translocation was evaluated using a fluorescent microscope after 8 hours of treatment with PB, revealing a clear translocation of CAR in both serum-free and serum-containing induction media.
Discussion
More than 300 publications have reported studies with HepaRG cells, with the majority of research focused on freshly differentiated HepaRG rather than the newer cryopreserved format (cryo-HepaRG). Cryo-HepaRG, although significantly more accessible and experimentally flexible, differ from freshly differentiated HepaRG with respect to their detachment from freshly differentiated monolayers (disrupting cell-cell/cell-matrix interactions important for hepatocyte function), cryopreservation, thawing, and reattachment. Although it has been claimed that HepaRG cell cultures (fresh and cryopreserved) are comparable to SC-PHH, comparisons have been limited in functional coverage and broader contextualization to ranges of interindividual variation. In this report we address these limitations and identify an initial adaptation period in which cryo-HepaRG recover functionality. We provide broader contextualization of cryo-HepaRG metabolic competence, uptake transport, and liver enzyme inducibility to ranges of interindividual variation observed with PHH, as well as genotyping analysis of pharmacologically important drug clearance alleles. To our knowledge, this is the first report of the effect that extracellular matrix overlay has on cryo-HepaRG drug metabolism, transport, and liver enzyme induction. Finally, we demonstrate for the first time that cryo-HepaRG can sequester CAR in the cytosol of hepatocytes and translocate CAR to hepatocyte nuclei upon PB treatment, analogously to CAR disposition in liver and PHH.
It is well known that hepatocyte cell-cell interactions are key drivers of hepatocyte functionality with PHH that are disrupted during cell isolations from liver (Hamilton et al., 2001). This can result in dedifferentiation and reduced drug-metabolizing enzyme expression/activity, attributed to the loss of cell-cell interactions and cell-matrix interactions shifting these epithelial cells to more proliferative states. A similar process was observed with the cryo-HepaRG. Freshly differentiated HepaRG cultures disrupted during detachment/cryopreservation appeared to require a form of reorganization into organized, differentiated “epithelia” upon reattachment. However, unlike PHH, cryo-HepaRG were able to recover their fresh-like state for multiple weeks in culture. Both freshly differentiated HepaRG and cryo-HepaRG appeared to form two distinct cell types with “cords” of hepatocyte-like cells. These networks did not immediately reform but adaptively matured over 3–4 days, stabilizing by ∼7–10 days to fresh-like topologies that persisted for at least 22 days. Although the mechanisms of how cryo-HepaRG cultures recover fresh-like topologies are not clear, possible mechanisms might include migration of hepatocyte-like cells in culture or some form of cellular transdifferentiation (Cerec et al., 2007) whereby hepatocyte-like and cholangiocyte-like cells switch identities. After ∼10 days of recovery/redifferentiation, enzymatic activities with cryo-HepaRG were restored to levels comparable to cryo-HepaRG suspension activities that were substantially higher (∼7-fold on average) than day-4 activities across the enzyme panel. Since drug-metabolizing enzymes are established markers of hepatocyte differentiation, lower day-4 metabolic competence, coupled with cell-morphology observations, indicates an initial loss of cellular differentiation with cryo-HepaRG. A more detailed look at the temporal kinetics of CYP3A4/5 activity loss during the initial 72 hours revealed a “half-life” of ∼34 hours with cryo-HepaRG cultures (∼10-fold decrease) that is analogous to the initial loss of metabolic competence in PHH (∼1–3 hours half-life in suspensions, ∼20–30 hours in cultures) (Smith et al., 2012). However, PHH cultures do not recover their respective suspension enzyme activity levels under standard conditions (serum-free, no DMSO); instead, activity levels stabilize after the initial loss of metabolic competency to ∼10% of their suspension levels as they adapt to their new culture environment, which lasts until they begin to reach the end of their longevity (typically ∼1 week). Presumably, this lack of recovery in PHH is the result of the limitations of standard in vitro culture models that lack tissue-like three-dimensionality and dynamic flow (Godoy et al., 2013). In the case of cryo-HepaRG, the use of high concentrations of DMSO in metabolism/differentiation media probably contributes to the observed differences.
In the broader context, metabolic competence with both SC-PHH and cryo-HepaRG was lower than suspension PHH levels, which may limit the ability of these models to generate physiologically relevant metabolites or to model metabolic clearance rates. However, these models do effectively model liver enzyme induction. In vivo, liver enzymes are often zonally enriched, with higher constitutive levels in Zone 3 and lower, more inducible levels in Zone 2. With cryo-HepaRG, we observed a similar phenomenon with varied media supplementation that may reflect “zonal” modeling. It is not clear how this “zonality” with cryo-HepaRG relates to changes observed during their readaptation period.
One possible explanation for the recovery of metabolic competence with cryo-HepaRG is that cellular proliferation might be occurring. This is improbable, however, because we observed no evidence of increasing cell numbers and HepaRG are thought to be contact-inhibited (Gripon et al., 2002). Total protein levels confirmed a lack of evidence for profoundly increased cell numbers over time with cryo-HepaRG (Supplemental Fig. 2). Additionally, we observed disproportionately higher levels of certain P450s across the panel of enzymes over time, suggesting a simple proliferation mechanism was not responsible solely for the recovery of metabolic competence. This also suggests that metabolic competence with cryo-HepaRG was not a static, intrinsic property. The disproportionately high levels of CYP2B6, CYP3A4/5, and UGT with cryo-HepaRG (day-10) were seemingly superphysiologic compared with activities in SC-PHH. However, these levels were lower than median suspension PHH levels (Fig. 4). A probable explanation for these disproportionate levels of inducible enzymes is a combination of hepatocyte redifferentiation and induction by the cell culture media that mimics constitutively induced Zone 3 of a liver lobule. Previous reports have shown that higher levels of DMSO can induce CYP3A4 (Nishimura et al., 2003). Further research to explore the consequences of disproportionate metabolic competence on metabolite profiles is warranted.
Overlay of cryo-HepaRG cultures with Geltrex, an extracellular matrix commonly used to improve longevity and quality with PHH, yielded a surprising effect on cryo-HepaRG morphologies. We observed higher apparent hepatocyte-to-cholangiocyte ratios along with corresponding elevations in basal metabolic competence (∼2- to 3-fold). This “sandwich” culture approach is not required with HepaRG cells, but the high concentrations of DMSO that are used to differentiate HepaRG cells (Gripon et al., 2002) can also inhibit some P450 enzymes (e.g., CYP2E1). CYP2E1 is involved in many examples of metabolically activated toxicity (i.e., acetaminophen), and overlay of cryo-HepaRG may support more mature differentiation and require lower DMSO levels (Easterbrook et al., 2001; Trafalis et al., 2010). Recent reports have used HepaRG cells in three-dimensional culture configurations and observed similar increases in metabolic capacity/longevity. However, these models can be complex to generate, highly variable, and difficult to normalize (Malinen et al., 2014; Mueller et al., 2014). Overlaying cryo-HepaRG may provide a solution for screening and high-content imaging that obviates the need for confocal microscopy by creating a more coplanar environment.
Cryo-HepaRG cells are an attractive cell model for in vitro screening owing to their functionality and year-over-year availability from a single genetic background, but they pose a challenge for xenobiotic metabolism research as they exhibit poor metabolizer alleles for CYP2D6 and CYP3A5 (and, to a lesser extent, CYP2C9). Although these polymorphisms may limit the utility of current HepaRG models, they do reflect a substantial proportion of the population owing to the prevalence of these alternate alleles. Further efforts to genetically alter these alleles could provide additional tools for research that better reflect population diversity.
In our hands cryo-HepaRG cells responded to aflatoxin B1 [which produces a CYP3A-mediated genotoxic epoxide metabolite (Kirby et al., 1996)] analogously to freshly differentiated HepaRG (Josse et al., 2008). This appeared to be dependent on CYP3A metabolism, as an attenuation was observed with CYP3A inhibitor ketoconazole. This functionality coupled with the substantially reduced lot variation of cryo-HepaRG make this an attractive model for toxicology screening.
One of the hallmarks of a functional in vitro liver model is the ability to respond to prototypical hepatic receptor activators to induce liver enzymes. Cryo-HepaRG cells were clearly able to support prototypical responses to AhR, CAR, PXR, and FXR activators comparably to SC-PHH. One notable difference with cryo-HepaRG cultures was the reduced magnitude of CYP2B6 induction in response to rifampicin, and further research to explore this mechanism is warranted. Geltrex overlay had little if any effect on the fold induction of liver enzyme levels or the muted CYP2B6 rifampicin response but did elevate basal levels of metabolic capacity. CAR activators PB (indirect) and CITCO produced induction responses suggestive of a highly functional CAR pathway in cryo-HepaRG. Further exploration of this mechanism confirmed that cryo-HepaRG cultures support nuclear sequestering of CAR in the cytosol and translocation to hepatocyte nuclei upon PB treatment. CAR translocation was not observed in the cholangiocyte-like cells but was observed with and without serum.
In conclusion, this report provides extensive evidence demonstrating the functionality of cryo-HepaRG and contextualizes various hepatocyte functionalities to ranges of response observed with SC-PHH. The work identifies important temporal dynamics, reveals the beneficial effects of extracellular matrix overlay, provides important genotyping data, and provides the first evidence for a functional CAR translocation pathway with cryo-HepaRG.
Acknowledgments
The authors thank Manda Edwards and Ashley Ganoe for laboratory support with overlay experiments. The authors also thank Patricia (Miki) Pawlowski and Jasminder Sahi for contributions to reference primary hepatocyte uptake transport data; and acknowledge Alex Merrick, Sreenivasa Ramaiahgari, Bryan Mackowiak, Jessica Bonzo, Rafal Witek, and Mark Powers for the efforts in the preparation of this manuscript.
Authorship Contributions
Participated in research design: Jackson, Li, Chamberlain, Wang, Ferguson.
Conducted experiments: Jackson, Li, Chamberlain, Ferguson.
Performed data analysis: Jackson, Li, Wang, Ferguson.
Wrote or contributed to the writing of the manuscript: Jackson, Li, Chamberlain, Wang, Ferguson.
Footnotes
- Received February 1, 2016.
- Accepted June 22, 2016.
↵1 Current affiliation: J.P.J. currently with Qualyst Transporter Solutions (QTS).
↵2 Current affiliation: E.D.C. currently with Quest Pharmaceutical Services (QPS).
↵3 Current affiliation: S.S.F. currently with the National Toxicology Program Division, National Institute of Environmental Health Sciences (NIEHS).
The laboratory research was funded by Life Technologies (Thermo Fisher) research and development. Resources for data analysis and manuscript preparation were supported by the National Institutes of Health, National Institute of Environmental Health Sciences, the National Toxicology Program Division, and by Qualyst Transporter Solutions, Inc.
Some of the data presented were part of a poster abstract: Jackson JP, Edwards M, Chamberlain E, and Ferguson, SS (2011) Cryopreserved HepaRG cells: an alternative in vitro screening tool for human hepatic drug metabolism, induction of metabolism, and safety applications, at the 2011 ISSX meeting; Atlanta, GA. International Society for the Study of Xenobiotics, Atlanta GA.
↵
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
Abbreviations
- ABCB11
- ATP-binding cassette, sub-family B, member 11
- AhR
- aryl hydrocarbon receptor
- CAR
- constitutive androstane receptor
- CDCA
- chenodeoxycholic acid
- CDF
- 5-(and-6)-carboxy-2′, 7′-dichlorofluorescein
- CDFDA
- carboxy dichlorofluorescein diacetate
- CITCO
- 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl)oxime
- cryo-HepaRG
- cryopreserved HepaRG cells
- DMSO
- dimethyl sulfoxide
- ES
- estrone sulfate
- EYFP-hCAR
- enhanced yellow fluorescent protein–tagged hCAR
- FMO
- flavin monooxygenase
- FXR
- farnesoid X receptor
- HepaRG
- HepaRG cells
- LC-MS/MS
- liquid chromatography–tandem mass spectrometry
- MRP2
- multidrug resistance–associated protein 2
- OATP1B1/3
- organic anion–transporting polypeptides 1B1 and 1B3
- PB
- phenobarbital
- PBS
- phosphate-buffered saline
- P450
- cytochrome P450
- PHH
- primary human hepatocytes
- PXR
- pregnane X receptor
- RIF
- rifampin
- SC-PHH
- sandwich-cultured primary human hepatocytes
- SULT
- sulfotransferase
- UGT
- UDP-glucuronosyltransferase
- WEM
- Williams’ E media
- U.S. Government work not protected by U.S. copyright