Abstract
Genes coding for cytochrome P450 are regulated by endogenous hormones such as the growth hormone, corticosteroids, thyroid, and sex hormones. Secretion of these hormones is regulated by the respective hypothalamus–pituitary–secretory organ axes. Since the brain sends its serotonergic projections from the raphe nuclei to the hypothalamus, we have assumed that damage to these nuclei may affect the neuroendocrine regulation of cytochrome P450 expression in the liver. Thereby, 5,7-dihydroxytryptamine (5,7-DHT), a serotonergic neurotoxin, was injected into the dorsal and median raphe nuclei of male Wistar rats. Ten days after the neurotoxin injections, the brain concentrations of neurotransmitters, serum hormone, and cytokine levels, as well as the expression of cytochrome P450 in the liver were measured. Injection of 5,7-DHT decreased serotonin concentration in the brain followed by a significant rise in the levels of the growth hormone, corticosterone, and testosterone, and a drop in triiodothyronine concentration in the serum. No changes in interleukin (IL) levels (IL-2 and IL-6) were observed. Simultaneously, the activity and protein level of liver CYP1A, CYP3A1, and CYP2C11 rose (the activity of CYP2A/2B/2C6/2D was not significantly changed). Similarly, the mRNA levels of CYP1A1, CYP1A2, CYP2C11, and CYP3A1 were elevated. This is the first report demonstrating the effect of intracerebral administration of serotonergic neurotoxin on liver cytochrome P450. The obtained results indicate involvement of the brain serotonergic system in the neuroendocrine regulation of liver cytochrome P450 expression. The physiologic and pharmacological significance of the findings is discussed.
Introduction
The role of the nervous system in the regulation of cytochrome P450 (P450) expression has not yet been fully recognized. Since the secretion of hormones regulating cytochrome P450 genes (the growth hormone, corticosteroids, thyroid hormones, sex hormones) is controlled by the nervous system (Müller, 1989; McMahon et al., 2001), changes in brain neurotransmission may influence cytochrome P450 expression in a neurotransmitter-dependent way (Wójcikowski and Daniel, 2011).
The following axes play a key role in the hormonal regulation of hepatic cytochrome P450: the hypothalamic-pituitary-adrenal axis (which regulates cortisol/corticosterone level), the hypothalamic-pituitary-thyroidal axis (which controls triiodothyronine and thyroxine levels), and the so-called hypothalamic-pituitary-hepatic axis (which affects growth hormone level). These three axes are controlled by the brain nervous system, in particular by the densely and diversely innervated hypothalamus (Törk, 1990; Lechin et al., 2006). The hypothalamus contains the paraventricular nucleus (which produces the corticotropin-releasing hormone, the thyrotropin-releasing hormone, and the growth hormone release–inhibiting hormone somatostatin) and the arcuate nucleus (which synthesizes the growth hormone–releasing hormone), which affects the secretion of hormones from the anterior lobe of the pituitary gland, and these hormones are engaged in the regulation of P450 gene expression. CYP2C11 is the main male rat isoform in the liver, being stimulated by pulsatile growth hormone (GH) secretion. The expression of male rat CYP2A2, CYP2C13, CYP3A2, and CYP4A2, as well as CYP2B1/2, CYP3A2, and CYP3A18, dominant in males, also depends on pulsatile GH secretion (Waxman et al., 1995; Waxman and O’Connor, 2006). Corticosterone is a positive regulator of CYP1A and CYP3A (Gibson et al., 2002; Monostory et al., 2005), whereas thyroid hormones negatively affect the expression of different P450 isoforms (Yamazoe et al., 1989; Murayama et al., 1991; Liddle et al., 1998). Our recent studies have provided a vast body of direct evidence that brain dopaminergic (Wójcikowski et al., 2007, 2008) and noradrenergic (Bromek et al., 2013; Sadakierska-Chudy et al., 2013) systems regulate cytochrome P450 expression via a neuroendocrine mechanism.
The hypothalamus is innervated by projections originating from the serotonergic anterior raphe nuclei, mainly from the dorsal (DRN) and median (MRN) raphe nuclei. Serotonergic projections reach the paraventricular and arcuate nuclei (Sawchenko et al., 1983; Gruber et al., 1987; Willoughby and Blessing, 1987; Larsen et al., 1996). The effect of the brain serotonergic system on the regulation of pituitary hormones secretion has not been satisfactorily explained, since both stimulatory and inhibitory actions can be observed (Tuomisto and Männistö, 1985; Müller, 1989). A number of studies showed a positive effect of brain serotonin on the secretion of adrenocorticotropic hormone (Jørgensen, 2007). The stimulating effect of the brain serotonergic system on growth hormone secretion can be initiated by either growth hormone–releasing hormone release from the arcuate nucleus (Vijayan et al., 1978; Murakami et al., 1986; Willoughby et al., 1987) or the suppression of somatostatin release from the paraventricular nucleus (Mota et al., 1995; Valverde et al., 2000). A majority of data indicate a complex mechanism of the serotonin-regulated thyroid-stimulating hormone (TSH) secretion which may occur at the level of the hypothalamus (inhibition of thyrotropin-releasing hormone release or somatostatin secretion) or pituitary gland (stimulation of TSH secretion) (Abrahamson et al., 1987; Toivonen et al., 1990; Masalova and Sapronov, 2009).
Our preliminary studies into the general damage of the serotonergic system (central and peripheral) indicate involvement of this system in the regulation of cytochrome P450. Intraperitoneal administration of the neurotoxin p-chloroamphetamine (PCA) or the serotonin synthesis inhibitor p-chlorophenylalanine (PCPA) leads to an increase in CYP1A activity and a decrease in CYP2C11 and CYP3A activities (Kot and Daniel, 2011). On the other hand, a 3-week tryptophan-free diet enhances the activity of many P450 isoforms (CYP1A, 2A, 2B, 2C6, 2D, 3A) and diminishes CYP2C11 activity (Kot et al., 2012). Such changes in cytochrome P450 activity may be due to the reduction in serotonin level in both the brain and periphery and, possibly, due to some additional effects in the liver produced by the applied substances or the diet. To recognize the effect of the brain serotonergic system alone on the activity of liver cytochrome P450 and engagement of central neuroendocrine regulation, all of the aforementioned peripheral influences of neurotoxins or diet have to be excluded.
The aim of the present study was to assess the role of the brain serotonergic system in the regulation of liver cytochrome P450. To this end, we injected the specific serotonergic neurotoxin 5,7-dihydroxytryptamine (5,7-DHT) into the DRN and MRN of rat brain, which project to the hypothalamus. Afterward, brain serotonin, serum hormones, and liver cytochrome P450 expression were examined.
Materials and Methods
Animals.
Adult male Wistar Han rats (Charles River Laboratories, Sulzfeld, Germany) weighing 280–300 g were kept individually under standard laboratory conditions (12:12-hour light/dark cycle; temperature of 22 ± 2°C; room humidity of 55 ± 5%). The animals had free access to food and tap water, but 18 hours before decapitation, they were deprived of food (both control and neurotoxin-treated animals) because the digestive process might have affected enzymatic activity. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Bioethical Committee at the Institute of Pharmacology, Polish Academy of Sciences, Kraków.
Drugs and Chemicals.
The following compounds were used for the study: noradrenaline, dopamine, serotonin (5-hydroxytryptamine), 5-hydroxyindoleacetic acid (5-HIAA), 5,7-DHT (a creatinine sulfate salt), ascorbic acid, NADPH, NADP, glucose-6-phosphate-dehydrogenase and glucose-6-phosphate, and caffeine and its metabolites (theobromine, paraxanthine, theophylline, and 1,3,7-trimethyluric acid), which were purchased from Sigma-Aldrich (St. Louis, MO). Testosterone and its metabolites were provided by Steraloids (Newport, RI). Warfarin was donated by Merck (Darmstadt, Germany), whereas 7-hydroxywarfarin was synthesized at our institute (Daniel et al., 2006). Bufuralol and 1-hydroxybufuralol were a gift from Dr. Y. Funae of the Osaka University (Osaka, Japan). The polyclonal primary anti-rat CYP1A1 antibody, a secondary antibody (anti-IgG), and rat cDNA-expressed P450s were obtained from Gentest Corp. (Woburn, MA). The polyclonal primary anti-rat CYP2C11 antibody was purchased from Abcam (Cambridge, UK); the anti-rat CYP3A1 and CYP3A2 antibodies were obtained from Millipore (Temecula, CA). The chemiluminescence reagent LumiGlo kit came from KPL (Gaithersburg, MD). Enzyme-linked immunosorbent assay (ELISA) kits for the serum hormones (growth hormone and testosterone) were purchased from DRG MedTek (Warsaw, Poland), and for corticosterone, triiodothyronine (T3), and thyroxine (T4), from Endocrine Technologies (Newark, CA). ELISA kits for interleukin-2 (IL-2) and IL-6 were obtained from R&D Systems (Minneapolis, MN). All of the organic solvents were of high-performance liquid chromatography (HPLC) purity and were supplied by Merck. Ketamine (ketamine hydrochloride) and Sedazin (xylazine hydrochloride) were obtained from Biowet (Puławy, Poland).
Surgery and Lesion of Brain Serotonergic System.
The rats were anesthetized with ketamine (65 mg/kg i.p.) and xylazine (10 mg/kg i.p.) and were placed in a Kopf (Tujunga, CA) stereotaxic apparatus. All solutions were freshly prepared on the days of experimentation. 5,7-DHT (a toxin specific to serotonergic neurons) was dissolved in a 0.9% NaCl with 0.05% ascorbic acid and injected into the DRN and MRN of the brain at a concentration of 10 μg/μl (1 μl infused at a rate of 1 μl/min) into both raphe nuclei (10 μg per raphe nucleus). The following coordinates were used (Paxinos and Watson, 2007): AP (anterior-posterior) –7.9, L (lateral) 0.0 from the bregma, and V (ventral) –7.9 (MRN), –5.9 (DRN) from the surface of the dura (one after the other, respectively). The needle was left in place for 5 minutes after injection before it was slowly withdrawn. Control rats (sham-operated animals, n = 7) were subjected to the same procedure as the 5,7-DHT–treated group (n = 7), except for the fact that they received vehicle treatment (a 0.9% NaCl + 0.05% ascorbic acid) instead of 5,7-DHT. The placement of the needle was histologically verified in a preliminary experiment with three rats. Moreover, the correctness of injections was confirmed by measuring serotonin levels in brain structures. An adequate decrease (below 50% of the control level) in the serotonin concentration of 5,7-DHT–injected animals was observed.
Sample Collection.
Ten days after the 5,7-DHT lesion, the rats were decapitated (10—10:30 a.m.), and their livers and brains were immediately removed. The brains were dissected into the cerebellum, hypothalamus, thalamus, nucleus accumbens, striatum, hippocampus, frontal cortex, cortex, brain stem, and medulla oblongata. The liver and brain tissues were frozen on dry ice and stored at –70°C until they were further analyzed. The blood was collected into tubes and the serum was separated by centrifugation and stored at –20°C. Liver microsomes were prepared by differential centrifugation in 20 mM Tris/KCl buffer (pH 7.4) including washing with 0.15 M KCl, as described previously (Kot and Daniel, 2011).
Analysis of Serotonin, Its Metabolite 5-HIAA, and Catecholaminergic Neurotransmitters in the Brain.
Concentrations of endogenous serotonin and its metabolite 5-HIAA were measured by a high-performance liquid chromatography with electrochemical detection according to the previously described method (Bromek et al., 2013). Moreover, to check the specificity of lesion of the brain serotonergic system by the neurotoxin 5,7-DHT, concentrations of the catecholaminergic neurotransmitters noradrenaline and dopamine were also simultaneously estimated. In brief, the brain structures were homogenized in ice-cold 0.1 M HClO4. The obtained homogenates were centrifuged (15,000 × g) and the supernatants were filtered off and injected into the HPLC system. A GOLD-Hypersil analytical column (3 μm, 100 × 3 mm; Thermo Scientific, Waltham, MA), kept at 30°C, was used. The eluent consisted of 0.1 M KH2PO4, 0.5 mM Na2EDTA, 80 mg/l sodium 1-octanesulfonate, and a 4% methanol, and was adjusted to pH 3.7. The flow rate of the eluent was 0.6 ml/min.
Determination of Cytochrome P450 Isoform Activities in Liver Microsomes.
The activity of rat CYP1A was studied by measuring the rate of caffeine metabolism (C-8-hydroxylation and N-demethylation) at a substrate concentration of 100 μM, 1 mg of microsomal protein/ml, and incubation time of 50 minutes. Caffeine and its metabolites were analyzed by HPLC with UV detection, as described previously (Kot and Daniel, 2008). The activity of CYP2C6 was studied by measuring the rate of warfarin 7-hydroxylation at a substrate concentration of 60 μM, 1 mg/ml microsomal protein, and incubation time of 15 minutes. Warfarin and its metabolite were analyzed by HPLC with fluorescence detection, as described previously (Daniel et al., 2006). The activities of CYP2A, CYP2B, CYP2C11, and CYP3A were examined by measuring the rate of P450 isoform–specific reactions: the 7α-, 16β-, 2α- and 16α-, 2β- and 6β-hydroxylation of testosterone, respectively, at a testosterone concentration of 100 μM, 1 mg/ml microsomal protein, and incubation time of 15 minutes. The metabolites formed were analyzed by HPLC with UV detection, as described previously (Haduch et al., 2006). The activity of CYP2D was studied by measuring the rate of bufuralol 1′-hydroxylation at a substrate concentration of 10 μM, 0.5 mg/ml microsomal protein, and incubation time of 10 minutes. Bufuralol and its metabolite were analyzed by HPLC with fluorescence detection, as described previously (Hiroi et al., 1998).
Western Blot Analysis and ELISA.
The protein levels of cytochrome P450 isoforms in the liver microsomes of control and 5,7-DHT–treated rats were estimated using a Western immunoblot analysis. Microsomal proteins, 10 μg (CYP3A1, CYP3A2, and CYP2C11) or 20 μg (CYP1A), were separated by an SDS polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes, and then immunodetected and visualized by chemiluminescence, as previously described (Sadakierska-Chudy et al., 2013). The following primary antibodies were used: a polyclonal goat anti-rat antibody, raised against CYP1A1 (Gentest), which also recognized the CYP1A2 isoform; a polyclonal rabbit anti-rat CYP2C11 antibody (Abcam); a polyclonal rabbit anti-rat CYP3A1 and anti-rat CYP3A2 antibodies (Millipore). After incubation with a primary antibody, the blots were incubated with a secondary antibody, i.e., a horseradish peroxidase–conjugated anti-IgG (Gentest). The rat cDNA-expressed proteins CYP1A2 (5 μg), CYP2C11 (5 μg), CYP3A1 (1 μg), and CYP3A2 (5 μg) (Supersomes; Gentest) were used as standards. P450 protein bands were quantified with the Luminescent Image analyzer LAS-1000 using the Image Reader LAS-1000 (Fuji Film, Tokyo). Serum hormone and cytokine levels were measured using ELISA kits: growth hormone and testosterone kits (DRG; MedTek); corticosterone, T3, and T4 ELISA kits (Endocrine Technologies); and IL-2 and IL-6 ELISA kits (R&D Systems). A Synergy Mx Monochromator–based Multi-Mode Microplate Reader (Biotek, Winooski, VT) was used to measure the absorbance.
RNA Isolation and Quantitative Reverse-Transcription Polymerase Chain Reaction Polymerase Chain Reaction.
Frozen liver (25 mg) was homogenized using the TissueLyser II (2 × 2 minutes at 30 Hz; Qiagen Valencia, CA), and the total RNA was extracted using a mirVana isolation kit (Life Technologies, Carlsbad, CA) following the manufacturer’s instructions. RNA was eluted with 50 μl of RNase-free H2O (Sigma-Aldrich). The quantity and the quality of RNA were assessed using a NanoDrop 8000 Spectrophotometer (Thermo Scientific) and agarose gel electrophoresis. RNA samples were stored at −70°C until they were further used. The first-strand cDNA products were generated using a Transcriptor High Fidelity cDNA Synthesis Kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s recommendations. In brief, a reverse transcription was performed using 2 µg of the total RNA and oligo(dT) primers at a total volume of 20 µl. cDNA synthesis was carried out at 55°C for 30 minutes and at 85°C for 5 minutes to inactivate the enzyme. Following the reverse transcription, samples were diluted with 20 µl of RNase-free water and stored at −20°C until subsequent testing. The expression of genes encoding cytochrome P450 enzymes (CYP2C11, CYP3A1, CYP3A2, CYP1A1, and CYP1A2) and GAPDH and β-actin as reference genes was detected by a real-time polymerase chain reaction (PCR). The reaction mixture, 10 µl, consisted of 4.5 μl of cDNA, 5 μl of TaqMan Gene Expression Master Mix, and 0.5 µl of TaqMan assay (Life Technologies, Carlsbad, CA). Negative control samples were processed likewise, except for the fact that the template was omitted. Real-time PCR runs were performed using the CFX96 PCR system (Bio-Rad, Hercules, CA), and standard thermal cycling conditions were used (50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 minutes and 60°C for 1 minute). The PCR reaction of target and reference genes was run in duplicate.
Real-Time PCR Data Processing.
To obtain Ct values (Ct is defined as the cycle number at which the fluorescence crossed the fixed threshold), appropriate thresholds were drawn manually (3000). The mean Ct and a standard deviation were calculated for the replicates of each reaction. Samples with replicate standard deviations >0.4 were repeated or excluded from the analysis. The level of P450 transcripts was normalized to the GAPDH and β-actin expression in each sample, and relative quantification was obtained using the comparative delta-delta Ct method (2−∆∆Ct). The relative amount of target transcript was expressed as a fold change in the expression level relative to the calibrator (i.e., the average ΔCt of the control group).
Data Analysis.
The obtained values are the mean ± S.E.M. of five to seven animals. Changes in brain neurotransmitter levels, serum hormone, and interleukin concentrations, as well as liver cytochrome P450 isoform activities, protein, and mRNA levels were statistically assessed using a two-tailed Student’s t test. The results were regarded as statistically significant when P < 0.05.
Results
The Effect of Intracerebral Injection of the Neurotoxin 5,7-DHT on the Concentration of Serotonin and Its Metabolite 5-HIAA in the Brain.
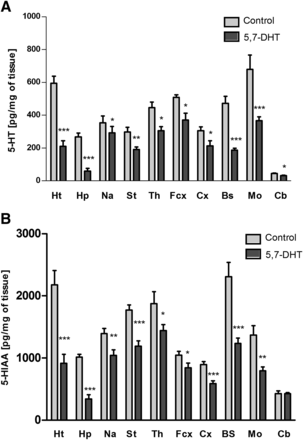
Injection of the serotonergic neurotoxin 5,7-DHT into the anterior raphe nuclei DRN and MRN of the brain specifically and significantly decreased the level of serotonin and its metabolite 5-HIAA in the brain structures tested (Fig. 1) except for the cerebellum, in which 5-HIAA was not changed. The neurotoxin also slightly reduced dopamine levels in the medulla oblongata (Fig. 2A) and noradrenaline levels in the brain stem (Fig. 2B).
The effect of injection of 5,7-DHT into the median and dorsal raphe nuclei of rat brain on the levels of serotonin (5-HT) (A) and its metabolite 5-HIAA (B) in the following brain structures: the hypothalamus (Ht), hippocampus (Hp), nucleus accumbens (Na), striatum (St), thalamus (Th), frontal cortex (Fcx), cortex (Cx), brain stem (Bs), medulla oblongata (Mo), and cerebellum (Cb). The data are expressed as the mean ± S.E.M. of the control (n = 7) and the 5,7-DHT (n = 6) group. Statistical significance was assessed by Student’s t test and shown as *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 compared with the control. The control values (pg/mg of tissue) are as follows: 594.7 ± 42.2 (Ht), 267.2 ± 68.5 (Hp), 352.3 ± 41.7 (Na), 297.4 ± 28.8 (St), 446,4 ± 33.3 (Th), 514.5 ± 16.5 (Fcx), 305.3 ± 22.7 (Cx), 472.5 ± 47.7 (Bs), 678.5 ± 87.7 (Mo), and 44.2 ± 3.1 (Cb) (A); 2177.1 ± 228.57 (Ht), 1014.8 ± 45.3 (Hp), 1394.3 ± 82.5 (Na), 1772.0 ± 80.6 (St), 1875.4 ± 192.3 (Th), 1045.2 ± 63.2 (Fcx), 893.7 ± 47.6 (Cx), 2309.9 ± 226.5 (Bs), 1241.3 ± 148.9 (Mo), and 426.9 ± 11.4 (Cb) (B).
The effect of injection of 5,7-DHT into median and dorsal raphe nuclei of rat brain on dopamine (DA) (A) and noradrenaline (NA) (B) levels in the following brain structures: hypothalamus (Ht), hippocampus (Hp), nucleus accumbens (Na), striatum (St), thalamus (Th), frontal cortex (Fcx), cortex (Cx), brain stem (BS), medulla oblongata (MO), and cerebellum (Cb). The data are expressed as the mean ± S.E.M. of the control (n = 7) and the 5,7-DHT (n = 6) group. Statistical significance was assessed by Student’s t test and shown as *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 compared with the control. The control values (pg/mg of tissue) are as follows: 241.2 ± 24.0 (Ht), 14.4 ± 4.9 (Hp), 4535.8 ± 251.4 (Na), 6553.8 ± 331.6 (St), 115.9 ± 22.6 (Th), 291.7 ± 55.4 (Fcx), 377.4 ± 43.1 (Cx), 116.1 ± 13.5 (Bs), and 36.4 ± 4.4 (Mo) (A); 2368.4 ± 120.5 (Ht), 286.5 ± 17.4 (Hp), 311.2 ± 49.6 (Na), 144.1 ± 13.5 (St), 422.7 ± 37.6 (Th), 215.4 ± 12.8 (Fcx), 169.6 ± 14.5 (Cx), 501.5 ± 26.3 (Bs), 467.3 ± 53.3 (Mo), and 139.0 ± 9.9 (Cb) (B).
The Effect of Intracerebral Injection of the Neurotoxin 5,7-DHT on P450 Isoform Activities in the Liver.
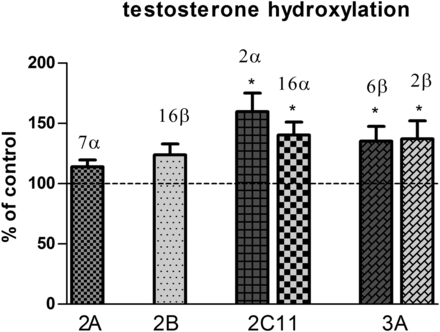
Intracerebral injection of the neurotoxin 5,7-DHT produced statistically significant increases in the activity of liver CYP2C11 and CYP3A (measured as a rate of testosterone hydroxylation in position 2α and 16α, or 2β and 6β, respectively); a similar tendency was reported in the case of CYP2B (Fig. 3). The activity of CYP1A increased as the rate of the C-8 hydroxylation of caffeine went up (Fig. 4). Such a reaction is specific to rat CYP1A, since at the substrate concentration used (100 µM) the contribution of CYP1A to this reaction amounts to ca. 73%, whereas that of CYP3A amounts only to ca. 15% (Kot and Daniel, 2008). Furthermore, CYP1A contributes to 1-N-, 3-N-, and 7-N-demethylation, although to a lower degree, i.e., to ca. 44%, 48%, and 17%, respectively (in the latter case, CYP2C contributes to 65%). The rate of all N-demethylation reactions increased after 5,7-DHT. However, in the case of 1-N- and 3-N-demethylation, those increases did not reach the level of statistical significance (P = 0.11 and P = 0.06, respectively). The activity of CYP2A, CYP2C6, and CYP2D remained practically unchanged (Figs. 3 and 4).
The effect of injection of 5,7-DHT into the median and dorsal raphe nuclei of rat brain on different P450 isoform activites, measured as a rate of specific metabolic reactions of testosterone in liver microsomes: testosterone 7α- (CYP2A), 16β- (CYP2B), 2α- and 16α- (CYP2C11), and 6β- and 2β-hydroxylation (CYP3A). All values are the mean ± S.E.M. of the control (n = 7) and 5,7-DHT (n = 6) group. Statistical significance was assessed by Student’s t test and indicated as *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 compared with the control. The control values (nmol/mg protein/min) are as follows: 152.5 ± 9.8, 14.0 ± 1.1, 922.4 ± 119.7, 492.1 ± 80.1, 47.6 ± 9.3, and 48.5 ± 65.2 (testosterone 7α-, 16β-, 2α-, 16α-, 2β-, and 6β-hydroxylation, respectively).
The effect of injection of 5,7-DHT into the median and dorsal raphe nuclei of rat brain on different P450 isoform activities, measured as a rate of P450 isoform–catalyzed reactions in liver microsomes: caffeine 1-N-demethylation (CYP1A, CYP2C, and CYP3A), 3-N-demethylation (CYP1A and CYP2C), 7-N-demethylation (CYP1A, CYP2C11, and CYP3A), 8-hydroxylation (CYP1A and CYP3A), warfarin 7-hydroxylation (CYP2C6), and bufuralol 1′-hydroxylation (CYP2D). All values are the mean ± S.E.M. of the control (n = 7) and the 5,7-DHT group (n = 7). Statistical significance was assessed by Student’s t test and is shown as *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 compared with the control. The control values (pmol/mg protein/min) are as follows: 0.8 ± 0.09 (caffeine 1-N-demethylation), 1.8 ± 0.14 (caffeine 3-N-demethylation), 1.9 ± 0.19 (caffeine 7-N-demethylation), 17.2 ± 1.02 (caffeine 8-hydroxylation), 3.6 ± 0.38 (warfarin 7-hydroxylation, 7-OH), and 1.6 ± 0.64 (bufuralol 1′-hydroxylation, 1′-OH).
The Effect of Intracerebral Injection of the Neurotoxin 5,7-DHT on P450 Isoform Expression in the Liver.
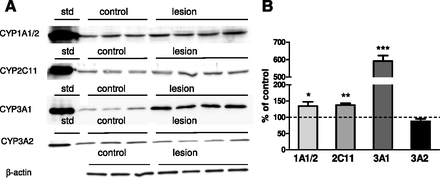
The increases in the activity of CYP1A, CYP2C11, and CYP3A positively correlated with those in P450 protein levels (Fig. 5). Intracerebral 5,7-DHT significantly increased the protein levels of CYP1A, CYP2C11, and CYP3A1 up to 134%, 138%, and 592% of the control, respectively, although not affecting that of CYP3A2, which corresponded to mRNA level. The quantitative real-time PCR analyses of P450 genes showed a significant elevation in the mRNA levels of CYP1A1, CYP1A2, CYP2C11, and CYP3A1 (Table 1).
The effect of injection of 5,7-DHT into the median and dorsal raphe nuclei of rat brain on the protein levels of CYP1A1/2, CYP2C11, and CYP3A1/2 isoforms in liver microsomes. Microsomal protein (20 µg in the case of CYP1A1/2 or 10 µg in the case of CYP2C11, CYP3A1, and CYP3A2) was subjected to a Western immunoblot analysis. The rat cDNA-expressed CYP1A2, CYP2C11, CYP3A1, and CYP3A2 proteins (Supersomes) were used as standards (std). (A) The presented results are typical of three (in the case of the control) or four (in the case of 5,7-DHT–treated rats) separate animals. (B) The data are expressed as the mean ± S.E.M. of control (n = 7) and the 5,7-DHT (n = 6) group. Statistical significance was assessed by Student’s t test and is shown as *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 compared with the control.
The effect of intracerebral injection of 5,7-DHT on the mRNA expression level of P450 genes in rat liver
The results are expressed as a fold change in relation to 2 housekeeping genes: the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene and β-actin gene controls. All values are the mean fold change calculated by the comparative 2−∆∆Ct method for control (n = 7) and the 5,7-DHT (n = 6) group.
The Effect of Intracerebral Injection of the Neurotoxin 5,7-DHT on the Serum Concentration of Hormones and Cytokines.
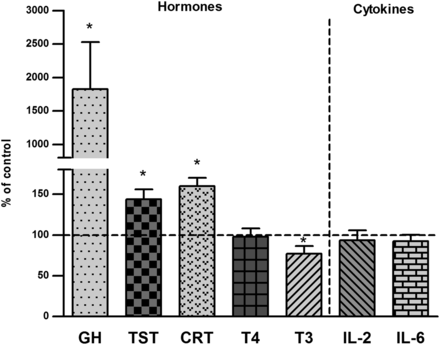
The ELISA analysis revealed significant increases in the serum concentration of the growth hormone, corticosterone, and testosterone, as well as a decrease in the thyroid hormone T3 level evoked by intracerebral injection of the neurotoxin 5,7-DHT. The concentrations of T4, IL-2, and IL-6 were not changed by 5,7-DHT (Fig. 6).
The effect of injection of 5,7-DHT into the median and dorsal raphe nuclei of the brain on hormone and cytokine concentrations in rat blood serum. All values are the mean ± S.E.M. of the control (n = 7) and the 5,7-DHT (n = 6) group. Statistical significance was assessed by Student’s t test and indicated as *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 compared with the control. The absolute control values were 2.43 ± 1.13 ng/ml, 3.87 ± 0.21 ng/ml, 43.48 ± 9.85 ng/ml, 72.61 ± 9.63 ng/ml, 1.54 ± 0.098 ng/ml, 17.89 ± 1.57 pg/ml, and 48.33 ± 2.05 pg/ml for the GH, testosterone (TST), corticosterone (CRT), T4, T3, IL-2, and IL-6, respectively.
Discussion
Our earlier studies carried out after intracerebral administration of catecholaminergic (dopaminergic and noradrenergic) neurotoxins showed that the brain nervous system may influence liver cytochrome P450 expression via central neuroendocrine regulation involving the hypothalamus and pituitary (Wójcikowski et al., 2007, 2008; Bromek et al., 2013; Sadakierska-Chudy et al., 2013; Kot et al., 2015). Our recent studies into general damage to the central and peripheral serotonergic (indoleaminergic) systems have shown that serotonin is also important for the regulation of liver cytochrome P450. Intraperitoneal administration of the serotonergic neurotoxin PCA or the serotonin synthesis inhibitor PCPA, or a 3-week tryptophan-free diet affects cytochrome P450 activity in the liver (Kot and Daniel, 2011; Kot et al., 2012).
The results presented herein, obtained after intracerebral injection of the serotonergic neurotoxin 5,7-DHT, indicate that the brain serotonergic system is involved in the regulation of cytochrome P450 expression in the liver. Damage to the DRN and MRN of the brain that project to the forebrain selectively decreases serotonin levels in all brain structures studied, including the hypothalamus, followed by changes in the serum level of hormones regulating (via their receptors) cytochrome P450 expression, which, consequently, leads to an increase in mRNA and protein levels and the activity of hormone-dependent P450 isoforms in the liver (Fig. 7). This is the first report showing that selective damage to the brain serotonergic system affects liver cytochrome P450.
The effect of local 5,7-DHT lesion of the raphe nuclei on serotonin concentration in the hypothalamus, serum hormone levels, and the expression of liver cytochrome P450 isoforms (scheme). The dorsal and the median raphe nuclei of the brain send their serotonergic projections to the hypothalamus. The injection of the serotonergic neurotoxin 5,7-DHT to the raphe nuclei decreases serotonin level in the hypothalamus, which affects the hypothalamic endocrine system and leads to an enhanced concentration of the GH and corticosterone and a diminished level of thyroid hormone (T3) in the blood (serum). This, in turn, stimulates the expression of cytochrome P450 in the liver (CYP1A/2C11/3A). ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; GHRH, growth hormone–releasing hormone; SRIH, somatostatin; TRH, thyrotropin-releasing hormone; . The circles (dots) in the raphe nuclei and hypothalamus represent serotonin molecules.
As has been mentioned earlier, after 5,7-DHT lesion of the raphe nuclei, the concentration of serotonin in the hypothalamus substantially decreases. In turn, this phenomenon produces a rise in the serum levels of the growth hormone and corticosterone, which positively regulate liver cytochrome P450 expression (Waxman et al., 1995; Waxman and O’Connor, 2006; Dvorak and Pavek, 2010). On the other hand, the lesion decreases the concentration of triiodothyronine (the most effective thyroid hormone in the regulation of target gene transcription in the cell nucleus), but does not affect serum interleukins (IL-2 and IL-6). Both triiodothyronine and the investigated interleukins are known to negatively regulate different P450 isoforms (Yamazoe et al., 1989; Murayama et al., 1991; Liddle et al., 1998; Zidek et al., 2009). As a consequence, the expression of CYP1A, CYP2C11, and CYP3A1 (mRNA and protein level), as well as the enzyme activity (measured as a rate of the metabolism of caffeine or testosterone) are augmented. These findings may suggest that the aforementioned P450 isoforms are induced at the transcription level.
The elevated growth hormone is the chief regulator of the male-specific gene CYP2C11 and plays an important role in the regulation of CYP3A gene transcription (Waxman et al., 1995; Waxman and O’Connor, 2006). Furthermore, the enhanced concentration of testosterone may indirectly contribute to the growth hormone–induced induction of these P450 isoforms (Waxman and Holloway, 2009). Importantly, the direction of changes in serum GH level and GH-governed CYP2C11 expression (activity, protein, and mRNA) is consistent.
The increased expression of CYP3A1 isoform may also be induced by the elevated serum corticosterone. CYP3A1 is most sensitive to hormonal regulation, whereas the male-specific CYP3A2 isoform is expressed at the highest constitutive level among CYP3A subfamily enzymes (Gibson et al., 2002; Jan et al., 2006). The increased expression of CYP1A1/2 isoforms may also be ascribed to the enhancement of serum corticosterone level. CYP1A genes are transcriptionally governed by an aryl hydrocarbon receptor (AhR), which is positively modulated by the physiologic concentrations of glucocorticoids in the rat (Monostory et al., 2005). Moreover, the observed increase in the expression of CYP1A isoforms (and of other investigated P450s) may also be partly due to the reduced concentration of thyroid hormone (T3), which negatively regulates these enzymes (Yamazoe et al., 1989). The signaling pathways of AhR and the thyroid hormone receptor share several coactivators and transcription factors (e.g., retinoid X receptor), a phenomenon that may lead to a transcriptional cross-talk between these pathways and alterations in CYP1A gene expression (Brtko and Dvorak, 2011). The present results confirm our earlier observations that the transcription of the CYP1A1 gene in the rat is more responsive to hormonal modulation than is that of the CYP1A2 gene (Sadakierska-Chudy et al., 2013). Our previous study, carried out after intracerebral administration of the noradrenergic neurotoxin DSP-4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine), showed that the neurotoxin increased serum corticosterone concentration and decreased thyroid T4 levels, with both effects being followed by enhanced CYP1A1 expression (an increase in mRNA level). CYP1A2 expression was not significantly changed. We assume that increases in corticosterone concentration are mainly involved in the observed stimulation of CYP1A genes after lesion of the brain monoaminergic systems studied, since corticosterone is known to be an important modulator of the gene expression regulated by AhR (Monostory et al., 2009); moreover, changes observed in this hormone are more pronounced compared with those in thyroid hormones.
The present results, obtained after intracerebral injection of the serotonergic neurotoxin 5,7-DHT, showing an increased expression of CYP1A, CYP2C11, and CYP3A, differ from those reported earlier after peripheral administration of a serotonergic neurotoxin or after a tryptophan-free diet. Intraperitoneal administration of the neurotoxin PCA or the serotonin synthesis inhibitor PCPA increased CYP1A activity and decreased CYP2C11 and CYP3A activities (Kot and Daniel, 2011), whereas a 3-week tryptophan-free diet (i.e., the serotonin precursor–free diet) enhanced the activity of a number of P450 isoforms (CYP1A, 2A, 2B, 2C6, 2D, and 3A) and simultaneously diminished CYP2C11 activity (Kot et al., 2012). However, the aforementioned experimental models involved both the central and the peripheral serotonergic system. It is noteworthy that 90% of serotonin is produced in enterochromaffin cells located in the gastric mucosa and taken up by platelets that transport it to an appropriate site, e.g., the adrenal and thyroid glands or the liver, where it can affect the proliferative capacity of hepatocytes (Ruddell et al., 2008). Therefore, systemic administration of the aforementioned substances (PCA or PCPA) or a tryptophan-free diet must have also affected the peripheral serotonin pool and its action on the peripheral secretory glands and the liver. In addition, the applied agents or their reactive metabolites may act directly on the liver via a nonserotonin mechanism (Kot and Daniel, 2011). On the other hand, the tryptophan-free diet deprived the organism of the essential amino acid, which might have affected the synthesis of enzyme protein (Kot et al., 2012). Since our present results, obtained after damage to the brain serotonergic system, are different from those reported after general serotonin reduction, it is suggested that peripheral serotonin may also contribute to the regulation of cytochrome P450 in the liver by affecting the enzyme in a different way. It has been found that serotonin receptors are also present in the pituitary (Abrahamson et al., 1987; Dinan, 1996; Balsa et al., 1998; Papageorgiou and Denef, 2007), adrenal gland (Lefebvre et al., 1998), thyroid gland (Csaba and Richter, 1975; Lychkova, 2013), and the liver (Ruddell et al., 2008), where they may affect the release of pituitary hormones (GH, adrenocorticotropic hormone, TSH), corticosterone, or thyroid hormones (T3, T4), and thus regulate liver biology, respectively.
In conclusion, the results obtained after intracerebral injection of the serotonergic neurotoxin 5,7-dihydroxytriptamine into the raphe nuclei (DRN and MRN) projecting to the forebrain indicate that the brain serotonergic system contributes to the regulation of liver cytochrome P450 via a neuroendocrine mechanism involving the growth hormone, corticosterone, triiodothyronine, and testosterone. The effect of the brain serotonergic system on the regulation of liver cytochrome P450 expression seems to be different from that observed for brain catecholaminergic systems. Although changes in CYP1A are similar, increases in the expression of CYP2C11 and CYP3A isoforms, produced by a lesion of the brain serotonergic system, are in opposition to decreases in the expression of these P450 isoforms found after damage to brain dopaminergic or noradrenergic systems (Wójcikowski et al., 2007; Sadakierska-Chudy et al., 2013). The observed opposite changes in enzyme activities are consistent with the different profile of changes in blood hormone levels, produced by respective lesions of the investigated neurotransmitter systems (Wójcikowski and Daniel, 2008; Sadakierska-Chudy et al., 2013).
Further studies are in progress to identify the role of individual serotonergic nuclei and their projections to specific hypothalamic structures, as well as different types of serotonin receptors involved in the central neuroendocrine regulation of cytochrome P450. Clarification of this neuroendocrine regulatory mechanism of cytochrome P450 expression seems important because of its possible practical application to pharmacology and pharmacotherapy. The isoforms CYP1A, 2C, and 3A, affected by the brain serotonergic system, constitute over 50% of the total pool of rat or human cytochrome P450 and are engaged in the metabolism of endogenous steroids and numerous drugs of different clinical applications. A further explanation of the issue in question should help to foresee changes in cytochrome P450 activity in the liver, induced by drugs affecting serotonergic neurotransmission (such as antidepressants, neuroleptics, and antianxiety and antiobesity drugs), and to facilitate drawing conclusions about possible changes in cytochrome P450 activity in pathologic states when the functioning of the serotonergic system is altered. It may also be possible to anticipate interactions between neuroactive drugs with a serotonin profile and endogenous or exogenous substances (including drugs) at the level of the serotonergic regulation of cytochrome P450.
Authorship Contributions
Participated in research design: Daniel.
Conducted experiments: Rysz, Bromek, Haduch, Sadakierska-Chudy.
Performed data analysis: Rysz, Daniel.
Wrote or contributed to the writing of the manuscript: Daniel.
Footnotes
- Received April 17, 2015.
- Accepted June 9, 2015.
This work was financially supported by the Interdisciplinary PhD Studies project “Molecular sciences for medicine” (cofinanced by the European Social Fund within the Human Capital Operational Programme) and by statutory funds from the Institute of Pharmacology, Polish Academy of Sciences.
Abbreviations
- AhR
- aryl hydrocarbon receptor
- 5,7-DHT
- 5,7-dihydroxytryptamine
- DRN
- dorsal raphe nucleus
- ELISA
- enzyme-linked immunosorbent assay
- GH
- growth hormone
- 5-HIAA
- metabolite 5-hydroxyindoleacetic acid
- HPLC
- high-performance liquid chromatography
- IL
- interleukin
- MRN
- median raphe nucleus
- P450
- cytochrome P450
- PCA
- p-chloroamphetamine
- PCPA
- p-chlorophenylalanine
- PCR
- polymerase chain reaction
- T3
- triiodothyronine
- T4
- thyroxine
- TSH
- thyroid-stimulating hormone
- Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics