Abstract
Serum creatinine is commonly used as a marker of renal function, but increases in serum creatinine might not represent changes in glomerular filtration rate (GFR). INCB039110 (2-(3-(4-(7H-pyrrolo[2,3-day]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(1-(3-fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile) is an inhibitor of the Janus kinases (JAKs) with selectivity for JAK1. In a phase 1 study, a modest and reversible increase in serum creatinine was observed after treatment with INCB039110. However, a dedicated renal function study with INCB039110, assessed by iohexol plasma clearance, conducted in healthy volunteers indicated no change in GFR. In vitro studies were therefore conducted to investigate the interaction of INCB039110 with five transporters that are likely involved in the renal clearance of creatinine. Cell systems expressing individual or multiple transporters were used, including a novel quintuple-transporter model OAT2/OCT2/OCT3/MATE1/MATE2-K. INCB039110 potently inhibited OCT2-mediated uptake of creatinine as well as MATE1-/MATE2-K-mediated efflux of creatinine. Given the interactions of INCB039110 with multiple transporters affecting creatinine uptake and efflux, an integrated system expressing all five transporters was sought; in that system, INCB039110 caused a dose-dependent decrease in transcellular transport of creatinine with weaker net inhibition compared with the effects on individual transporters. In summary, a molecular mechanism for the increase in serum creatinine by INCB039110 has been established. These studies also underline the limitations of using serum creatinine as a marker of renal function.
Introduction
Creatinine is a cyclic anhydride of creatine and an end-product of muscle metabolism. Creatinine clearance based on serum creatinine is used to estimate glomerular filtration rate (GFR) and is the most commonly used clinical indicator of renal function (Perrone et al., 1992). Creatinine is predominantly eliminated by glomerular filtration, but it also undergoes active tubular secretion, which can account for 10%–40% of creatinine clearance (Levey et al., 1988). Although the mechanisms underlying the renal tubular transport of creatinine have not been fully elucidated, it is known that multiple transporters are involved. Renal tubular secretion of creatinine was reported to be driven by uptake via human organic cation transporter 2 (OCT2) (Urakami et al., 2004; Koteff et al., 2013), organic cation transporter 3 (OCT3) (Imamura et al., 2011), and organic anion transporter 2 (OAT2) (Lepist et al., 2014). In addition, multidrug and toxin extrusion protein 1 (MATE1) and 2-K (MATE2-K), expressed on the apical side of the renal tubular cells, have been shown to play a role in the tubular efflux of creatinine (Tanihara et al., 2007). Hence, drugs can interact with one or more of these transporters to cause nonpathologic elevation of serum creatinine, and such elevations have been reported with several drugs, for example, cimetidine (Hilbrands et al., 1991), trimethoprim (Naderer et al., 1997), tofacitinib (Lawendy et al., 2009), and dolutegravir (Koteff et al., 2013). During the clinical development of INCB039110 (2-(3-(4-(7H-pyrrolo[2,3-day]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(1-(3-fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile), a Janus kinase 1 (JAK1) inhibitor, transient and reversible increases of serum creatinine were observed. In vitro studies were conducted to understand the mechanisms involved, including experiments using a novel multitransporter cell system; the results from those investigations are presented in this article.
Materials and Methods
[14C]-creatinine was purchased from Moravek Biochemicals (Brea, CA). Cell culture media and buffer solution were purchased from Corning-Cellgro (Manassas, VA). Porous membrane insert plates were purchased from Millipore (Billerica, MA). INCB039110 and placebo tablets were supplied by Incyte Corporation (Wilmington, DE). Iohexol was purchased commercially as Omnipaque from GE Healthcare (Princeton, NJ). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO). The purified subclone of Madin-Darby canine kidney II cells (MDCK-II) used in these experiments was isolated at Optivia Biotechnology (Menlo Park, CA).
Clinical Study Design.
A definitive renal function study using INCB039110 was conducted at Prism Research (St. Paul, MN) in full accordance with the Declaration of Helsinki, Good Clinical Practice, as required by and described in 21 Code of Federal Regulations parts 50, 54, 56, 312 subpart D, and 314. This was a randomized, placebo-controlled, two-way crossover study in healthy adult subjects aged 18–55 years. A total of 24 healthy male adult subjects were randomized to two treatment sequences (12 subjects per sequence), either active treatment first followed by placebo or placebo first followed by active treatment. Subjects received 6 × 100-mg INCB039110 tablets or matching placebo along with a standardized medium-fat meal, twice daily for 8 days. After a washout period of 13 days, the alternate treatment was administered. Iohexol 350 mg/ml was administered as a 20-ml i.v. infusion over 15 minutes and was given 2 hours after the INCB039110 or placebo morning dose on days –1 (baseline), 8, 21 (baseline), and 29. Serum concentrations of creatinine and plasma concentrations of INCB039110 and iohexol were evaluated in accordance with the schedule of assessments. Iohexol and INCB039110 were extracted via protein precipitation or liquid/liquid extraction, respectively, followed by liquid chromatography/tandem mass spectometry analysis using Sciex API-4000 (AB Sciex LLC, Foster City, CA) and multiple reaction monitoring. Standard noncompartmental pharmacokinetic methods were used to analyze iohexol and INCB039110 plasma concentration using Phoenix WinNonlin version 6.0 (Pharsight Corporation, Mountain View, CA). GFR at the end of treatment was measured by iohexol plasma clearance and was baseline corrected. The geometric mean ratio for active versus placebo treatment and its 90% confidence interval (CI) were determined using a two-way crossover linear mixed-effect analysis of variance model.
Cellular Uptake Study in OAT2, OCT2, or OCT3 Transfected MDCK-II Cells.
Fully confluent MDCK-II cell monolayers were transfected with DNA plasmids encoding one of the three uptake transporters: OAT2 (SLC22A7), OCT2 (SLC22A2), or OCT3 (SLC22A3) or green fluorescent protein (GFP) as a control, at a final concentration of 30 ng/μl (Optivia Biotechnology, Menlo Park, CA). The transiently transfected MDCK-II cells were incubated for approximately 48 hours to allow the cells to become polarized and transporters to be appropriately localized. Preincubation was carried out with Hanks’ balanced salt solution (HBSS) (37°C) in both apical and basolateral compartments for 15 minutes. Uptake was initiated by replacing the buffer with HBSS containing 100 µM 14C-creatinine (specific activity 0.05 Ci/mmol) and either reference inhibitors or INCB039110 in the basolateral compartment, followed by a 5-minute incubation at 37°C. The reference inhibitors used were cimetidine (for OCT2), indomethacin (for OAT2), and quinidine (for OCT3). At the end of incubation, cells were washed with ice-cold phosphate-buffered saline and permeabilized by incubation with 50% acetonitrile in water. Radioactivity was counted on a Wallac 1450 Microbeta (PerkinElmer, Waltham, MA).
Transcellular Transport Study in a Dual or Quintuple Transporter Model.
The dual-transporter model for MATE1 (SLC47A1) and MATE2-K (SLC47A2) was created by transfecting fully confluent MDCK-II cell monolayers with a DNA mixture containing the plasmids encoding MATE1 and MATE2-K at concentrations of 15 and 10 ng/µl, respectively. The quintuple-transporter model consists of OAT2, OCT2, and OCT3, expressed on the basolateral cell membrane, and MATE1 and MATE2-K, expressed on the apical side of the cells. This model was created using similar procedure as described above for the dual transporter system with transfection carried out using a DNA mixture containing the plasmids encoding OCT2, OAT2, OCT3, MATE1, and MATE2-K transporters at concentrations of 20, 20, 10, 15, 10 ng/µl, respectively. Approximately 48 hours after transfection, cells of both dual and quintuple models were washed with HBSS. A 30-minute preincubation at 37°C with HEPES-buffered HBSS (HBSS-HEPES, pH 7.4) containing either reference inhibitors or INCB039110 in both the apical and basolateral compartments was carried out. The buffer in the apical compartment was then replaced with Bis-Tris buffered HBSS (HBSS-Bis-Tris, pH 6.0) containing either the reference inhibitor or INCB039110. The buffer in the basolateral compartment was replaced with HBSS-HEPES buffer (pH 7.4) containing 100 µM 14C-creatinine with either reference inhibitors or INCB039110. After the 60-minute incubation, aliquots of the apical chambers were sampled and radioactivity was counted on a Wallac 1450 Microbeta to measure transcellular flux.
Analysis of Data from Transport Studies.
Transport studies were conducted in triplicate in both transporter-expressing cells and in GFP control cells. The percent inhibition was calculated by dividing the net transporter-mediated transport of creatinine in the presence of inhibitor by the corresponding value for vehicle.
The IC50 value was calculated using eq. 1:
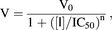 (1)
(1)where V0 is the mean transporter-mediated flux or accumulation in the presence of vehicle (0.5% DMSO), V is the transporter-mediated flux or accumulation in the presence of inhibitor, [I] is the inhibitor concentration, and n is a Hill coefficient. Unlike the IC50 values determined for the individual uptake transporters, the IC50 values determined for basolateral to apical transport in the multiple transporter models reflects net inhibition.
Results and Discussion
Inconsequential elevations in serum creatinine without an impairment of renal function have been reported as early as in the 1970s, when patients were treated with trimethoprim and sulfamethoxazole (or Bactrim) (Berglund et al., 1975). Recent studies suggested that several transporters, such as OCT2 (Urakami et al., 2004; Koteff et al., 2013), OCT3 (Imamura et al., 2011), OAT2 (Lepist et al., 2014), MATE1, and MATE2-K (Tanihara et al., 2007), are likely involved in the movement of creatinine from the blood into the kidney proximal tubule cells and then into the urine. Thus, there are multiple points of interaction that can lead to a change in serum creatinine. Although studies have demonstrated the importance of individual renal transporters in creatinine renal secretion using single transporter transfected cell lines, the combined net effect of these transporters on the renal tubular secretion of creatinine is best studied using a dynamic multitransporter system where the interplay of these transporters can be assessed.
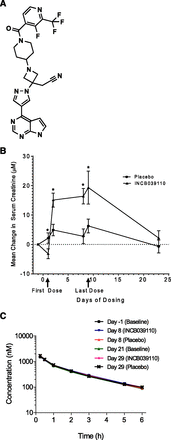
INCB039110 is a JAK1 inhibitor in clinical development for multiple oncology indications (structure shown in Fig. 1A). Multiple-dose studies in healthy volunteers showed small, reversible, and dose-dependent increases in serum creatinine. To understand any pathologic impact from multiple dosing of INCB039110, a definitive renal function study was conducted in healthy male adult volunteers using iohexol as a marker of glomerular filtration. The changes in mean serum creatinine after multiple oral dosing of INCB039110 in that study are shown in Fig. 1B. The highest mean serum creatinine change of 19 μM was noted on day 9 of INCB039110 treatment compared with 6 μM for placebo, resulting in a net increase of 13 μM of serum creatinine with INCB039110 treatment. At this time point, two subjects treated with INCB039110 had serum creatinine values above the upper limit of normal, although the mean values were within the normal range. At the follow-up visit, all INCB039110 subjects had serum creatinine measurements within the normal range, consistent with the findings from an earlier clinical study. The impact on renal function was then assessed by change in iohexol clearance after INCB039110 treatment compared with placebo treatment. The mean iohexol clearance values were 6.99 l/h and 7.00 l/h at the end of treatment in the INCB039110 and placebo groups, respectively, on day 8 and 6.99 l/h for both groups on day 29. The plasma concentration versus time curves were superimposable on days −1, 8, 21, and 29 (Fig. 1C). The geometric mean ratio of baseline-corrected GFR was 1.00 with a 90% confidence interval of 0.956–1.05; that is, INCB039110 was not significantly different from placebo. These results clearly indicate that INCB039110 treatment does not affect renal function, and therefore the small increases in serum creatinine likely resulted from modulation of transporters involved in creatinine disposition.
(A) Chemical structure of INCB039110. (B) Mean change of serum creatinine by study day after 600-mg twice daily dose of INCB039110 for 8 days. Twenty-four healthy male adult subjects were randomized to one of two treatment sequences (12 subjects per sequence) that included two consecutive treatment periods separated by a 13-day washout period. Data represent the mean and standard error of 18 to 24 subjects that were on file at each time point. The baseline serum creatinine for all 24 subjects was 85 ± 9 µM (mean ± S.E.). *P < 0.05 versus placebo. (C) Iohexol plasma concentration-time profiles in the subjects who completed the study (N = 17, mean ± S.E.). One subject receiving INCB039110 treatment in period 1 had an unusually high GFR value on day 21. Statistically, this abnormally high GFR value was > 3 × S.D. greater than the group mean of the 17 subjects on day 21 and outside the whisker of the box plot (data not shown). Therefore, it was treated as a statistical outlier and excluded from the final statistical analysis.
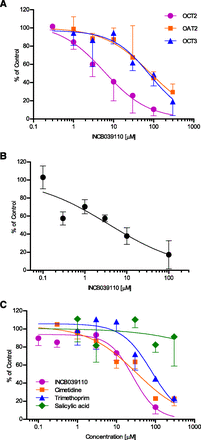
To understand the potential mechanism behind the increase in serum creatinine observed with INCB039110 treatment, the interaction of INCB039110 with the five known transporters involved in the uptake or efflux of creatinine was evaluated using in vitro systems expressing a single or multiple transporters. In the case of single-transporter systems, the functional activity of each transporter transfected into MDCK-II cells, that is, OCT2, OCT3, and OAT2, was confirmed by comparing the accumulation of probe substrates in the transfected cells versus GFP-transfected mock control. Compared with the mock control cells, varying degrees of creatinine accumulation were observed in transporter transfected cells, 7.2-fold in the case of OAT2, 2.4-fold with OCT3, and 2.2-fold with OCT2 (data on file). Addition of positive control inhibitors decreased the transport of creatinine into these cells, confirming transporter-mediated uptake of creatinine in the cell systems used. The basolateral uptake of creatinine mediated by OCT2, OCT3, and OAT2 was inhibited in the presence of various concentrations of INCB039110 in a concentration-dependent manner. The corresponding inhibition IC50 values were 5.8 μM, 84 μM, and 80 μM by OCT2, OAT2, and OCT3, respectively (Fig. 2A; Table 1).
(A) Effects of INCB039110 on the basolateral uptake of creatinine mediated by OCT2 (purple), OCT3 (blue), or OAT2 (orange). INCB039110 was studied in the concentration range of 0.1–300 µM. The percent inhibition (% of control) was calculated by dividing the net transporter-mediated transport of creatinine in the presence of INCB039110 by the net transporter-mediated transport of creatinine in the presence of vehicle. The IC50 value was determined by a nonlinear regression using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). Data represent the mean and S.D. of triplicate samples. (B) Inhibition of efflux of creatinine by INCB039110 in the dual MATE1/MATE2-K model. INCB039110 was studied in the concentration range of 0.1–100 µM. The percent inhibition (% of control) was calculated by dividing the net MATE1/MATE2-K-mediated transport of creatinine in the presence of INCB039110 by the net MATE1/MATE2-K-mediated transport of creatinine in the presence of vehicle. The IC50 value was determined by a nonlinear regression using GraphPad Prism. Data represent the mean and S.D. of triplicate samples. (C) Inhibition of transcellular creatinine transport in the quintuple OAT2/OCT2/OCT3/MATE1/MATE2-K model. The IC50 values were determined by nonlinear regression using GraphPad Prism and were 32 µM for cimetidine (orange), 80 µM for trimethoprim (blue), 27 µM for INCB039110 (purple), and not calculable because of insufficient inhibition for salicylic acid (green). Data represent the mean and S.D. of triplicate samples.
Inhibition of creatinine transport by INCB039110 or by a reference inhibitor
IC50 values in μM, mean ± S.D.; N = 3.
INCB039110 also exhibited an inhibitory effect on the efflux of creatinine in the dual MATE1/MATE2-K coexpressing MDCK-II cells with an IC50 of 3.7 μM (Fig. 2B; Table 1). Thus, INCB039110 can simultaneously inhibit both uptake and efflux of creatinine; therefore, an integrated model that captures the interaction with all five transporters was sought. It was indeed feasible to transfect MDCK-II monolayers with all five transporters and is herein referred to as the quintuple model. The relative amounts of DNAs used in transfection were aimed to approximate the relative expression levels of the transporters in human kidney (Nishimura and Naito, 2005; Cheng et al., 2012; Morrissey et al., 2012). Reports on the relative mRNA levels of OAT2 and OCT2 are conflicting; therefore, these two transporters were expressed at similar levels in the quintuple transporter model. INCB039110 exhibited a dose-dependent decrease in the transcellular transport of creatinine in the quintuple model with a maximum inhibition of 87% at 100 μM and an IC50 of 27 μM (Fig. 2C; Table 1).
Next, an attempt was made to correlate the in vitro inhibitory data to observed in vivo changes in serum creatinine when treated with such inhibitors. Based on the observed mean unbound steady-state plasma concentration of INCB039110 (∼1 µM between 3 and 4 hours postdose) and its inhibitory potency for creatinine transport in the quintuple transporter model, only a modest impact on creatinine secretion by INCB039110 is expected, which is consistent with the small increases (∼20%) observed in serum creatinine in the clinical study involving multiple doses of INCB039110. Drugs known to interact with creatinine secretion clinically via inhibition of transport (cimetidine and trimethoprim) were studied in this integrated cell system and compared with a negative control (salicylic acid). Cimetidine is a well-known competitive inhibitor of OCT2 (Imamura et al., 2011) and MATE1 (Matsushima et al., 2009) transporters. In single-transporter transfected cell lines, cimetidine inhibited all five transporters with varying inhibition potencies ranging all the way from 1.5 µM (potent) to 140 µM (weak) (Lepist et al., 2014). In the current study using the quintuple transporter model, the IC50 for cimetidine was 32 µM (Fig. 2C; Table 2). Clinical studies have shown up to a 33 μM increase in serum creatinine after oral dosing of cimetidine (Hilbrands et al., 1991). In the case of trimethoprim, the observed IC50 value for trimethoprim in the quintuple cell model was 80 µM (Fig. 2C; Table 2), and in a clinical study, an increase of 18 μM in serum creatinine was observed (Berglund et al., 1975). It has been reported that the in vitro inhibition of creatinine secretion by trimethoprim may be primarily through blocking apical efflux mediated by MATE1 and MATE2-K (Lepist et al., 2014). As a negative control, salicylic acid, which is known to elevate serum creatinine levels through glomerular damage (Caspi et al., 2000), did not inhibit creatinine transport in the quintuple model (Fig. 2C). To compare the in vitro data with changes in observed serum creatinine levels, the ratios of unbound steady-state plasma concentration [I] and in vitro IC50 values obtained in the quintuple model, [I]/IC50, of cimetidine, trimethoprim, and INCB039110 were calculated, as well as the relative [I]/IC50 normalized to that of cimetidine (Table 2). Although the data set is limited, an apparent rank order exists among these three agents, suggesting that an in vitro-in vivo correlation may be possible using this multitransporter model as more data are generated with additional agents that interact with these transporters. In particular, the availability of more potent inhibitors would be important in establishing robust correlations, which in turn may alleviate the need for dedicated renal function studies for some compounds.
Comparison between the in vitro creatinine transport and in vivo serum creatinine elevation on inhibition of renal transporters
In conclusion, small increases in serum creatinine were seen with the treatment of INCB039110; however, it did not affect the clearance of iohexol, a marker of glomerular filtration, in a dedicated renal function study. A molecular basis for the transient serum creatinine elevation observed after INCB039110 treatment is proposed that involves multiple transporters that play a role in both uptake and efflux of creatinine. The results from these studies demonstrate the utility of a cell system expressing all five transporters in quantitating the impact on creatinine clearance. Further, the conclusions drawn from the use of serum creatinine as a marker of renal function should be made with caution, acknowledging the possibility of artifactual increases through modulation of transporters involved in its clearance.
Authorship Contributions
Participated in research design: Y. Zhang, Warren, X. Zhang, Diamond, Williams, Punwani, Y. Huang, Yeleswaram.
Conducted experiments: X. Zhang, Warren, Williams, Punwani.
Performed data analysis: Y. Zhang, X. Zhang, Warren, Williams, Punwani.
Wrote or contributed to the writing of the manuscript: Y. Zhang, Warren, X. Zhang, Diamond, Williams, J. Huang, Y. Huang, Yeleswaram.
Footnotes
- Received August 16, 2014.
- Accepted January 20, 2015.
No financial support was received for this study. Y. Zhang, Diamond, Williams, Punwani, and Yeleswaram are employed by and are shareholders of Incyte Corporation. Warren, X. Zhang, J Huang, and Y. Huang are employed by Optivia Biotechnology, a contract service provider of transporter-related assays.
Abbreviations
- GFP
- green fluorescent protein
- GFR
- glomerular filtration rate
- HBSS
- Hanks’ balanced salt solution
- INCB039110
- 2-(3-(4-7H-pyrrolo[2,3-day]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(1-(3-fluoro-2-(trifluoromethyl)isonicotinoyl)piperidin-4-yl)azetidin-3-yl)acetonitrile
- JAK
- Janus kinase
- MATE
- multidrug and toxin extrusion protein
- MDCK-II
- Madin-Darby canine kidney II
- OAT
- organic anion transporter
- OCT
- organic cation transporter
- Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics