Abstract
Olmesartan medoxomil (OM) is a prodrug-type angiotensin II type 1 receptor blocker (ARB). We recently identified carboxymethylenebutenolidase homolog (CMBL) as the responsible enzyme for OM bioactivation in humans. In the present study, we compared the bioactivating properties of OM with those of other prodrug-type ARBs, candesartan cilexetil (CC) and azilsartan medoxomil (AM), by focusing on interspecies differences and tissue specificity. In in-vitro experiments with pooled tissue subcellular fractions of mice, rats, monkeys, dogs, and humans, substantial OM-hydrolase activities were observed in cytosols of the liver, intestine, and kidney in all the species tested except for dog intestine, which showed negligible activity, whereas lung cytosols showed relatively low activities compared with the other tissues. AM-hydrolase activities were well correlated with the OM-hydrolase activities. In contrast, liver microsomes exhibited the highest CC-hydrolase activity among various tissue subcellular fractions in all the species tested. As a result of Western blot analysis with the tissue subcellular fractions, the band intensities stained with anti-human CMBL and carboxylesterase 1 (CES1) antibodies well reflected OM- and AM-hydrolase activities and CC-hydrolase activity, respectively, in animals and humans. Recombinant human CMBL and CES1 showed significant AM- and CC-hydrolase activities, respectively, whereas CC hydrolysis was hardly catalyzed with recombinant carboxylesterase 2 (CES2). In conclusion, OM is bioactivated mainly via intestinal and additionally hepatic CMBL not only in humans but also in mice, rats, and monkeys, while CC is bioactivated via hepatic CES1 rather than intestinal enzymes, including CES2. AM is a substrate for CMBL.
Introduction
Historically, many ester-based prodrugs have been developed with the aim of overcoming a number of barriers to drug-like properties (Liederer and Borchardt, 2006; Huttunen et al., 2011). Esterases, which are involved in bioactivation of ester-based prodrugs and inactivation of clinical drugs as well, are typically represented by carboxylesterases (CES) (Satoh and Hosokawa, 2006; Hosokawa, 2008), cholinesterases (Taylor, 1991; Taylor and Radic, 1994), and paraoxonases (Draganov and La Du, 2004). In addition, recent works revealed several valuable aspects of other esterases responsible for drug hydrolysis: valacyclovirase as a bioactivating hydrolase for the amino-acid ester prodrugs valacyclovir and valganciclovir (Kim et al., 2003; Lai et al., 2008); arylacetamide deacetylase as a hydrolase being associated with organ toxicities of clinical drugs such as flutamide, phenacetine, and rifamycins (Watanabe et al., 2009; Watanabe et al., 2010; Nakajima et al., 2011); and α/β hydrolase domain-containing 10 (ABHD10), which functions as the detoxification enzyme of mycophenolic acid acyl-glucuronide (Iwamura et al., 2012).
Although esterases are attractive targets for prodrug bioactivating enzymes due to their wide distribution in the blood, liver, intestine, and many other biologic fluids and tissues (Berry et al., 2009; Fukami and Yokoi, 2012), interspecies differences in tissue-specific esterase expressions at times challenge extrapolation from animal data to humans. For instance, many examples have shown higher hydrolase activity in rodent species than humans, while there is minimal hydrolase activity in dog small intestine (Liederer and Borchardt, 2006; Satoh and Hosokawa, 2010). These observations suggest the importance of selecting appropriate animal species that most closely represent humans in drug development processes, based on comprehensive understanding of the species differences.
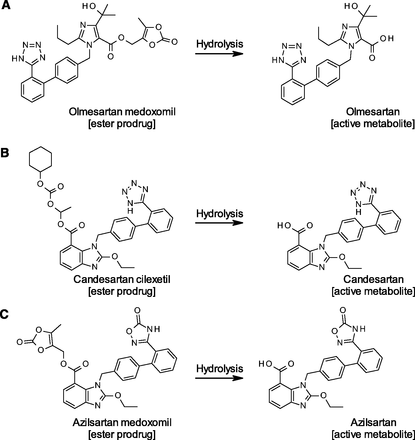
Angiotensin receptor blockers (ARBs) that directly inhibit the binding of angiotensin II to angiotensin II type 1 receptor provide an effective pharmacologic strategy in the management of hypertension, cardiovascular disease, and renal disease (De Gasparo et al., 2000). Among eight market-available ARBs, candesartan cilexetil (CC), olmesartan medoxomil (OM), and azilsartan medoxomil (AM) are ester-based prodrugs that require hydrolysis to be converted into their respective active metabolites. As shown in Fig. 1, the prodrug moieties of OM and AM are identical, where the medoxomil moiety links to the carboxylic acid of the drug via an ester bond (Laeis et al., 2001; Kawaguchi et al., 2013), while the CC is a cyclohexyloxycarbonyloxy ethyl ester of the carboxylic acid of candesartan (Nishikawa et al., 1997). We recently reported that a novel human hydrolase, carboxymethylenebutenolidase homolog (CMBL), is responsible for the OM bioactivation (Ishizuka et al., 2010, 2012); however, responsible enzymes for CC- and AM-bioactivations have not been clearly identified thus far.
Bioactivation of the prodrug-type ARBs. Hydrolytic reactions of olmesartan medoxomil (A), candesartan cilexetil (B), and azilsartan medoxomil (C) are shown.
This study was designed to investigate the difference in the bioactivating properties of the prodrug-type ARBs by comparing CC- and AM-hydrolase activities with that of OM, using human and animal tissue subcellular fractions and recombinant proteins of candidate human hydrolases. Also, this work provides new insights into interspecies differences in tissue-specific expressions and activities of the novel hydrolase CMBL between humans and other animal species.
Materials and Methods
Olmesartan medoxomil (OM), olmesartan, candesartan, and RNH-6272 [2-butyl-4-(1-hydroxy-1-methylethyl)-1-[2′-(1H-tetrazole-5-yl)-4-biphenylyl]-1H-imidazole-5-carboxylic acid] were synthesized in Daiichi Sankyo (Tokyo, Japan). RNH-6272 is a structural analog of olmesartan and was used as an internal standard for determination of prodrug active metabolites (olmesartan, candesartan, and azilsartan). Candesartan cilexetil (CC) was synthesized at Chemtech Laboratory, Inc. (Tokyo, Japan). Azilsartan medoxomil (AM) and azilsartan were purchased from Cosmo Bio (Tokyo, Japan) and Sigma-Aldrich Japan (Tokyo, Japan), respectively. Bis-p-nitrophenylphosphate (BNPP) was purchased from Tokyo Chemical Industry (Tokyo, Japan). All the other chemicals and reagents were of the highest grade available and were obtained from standard commercial sources.
Tissue Subcellular Fractions and Plasma.
Pooled male CD1 mouse, male Sprague-Dawley rat, male cynomolgus monkey, male beagle dog, and mixed gender human tissue (liver, intestine, kidney, and lung) cytosolic and microsomal fractions were used in the study. Mouse intestinal, human kidney, rat, monkey, dog, and human lung cytosolic fractions were separated from commercially available 9000g supernatant (S9) fractions of respective tissues (XenoTech, LLC, Lenexa, KS) as supernatant by ultracentrifugation at 105,000g at 4°C for 1 hour. Mouse lung microsomal fraction was also prepared from a mouse lung S9 fraction (XenoTech, LLC). The pellet obtained by ultracentrifugation of the S9 fraction at 105,000g at 4°C for 1 hour was washed and repelleted with 10 mM potassium phosphate buffer (pH 7.4) containing 1.15% KCl by ultracentrifugation with the same conditions as above. The obtained pellet was resuspended with 10 mM potassium phosphate buffer (pH 7.4) containing 30% glycerol for use as the microsomal fraction. The other cytosolic and microsomal fractions were purchased from XenoTech, LLC. Pooled male CD1 mouse, male Sprague-Dawley rat, male cynomolgus monkey, male beagle dog, and male human plasma were used in the study. Blood was collected from three or more individuals and centrifuged to separate plasma.
Recombinant Human Carboxylesterase.
To construct a destination vector containing a FLAG epitope tag, the synthesized oligonucleotides encoding FLAG-tag (amino acid sequence: DYKDDDDK) and reading frame A (Invitrogen, Carlsbad, CA) were inserted into pcDNA3.1(+) (Invitrogen) as a NheI/HindIII and EcoRV fragment, respectively. The resulting vector pcDNA3.1-CFLAG-GW was verified by sequencing. pDONR-human CES1 and pDONR-human CES2 entry clones, which contain human CES1 and CES2 genes without their native stop codon, respectively (produced by Invitrogen), were recombined with the destination vector pcDNA3.1-CFLAG-GW using LR clonase II (Invitrogen) to generate the expression vectors pcDNA3.1-CFLAG-CES1 and pcDNA3.1-CFLAG-CES2, which encode human CES1 and CES2, respectively, with a FLAG tag at the C terminus. The recombinant proteins were produced in mammalian FreeStyle 293-F Cells (Invitrogen). Gene-transfected cells were cultured for 7 days in FreeStyle 293 Expression Medium (Invitrogen). The conditioned media from the cells overexpressing human CES1 and CES2 were dialyzed against 20 mM Tris-HCl buffer (pH 8.0) and filtered with a polyethersulfone membrane filter (0.45 μm; Thermo Fisher Scientific, Rockford, IL). The overexpressed FLAG-tagged protein in the filtrate was purified with a two-step purification process: anion chromatography with HiTrap Q-XL (GE Healthcare Japan, Tokyo, Japan) followed by anti-FLAG M2 affinity gel (Sigma-Aldrich). The eluates were collected, desalted with PD MidiTrap G-25 (GE Healthcare Japan), and then the resultant protein was stored frozen at –80°C until use.
Hydrolase Activity Measurement in Tissue Subcellular Fractions.
The hydrolase activities for the three prodrug-type ARBs, OM, CC, and AM, in animal and human tissue subcellular fractions were measured with each 10-μM prodrug as a substrate (final solvent concentration: 2.5% acetonitrile) in duplicate. After 5-minute preincubation, the reaction was initiated by adding the substrate. OM and AM were incubated in 100 mM HEPES buffer (pH 7.4, incubation volume of 0.2 ml) with 100 μg cytosolic or microsomal protein/ml, and CC was incubated in 100 mM Tris-HCl buffer (pH 7.5, incubation volume of 0.2 ml) at 37°C for 1, 2, and 10 minutes with 10–100 μg cytosolic or microsomal protein/ml. The reaction was terminated by adding a 4-fold volume of ice-cold 87.5% acetonitrile containing RNH-6272 as an internal standard for the determination of the respective active metabolite concentrations and 0.25% formic acid for preventing the nonenzymatic degradation of the prodrugs. The mixture, with a volume of ca. 200 μl, was filtered using a Captiva 96-well filter plate.
For olmesartan measurement, the Captiva filtrate was mixed with 200 μl of 50% methanol containing 1% formic acid, and was analyzed by liquid chromatography–tandem mass spectrometry consisting of a Prominence LC-20A system (Shimadzu, Kyoto, Japan) and an API3200 (Applied Biosystems/MDS SCIEX, Foster City, CA) as previously reported (Ishizuka et al., 2013). For candesartan and azilsartan measurements, the filtrate was directly analyzed by liquid chromatography–tandem mass spectrometry consisting of a Prominence CBM-20A system (Shimadzu) and an API4000 (Applied Biosystems/MDS SCIEX). The two metabolites were separated with a reversed-phase ODS column (Shim-pack XR-ODS, 2.0-mm × 300-mm internal diameter; Shimadzu). The mobile phase total flow was set to 0.75 ml/min with binary gradient elution, using solvent A (5% acetonitrile, 0.2% formic acid, 100 mM ammonium acetate) and B (95% acetonitrile, 0.2% formic acid, 100 mM ammonium acetate). The gradient started with 45% B for 0.5 minutes and was increased to 100% B for 0.5 minutes. Elution was continued for 1 minute at 100% B, and then the total flow was increased to 5 ml/min to wash the column. Candesartan and azilsartan were determined by monitoring the ion transition of m/z 441 to m/z 263 and m/z 457 to m/z 233, respectively, with multiple reaction monitoring in the positive electrospray ionization mode.
The enzymatic activity was expressed as a metabolite formation rate (nanomole per minute per milligram protein) based on the production of respective active metabolite for the reaction by each enzyme source, from which the buffer control as nonenzymatic hydrolysis was subtracted. The incubation time and cytosolic/microsomal protein concentration were selected to be in the linear range for the metabolite formation.
Hydrolysis by Recombinant Human CES1 and CES2.
The OM-, CC- and AM-hydrolase activities by recombinant human CES1 and CES2 were measured with 10-μM prodrugs as substrates (final solvent concentration: 2.5% acetonitrile) with or without 1 mM BNPP (final solvent concentration: 1% dimethyl sulfoxide) in triplicate. After 5-minute preincubation, the reaction was initiated by adding the substrate. The prodrugs were incubated in 50 mM Tris-HCl buffer (pH 7.5, incubation volume of 0.2 ml) containing 5% (v/v) conditioned media from CES1- or CES2-overexpressed cells as an enzyme source at 37°C for 2 minutes. The enzymatic activity was expressed as a metabolite formation rate per reaction (nanomole per minute per reaction) based on the production of respective metabolites.
Hydrolysis by Recombinant Human CMBL.
The OM-, CC- and AM-hydrolase activities by recombinant human CMBL were measured with the 10-μM prodrugs as substrates (final solvent concentration: 2.5% acetonitrile) in triplicate. After 5-minute preincubation, the reaction was initiated by adding the substrate. The prodrugs were incubated in 100 mM HEPES buffer (pH 7.4, incubation volume of 0.2 ml) at 37°C for 5 minutes with 100 μg of the human CMBL-transfected mammalian cell lysate constructed in-house (Ishizuka et al., 2010) as an enzyme source. The enzymatic activity was expressed as a metabolite formation rate (v0: nanomole per min per milligram protein) based on the production of azilsartan, from which the vector control was subtracted as nonenzymatic hydrolysis.
In addition, the kinetic analysis for AM hydrolysis by recombinant human CMBL was carried out with a substrate range of 0.781–100 μM. The kinetic constant (Km) and maximum velocity (Vmax) for AM hydrolysis were estimated using WinNonlin Professional (version 1.4.1; Pharsight, Sunnyvale, CA) by a nonlinear least-squares regression analysis fitting to the Michaelis-Menten equation, v0 = Vmax × [S]/(Km + [S]), where v0 and [S] represent initial velocity and substrate concentration, respectively.
Western Blot Analysis of CMBL and CES1 Expression.
The following tissue subcellular fractions (liver, intestine, kidney, and lung cytosol and microsomes) and diluted plasma were subjected to Western blot analysis: mouse, rat and dog, 2 μg protein for each subcellular fraction and 10 μl of 100-fold diluted plasma; monkey and human, 0.5 μg protein for each subcellular fraction and 5 μl of 200-fold diluted plasma. As positive controls, 0.5 μg of human CMBL-overexpressed mammalian Freestyle 293-F cell lysate and 10 ng of purified recombinant protein of human CES1 were also analyzed. The sample proteins were separated by SDS-PAGE using 12% sodium dodecyl sulfate-polyacrylamide gel (Mini-PROTEAN TGX precast gel 12%; Bio-Rad, Hercules, CA) and were transferred electrophoretically onto a polyvinylidene difluoride membrane (Immun-Blot polyvinylidene difluoride membrane, 0.2 μm; Bio-Rad). The CMBL and CES1 proteins in animals and humans were stained with affinity-purified rabbit polyclonal IgG against a human CMBL peptide (1:5000 dilution; IBL, Shizuoka, Japan) and affinity-purified rabbit polyclonal IgG against a human CES1 peptide (ab52941, 1:1000 dilution; Abcam, Cambridge, MA) as primary antibodies, respectively, and ECL anti-rabbit IgG horseradish peroxidase–linked, from donkey (GE Healthcare Japan, 1:10000 dilution) as a secondary antibody. These immunoblots were visualized by chemiluminescence with an ECL Advance Western Blotting Detection Kit (GE Healthcare Japan). The immunoreactive signals were detected by a lumino-image analyzer (LAS-4000UV mini; FujiFilm, Tokyo, Japan) and the signal intensities were semiquantified by Science Laboratory 2005 Multi Gauge software (ver. 3.0; FujiFilm).
Protein Assay.
Protein concentration was determined by the Bradford method using bovine serum albumin as the reference standard.
Results
Prodrug Hydrolysis in Tissue Subcellular Fractions.
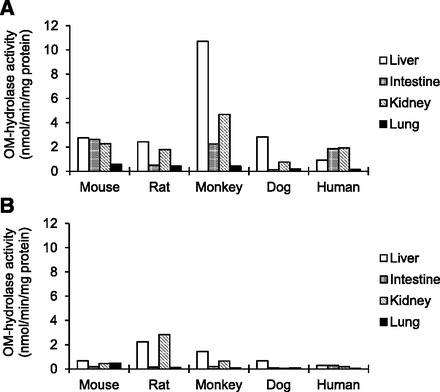
The OM-hydrolase activities with mouse, rat, monkey, dog, and human tissue cytosolic and microsomal fractions are shown in Fig. 2. The activity was generally higher in cytosols than in respective microsomes. Liver, intestine, and kidney cytosols exhibited substantial OM-hydrolase activities in all the species tested except for dog intestine, whereas lung cytosols showed relatively low activities. In rats, liver and kidney microsomes showed comparable activities to those of respective cytosols. In AM hydrolysis, similar patterns of the species- and tissue-specific activity to OM hydrolysis were observed in both cytosols and microsomes (data not shown). In contrast to OM hydrolysis, the CC-hydrolase activity was generally higher in microsomes than in respective cytosols as shown in Fig. 3. In all the species tested, liver microsomes exhibited the highest CC-hydrolase activity among various tissue subcellular fractions. The CC hydrolysis was mostly liver-specific in humans, whereas significant activities were observed in the kidney and lung along with liver in the other species. Minimal activity of CC hydrolysis was observed in the intestine in all the species tested.
OM-hydrolase activities with animal and human tissue subcellular fractions. Prodrug OM was incubated with animal and human tissue cytosolic (A) and microsomal (B) fractions, and the formed active metabolite olmesartan was measured by liquid chromatography–tandem mass spectrometry. Data represent the means of duplicate determinations.
CC-hydrolase activities with animal and human tissue subcellular fractions. Prodrug CC was incubated with animal and human tissue cytosolic (A) and microsomal (B) fractions, and the formed active metabolite candesartan was measured by liquid chromatography–tandem mass spectrometry. Data represent the means of duplicate determinations.
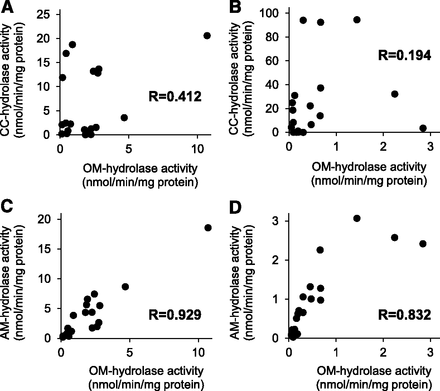
Using the above activity data, the CC-hydrolase and AM-hydrolase activities were plotted against the OM-hydrolase activity. Twenty data points for each cytosolic and microsomal fraction prepared from four different tissues (liver, intestinal, kidney, and lung) of five species (mouse, rat, monkey, dog, and human) were used. As shown in Fig. 4, no correlation was observed between the CC- and OM-hydrolase activities in both cytosolic and microsomal fractions, whereas the AM-hydrolase activities were well correlated with the OM-hydrolase activities in both cytosolic and microsomal fractions, with correlation coefficients of 0.929 and 0.832, respectively.
Correlation analysis of hydrolase activities for prodrug-type ARBs with animal and human tissue subcellular fractions. CC-hydrolase (A and B) and AM-hydrolase (C and D) activities are plotted against OM-hydrolase activity. Twenty data points for each cytosolic (A and C) and microsomal (B and D) fractions prepared from four different tissues (liver, intestinal, kidney, and lung) of five species (mouse, rat, monkey, dog, and human) were used. The prodrugs were incubated with animal and human tissue cytosolic and microsomal fractions and their respectively formed active metabolites were measured by liquid chromatography–tandem mass spectrometry. Data represent the means of duplicate determinations.
Hydrolysis by Recombinant Human CES1 and CES2.
To confirm the substrate specificities of the recombinant human CES1 and CES2 in conditioned media from the overexpressing cells, the hydrolase activities of p-nitrophenyl acetate, clopidogrel, and CPT-11, which are a general esterase substrate, CES1-specific and CES2-specific substrate, respectively, were measured. The CES1- and CES2-conditioned media showed comparable hydrolytic activity toward p-nitrophenyl acetate, and that CES1 and CES2 specifically catalyzed the clopidogrel hydrolysis, forming clopidogrel carboxylic acid and the CPT-11 hydrolysis forming SN-38, respectively (Supplemental Fig. 1).
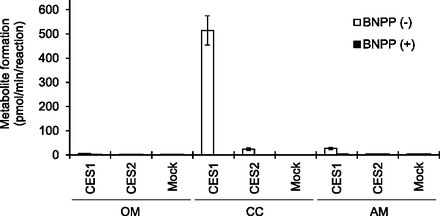
As shown in Fig. 5, CC was rapidly de-esterified with the recombinant CES1 and produced its active metabolite candesartan at a rate of 514 pmol/min per reaction, whereas the reaction was hardly catalyzed with the recombinant CES2 (24.2 pmol/min per reaction). Both activities were completely abolished by the addition of 1 mM BNPP, a carboxylesterase inhibitor. Although the recombinant CES1 showed the slight OM- and AM-hydrolase activities as being higher than that of the mock control, the activities were much lower than that for CC hydrolysis. The OM- and AM-hydrolase activities by the recombinant CES2 were comparable with the mock control with or without BNPP, suggesting that only nonenzymatic hydrolysis was slightly observed.
Hydrolysis of prodrug-type ARBs by recombinant human CES1 and CES2. Prodrugs OM, CC, and AM were incubated with 5% (v/v) conditioned media from human CES1- or CES2-overexpressed cell culture as an enzyme source under the conditions with or without 1 mM BNPP, and the formed active metabolites olmesartan, candesartan, and azilsartan were measured by liquid chromatography–tandem mass spectrometry. Data represent the means ± S.D. of triplicate determinations.
Hydrolysis by Recombinant Human CMBL.
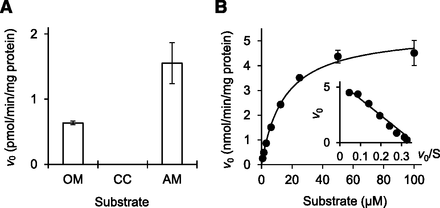
AM, which is another medoxomil-ester prodrug-type ARB, was substantially hydrolyzed by the recombinant human CMBL as well as OM (Fig. 6A) and exhibited simple Michaelis-Menten kinetics (Fig. 6B), with Km and Vmax values of 14.8 ±1.3 μM and 5.40 ± 0.16 nmol/min per milligram protein (the mean ± standard error of triplicate determinations). CC was not hydrolyzed by the recombinant CMBL (Fig. 6A).
Hydrolysis of prodrug-type ARBs by recombinant human CMBL. (A) OM-, CC-, and AM-hydrolase activities by recombinant human CMBL. Prodrugs OM, CC, and AM were incubated with recombinant CMBL-overexpressed cell lysate as an enzyme source, and the formed active metabolites, olmesartan, candesartan, and azilsartan, were measured by liquid chromatography–tandem mass spectrometry. Data represent the means ± S.D. of triplicate determinations. (B) Kinetic analysis of AM hydrolysis. The solid line is the best fit by nonlinear least-squares regression to the Michaelis-Menten equation. Data represent the means ± S.D. of triplicate determinations. The respective Eadie-Hofstee plot is presented in the inset.
Western Blot Analysis of CMBL and CES1 Expression.
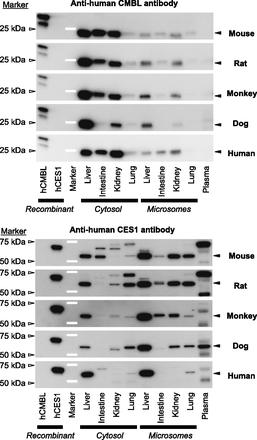
The immunoblots stained with polyclonal anti-human CMBL and anti-human CES1 antibodies were shown in Fig. 7. In the human liver, intestine, and kidney cytosols, the anti-human CMBL antibody intensely stained a single band at approximately 25 kDa, which was almost consistent with the theoretical mass 28 kDa, whereas it was weakly stained in human lung cytosol. Compared with the cytosols, the weaker CMBL expression was observed in the respective microsomes. Regarding the other animal species as well, liver, intestine, and kidney cytosols demonstrated strongly stained bands with approximately 25-kDa molecular mass except for dog intestine, whereas lung cytosols showed weak protein bands. The band intensity was generally lower in microsomes than in respective cytosols in the animal species, as was seen in humans. Collectively, these data indicate that the anti-human CMBL antibody recognized the CMBL orthologs in the animal species tested. No significant protein was detected in plasma samples of all the species tested including humans.
Western blot analysis of CMBL and CES1 expressions in animals and humans. The following tissue subcellular fractions (liver, intestine, kidney, and lung cytosol and microsomes) and diluted plasma were subjected to Western blot analysis: mouse, rat, and dog, 2 μg protein for each subcellular fraction and 10 μl of 100-fold diluted plasma; monkey and human, 0.5 μg protein for each subcellular fraction and 5 μl of 200-fold diluted plasma. As positive controls, 0.5 μg of human CMBL-overexpressed mammalian Freestyle 293-F cell lysate and 10 ng of purified recombinant protein of human CES1 were also analyzed. The proteins were separated by SDS-PAGE, transferred electrophoretically onto a polyvinylidene difluoride membrane, and stained by polyclonal anti-human CMBL and anti-human CES1 antibodies. The signal intensities of the protein bands in the subcellular fractions at the mobility indicated with closed triangles were semiquantified. White bars and open triangles represent molecular mass marker proteins.
The anti-human CES1 antibody used in the present study was reported to be CES1-specific, without cross-reactivity with CES2 proteins, by probing against cynomolgus monkey, dog, and human CES1 and CES2 enzymes (Williams et al., 2011). As previously reported (Satoh and Hosokawa, 2010; Williams et al., 2011), CES1 proteins were highly expressed in the liver in the three species, and were significantly expressed in monkey intestine and kidney, dog kidney, lung, and plasma, and human lung as a single band with a molecular mass approximately at 60 kDa. Regarding mouse and rat samples, single or multiple bands were detected in all the tissues and plasma.
The protein band intensities of CMBL in Western blot analysis mostly corresponded with the distribution of OM- and AM-hydrolase activities, and those of CES1 mostly corresponded with the distribution of CC-hydrolase activities in the eight different tissue subcellular fractions tested. The analysis plots for OM- and CC-hydrolase activities toward CMBL and CES1 protein expressions in humans were presented in Fig. 8 as exemplary cases. As shown in Table 1, the correlation coefficients between CMBL protein expression levels and OM- or AM-hydrolase activities were over 0.8 with one exception, where that between rat CMBL protein and OM-hydrolase activity was 0.477. The correlation coefficients between CES1 protein expression levels and CC-hydrolase activities were also over 0.8 without exception.
Correlation between protein expression levels of hydrolases and prodrug-hydrolase activities in humans. Data from eight different human tissue subcellular fractions (liver, intestine, kidney, and lung cytosolic and microsomal fractions) were employed to show the correlations between the protein expressions and activities. (A) CMBL versus OM-hydrolase activity, (B) CMBL versus CC-hydrolase activity, (C) CES1 versus OM-hydrolase activity, (D) CES1 versus CC-hydrolase activity.
Correlation coefficients between protein expression levels of hydrolases and prodrug-hydrolase activities
Data from protein expressions and activities of eight different subcellular fractions (liver, intestine, kidney, and lung cytosolic and microsomal fractions) were employed for each species. The protein levels were semiquantified as signal intensities of the immunoreactive bands at the mobility (indicated with closed triangles in Fig. 7).
Discussion
In the present study, we comparatively showed the difference between the bioactivating properties of prodrugs OM and CC, which belong to the same class of antihypertensive, ARB. We previously reported that the bioactivation of OM predominantly proceeds via intestinal CMBL in humans and that hepatic CMBL and plasma paraoxonase 1 are additionally involved (Ishizuka et al., 2010, 2012). On the contrary, present in vitro studies demonstrated that the contribution of intestinal enzymes to the CC bioactivation is relatively small and that hepatic CES1 is mainly involved. Although the responsible hydrolases and bioactivation sites were presented to be different between these two prodrugs, they were both reported to be rapidly and completely converted to their active metabolites through the first pass metabolism before they reach the systemic circulation and showed an increase of bioavailability as respective active metabolites (Atacand, 2000; Laeis et al., 2001), meaning that OM and CC are both good exemplary cases of absorbability improvement by esterification of the carboxylic acid group.
Also, we presented for the first time species- and tissue-specific OM hydrolysis catalyzed via CMBL—which was recently identified as an OM-bioactivating hydrolase and had not received much attention until then (Ishizuka et al., 2010)—using various tissue subcellular fractions of animals and humans. The kidney and lung were selected in addition to the liver and intestine, which were already demonstrated in humans as bioactivating organs for the prodrug OM (Ishizuka et al., 2010). Human kidney cytosol showed significant OM hydrolase activity comparable to that of liver and intestinal cytosol, whereas lung cytosol showed meaningfully lower activity. This tissue distribution pattern of the activity, namely high in the liver, intestine, and kidney and low in the lung, is consistent with the CMBL protein expression pattern demonstrated by Western blotting in the present study and also with the mRNA expression pattern previously reported (Ishizuka et al., 2010). The predominant expression of CMBL in the liver, intestine, and kidney implies that CMBL has primarily functioned in detoxification of xenobiotic compounds through the evolutional process from bacteria to humans. Bacterial CMBL, also called dienelactone hydrolase, has been reported as a catabolic enzyme enabling the use of chloroaromatic compounds (Schmidt and Knackmuss, 1980; Ngai et al., 1987), which are generally toxic to organisms.
Mice, rats, and monkeys, all commonly used experimental animal species, exhibited similar tissue distribution patterns to humans in the OM-hydrolase activity and CMBL protein expression. Based on the results, these animal species would provide dependable information to predict human bioactivation of ester compounds hydrolyzed by CMBL in the preclinical drug discovery and development settings. Although relatively high activities in monkey liver and kidney, and low activity in rat intestine appeared comparable with those in humans, the in vitro study results would be helpful when considering the quantitative difference of prodrug bioactivation between species. As a notable exception to the tissue distribution of CMBL, in the dog intestine slight OM-hydrolase activity or negligible CMBL protein expression was observed, while intestinal cytosols in humans and the other animal species exhibited significant activities and clear protein expressions. The absence of dog intestinal CMBL was observed despite highly conserved amino acid sequences with 87% homology between humans and dogs (GenBank Accession Numbers NP_620164.1 and XP_535793.1, respectively). This is a common feature of well-investigated esterases such as CES, paraoxonases, and cholinesterases (Williams et al., 2011) and shows interesting functional and evolutionary implications. Low capacity to detoxify esters in dog intestine potentially suggests the minor importance of this ability in carnivores, which would have infrequent exposure to toxic substances in plants.
Moreover, we also demonstrated that CMBL is a bioactivating enzyme for recently launched prodrug-type ARB AM (azilsartan medoxomil), whose prodrug moiety is identical to that of OM. Kawaguchi et al. (2013) recently reported a likely estimation that enzymes involved in the hydrolysis of AM would be the same as OM by considering the identical chemical structures of the prodrug moiety between the two ARBs and in vitro metabolic study results using liver and intestinal S9 fractions and plasma. As shown in the results of our in vitro study using recombinant human CMBL, obvious AM-hydrolase activity clearly demonstrated the involvement of CMBL in the reaction. Furthermore, the clear positive correlation between OM- and AM-hydrolase activities suggests the significant contribution of CMBL, not only in humans but also other tested animal species, to hepatic and intestinal AM bioactivation. Only limited information on the substrate specificity of CMBL is available at present: It specifically catalyzes ring-opening reaction of cyclic esters such as faropenem medoxomil and lenampicillin (Ishizuka et al., 2010), as well as OM and AM. What seems to be lacking, as a further interest, is to define the endogenous substrates and to elucidate the physiologic functions of CMBL proteins.
Also, we have shown that human CES1 rather than CES2 catalyzed the bioactivation of CC using recombinant proteins. It has often been reported that CC is converted to candesartan by carboxylesterases via intestinal and hepatic first-pass metabolism (Miwa et al., 1998; Easthope and Jarvis, 2002); however, which isozymes of CES1 or CES2 are a major contributor remained unknown until now. Although Laizure et al. (2013) supposed that CC is a CES2 substrate because of CC’s large alcohol group of the prodrug moiety that CES2 prefers as a substrate, in the present study, the recombinant CES2 exhibited only low CC-hydrolase activity in contrast to rapid hydrolysis by the recombinant CES1. Moreover, the intestinal subcellular fractions showed minimal CC-hydrolase activity in all the species tested including humans, indicating negligible involvement of CES2, which had been reported to be expressed in the intestine in mice, rats, monkeys, and humans (Taketani et al., 2007; Satoh and Hosokawa, 2010). Contrary to CC hydrolysis, the recombinant human CES1 showed slight OM- and AM-hydrolase activities, suggesting that human CES1 may partially catalyze OM- and AM-hydrolysis in the liver. However, higher activities were observed in cytosolic fraction of the liver rather than the microsomal fraction in which human CES1 is mostly distributed (Fig. 2 for OM hydrolysis). This fact indicates that the CES1 contribution in the liver to OM and AM hydrolysis is limited. CES2 showed only mock-control-level OM- and AM-hydrolase activities. Three prodrug-type ARBs, namely OM, CC, and AM, similarly require hydrolytic bioactivation to exert their antihypertensive effect; however, needless to say, the responsible hydrolases depend on the chemical structures of their prodrug moieties, OM and AM being substrates for CMBL while CC is a substrate for CES1.
In conclusion, we comparatively demonstrated the difference in the bioactivating properties of prodrug-type ARBs: OM is bioactivated mainly via intestinal and additionally hepatic CMBL, therefore the newly identified CMBL substrate AM is likely to be similarly bioactivated to OM, while CC is via hepatic CES1 rather than intestinal enzymes. Also, we presented species differences in hydrolytic activity and protein expression of the uninvestigated hydrolase CMBL in four major organs, namely liver, intestine, kidney, and lung, by comparison between humans and other animal species.
Acknowledgments
The authors thank Kaori Nakahara and Dr. Yuji Ogura for kindly providing the recombinant human CES1 and CES2 proteins.
Authorship Contributions
Participated in research design: Ishizuka, Yoshigae, Murayama.
Conducted experiments: Ishizuka.
Performed data analysis: Ishizuka.
Wrote or contributed to the writing of the manuscript: Ishizuka, Yoshigae, Murayama, Izumi.
Footnotes
- Received July 1, 2013.
- Accepted August 13, 2013.
↵
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
Abbreviations
- AM
- azilsartan medoxomil
- ARB
- angiotensin receptor blocker
- BNPP
- bis-p-nitrophenylphosphate
- CC
- candesartan cilexetil
- CES
- carboxylesterase
- CMBL
- carboxymethylenebutenolidase homolog
- Km
- Michaelis constant
- OM
- olmesartan medoxomil
- RNH-6272
- 2-butyl-4-(1-hydroxy-1-methylethyl)-1-[2′-(1H-tetrazole-5-yl)-4-biphenylyl]-1H-imidazole-5-carboxylic acid
- S9
- 9,000g supernatant
- Vmax
- maximum velocity
- Copyright © 2013 by The American Society for Pharmacology and Experimental Therapeutics