Abstract
Transporter expression, determined by quantitative proteomics, together with PBPK models is a promising approach for in vitro-to-in vivo extrapolation (IVIVE) of transporter-mediated drug clearance. OCT2-expressing HEK293 and MDCKII cells were used to predict in vivo renal secretory clearance (CLr,sec) of metformin. [14C]-Metformin uptake clearance in OCT2-expressing cells was determined and scaled to in vivo CLr,sec by using OCT2 expression in the cells versus the human kidney cortex. Through quantitative targeted proteomics, the total expression of OCT2 in HEK293, MDCKII cells, and human kidney cortex was 369.4 ± 26.8, 19 ± 1.1, and 7.6 ± 3.8 pmol/mg cellular protein, respectively. The expression of OCT2 in the plasma membrane of HEK293 and MDCKII cells, measured using an optimized biotinylation method followed by quantitative proteomics, was 30.2% and 51.6%, respectively. After correcting for percent of OCT2 expressed in the plasma membrane and the resting membrane potential (millivolts) difference between the OCT2-expressing cells and the renal epithelial cells, the predicted CLr,sec of metformin was 250.7 ml/min, a value within the range of the observed CLr,sec of metformin. These data demonstrate the promise of using quantitative proteomics for IVIVE of transporter-mediated drug clearance and highlight the importance of quantifying plasma membrane expression of transporters and utilizing cells that mimic the in vivo mechanism(s) of transport of drugs.
Introduction
Transporters play an important role in drug absorption, distribution, and elimination. When a drug under development or its active/toxic metabolite(s) has significant renal clearance, prediction of its in vivo renal clearance from in vitro studies (IVIVE) is important to predict the first in human dose, systemic clearance of the drug, renal drug-drug interaction, and kidney epithelial cell drug concentrations. While the filtration clearance of a drug can readily be predicted from the average glomerular filtration rate and the fraction of drug unbound in the plasma, IVIVE of the renal secretory clearance of a drug is challenging. While allometry (Paine et al., 2011) or quantitative structure-activity relationship (Dave and Morris, 2015) have been used to predict the renal clearance of drugs, these methods do not account for involvement of transporters and therefore lack the ability to predict in vivo transporter-mediated renal secretory clearance (CLr,sec) or renal transporter-mediated drug-drug interactions. Although tools (e.g., primary kidney epithelial cells) to predict CLr,sec of a drug are becoming available, their predictive abilities have yet to be tested. Thus, alternate methods are needed to predict CLr,sec of a drug. We (Ishida et al., 2018), and others (Bosgra et al., 2014; Vildhede et al., 2016) have shown that transport activity of a drug in cell lines overexpressing the transporter(s) of interest can be used to predict transporter-mediated drug clearance if a scaling factor to account for differences in transporter expression in the cell line and in the tissue (e.g., kidneys) is considered. Theoretically, since it is the transporters at the plasma membrane that are responsible for the cellular uptake and/or efflux of drugs, quantifying such expression may improve IVIVE of drug clearance (Kumar et al., 2017). Therefore, as a proof of concept, the goals of our study were to determine 1) if transporter-mediated in vivo CLr,sec of metformin can be predicted within the range of the observed values using protein expression and transport activity of organic cation transporter 2 (OCT2) in OCT2-expressing cell lines (HEK293 and MDCKII) as well as OCT2 protein expression in the human kidney cortex and 2) whether this prediction could be improved by incorporating plasma membrane expression of OCT2-expressing cells and the membrane potential of these cells versus human renal epithelial cells. We chose metformin as our model drug as literature evidence suggests (see Discussion) that its CLr,sec is determined primarily by OCT2.
Materials and Methods
Chemicals and Reagents
Synthetic signature peptides for OCT2 were obtained from New England Peptides (Boston, MA). The corresponding stable isotope labeled (SIL) peptides for OCT2 and Na+-K+ ATPase, total protein quantification bicinchoninic acid assay (BCA) kit, dithiothreitol (DTT), iodoacetamide (IAA), mass spectrometry grade trypsin, Dulbecco’s modified Eagles’ medium (DMEM) high glucose medium (Gibco), Dulbecco’s phosphate-buffered saline (DPBS), Krebs-Ringer-HEPES (KRH) buffer, and Pierce cell surface protein isolation kit were obtained from Thermo Scientific (Rockford, IL). Pierce cell surface protein isolation kit contains sulfosuccinimidyl-2-(biotinamido) ethyl-1, 3-dithiopropionate (sulfo-NHS-SS-biotin), quenching solution (100 mM glycine), lysis buffer, neutravidin agarose gel, wash buffer, column accessory pack, DTT, phosphate buffer, and Tris buffer. Metformin [biguanido-14C]hydrochloride (98 mCi/mmol) was purchased from Moravek Biochemicals, Inc. (Brea, CA). ScintiVerse BD Cocktail liquid scintillant and HPLC-grade acetonitrile were purchased from Fischer Scientific (Fair Lawn, NJ). Cimetidine, metformin (hydrochloride salt), and formic acid were purchased from Sigma-Aldrich (St. Louis, MO). All reagents were analytical grade.
Human Kidney Cortex and Transporter-Expressing Cell Lines
Transplant quality human kidney cortices (for demographics, see Supplementry Table 2S in Prasad et al., 2016) were obtained from Newcastle University, England (n = 5, randomly selected). OCT2-expressing HEK293 and MDCKII cells were created as described previously (Yin et al., 2015, 2016).
Measurement of OCT2 Transporter Uptake Activity in HEK293 and MDCKII Cells
Transport studies were conducted in OCT2-expressing MDCKII and HEK293 cells with [14C]-metformin with or without 1 mM cimetidine (OCT2 inhibitor). For transport assays, OCT2-expressing MDCKII and HEK293 cells were grown in 24-well poly-d-lysine coated plates, at a density of 75,000 cells per well with 1 ml of high glucose Dulbecco’s modified Eagles’ medium (changed daily). On day 3, cells were washed with 1 ml/well Dulbecco’s phosphate-buffered saline buffer and incubated at 37°C with KRH buffer (5.6 mM glucose, 125 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM CaCl2, 1.2 mM MgSO4, and 25 mM HEPES) containing 5.5 µM [14C]-metformin with or without 1 mM cimetidine (OCT2 inhibitor). After 2 minutes (when uptake is linear), the uptake (in triplicate) was terminated by washing the cells three times with ice-cold KRH buffer (1 ml each). Then, the cells were lysed with 0.5 ml 1 M NaOH at 37°C for 2 hours and neutralized with 0.5 ml 1 M HCl. Thirty microliters of this lysate solution was used for total protein estimation using the BCA method, and 200 µl was used to analyze total radioactivity by Tri-Carb Liquid Scintillation Counters (PerkinElmer, Waltham (MA)). OCT2-mediated uptake was determined by the difference in the metformin uptake in the presence/absence of 1 mM cimetidine. The in vitro OCT2-mediated clearance of metformin was calculated as the ratio of OCT2-mediated uptake and metformin concentration in the media.
LC-MS/MS Quantification of Total OCT2 Expression in Human Kidney Cortex as Well as Total and Plasma Membrane OCT2 Expression in HEK293 and MDCKII Cells
Plasma Membrane Isolation from OCT2-expressing HEK293 and MDCKII Cells.
Plasma membranes from OCT2-expressing HEK293 and MDCKII cells were isolated in three and two independent experiments, respectively, using a biotinylation methodology optimized in our laboratory (Kumar et al., 2017). Briefly, HEK293 and MDCKII cells were grown in 75 cm2 flasks until 80%–100% confluency and incubated with 10 ml of 0.78 mg/ml of sulfo-NHS-SS-biotin at 37°C for 1 hour to biotinylate extracellular free primary amines (lysine or arginine) or N-terminal primary amines of extracellular peptide residues in the plasma membrane. After cell lysis, biotinylated plasma membranes were isolated using neutravidin resin columns.
Determination of Percent Plasma Membrane Expression of OCT2 in OCT2-expressing Cells.
The expression of OCT2, Na+-K+-ATPase, and calreticulin in the lysate, the nonbiotinylated and the biotinylated fractions was quantified using LC-MS/MS (see below). Stable labeled internal standards of OCT2 and Na+-K+-ATPase unique peptides were used to determine the area ratio (analyte:SIL ratio) of the respective peaks in the samples obtained from the biotinylation experiment. For calreticulin, the stable labeled internal standard was not available, and therefore the stable labeled internal standard of Na+-K+-ATPase was used to estimate the calreticulin peak area ratio (Kumar et al., 2017).
OCT2 Total Protein Quantification in Kidney Cortex.
Sample preparation and quantitative proteomics were performed as described previously (Prasad et al., 2016). Total protein content (wt/wt) in kidney cortex homogenate was quantified by BCA assay. OCT2 expression was determined in triplicate for each kidney cortex homogenate. Briefly, 20 μl of 2.0 mg/ml (or lower concentration) of the human kidney cortex or transporter-expressing cell line or samples obtained through biotinylation experiments was treated with 15 μl of ammonium bicarbonate buffer (50 mM, pH 7.8), 18 μl of 3% sodium deoxycholate (wt/v), 6 μl DTT (100 mM), and 10 μl of human albumin (10 mg/ml). Proteins present in the sample were denatured, and disulfide bond between cysteine residues was reduced by incubation at 95°C for 5 minutes, followed by the addition of 6 μl of iodoacetamide (200 mM; an alkylating agent) and treatment with ice-cold methanol (0.5 ml), chloroform (0.2 ml), and water (0.45 ml) and centrifugation. Lastly, the protein sample was digested with 20 μl of trypsin followed by centrifugation at 5000 g for 5 minutes at 4°C, and 5 μl of the supernatant was introduced into the LC-MS/MS system. The calibration standards were prepared by spiking peptide standards into the extraction buffer II of the membrane protein extraction kit. Samples were analyzed as described before by Kumar et al. (2017). Briefly, AB Sciex 6500 triple-quadrupole mass spectrometer (Sciex, Framingham, MA) coupled to the Water Acquity UPLC system (Waters Corporation, Milford, MA) was operated in electrospray positive ionization mode for liquid chromatography-tandem mass spectroscopy (LC-MS/MS) analysis of the signature peptides (Supplemental Table 1). The transitions from doubly charged parent ion to singly charged product ions for the analyte peptides and their respective SIL peptides were monitored (Supplemental Table 1). The chromatographic separation and resolution were obtained on an Acquity UPLC HSS T3 Column, (1.8 µm, 2.1 × 100 mm) with a 0.2 µm inlet frits (Waters). Mobile phases (0.3 ml/min) consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The gradient program for UPLC method for determination of OCT2 in human kidney cortex and OCT2-expressing HEK293 and MDCKII cells was: 0–2 minutes: 3% B; 2–20 minutes: 3%–45% B; 20–23 minutes: 45%–90% B; 23 to 24 minutes: 90% B; 24–24.5 minutes: 90%–3%B; 24.5–28 minutes: 3%–3%B including washing and re-equilibration for 3.5 minutes. Seven calibration standards ranging from 0.14 to 70.4 fmol (on the column) were used to quantify OCT2.
Prediction of Metformin In-Vivo CLr,sec.
Metformin in vivo CLr,sec was scaled from experimentally determined metformin in-vitro clearance by using the expression of OCT2 in expressed cells and human kidney cortex (eq. 1, Table 1) and the plasma membrane potential difference between HEK293/MDCKII cells and human renal epithelial cells. To do so, we made the following assumptions: 1) OCT2-mediated metformin uptake into the human kidney is the rate determining step in metformin’s CLr,sec, 2) in vivo, OCT2 is expressed entirely in the plasma membrane of human kidney renal epithelial cells (Fisel et al., 2016), and 3) the human kidney cortex is 70% of total kidney by weight (Bouchet et al., 2003). In addition, we experimentally determined the total protein content in the human kidney cortex homogenate (wt/wt). Therefore: (1)Where [E] = Total OCT2 expression in human kidneys (in vivo) and in OCT2-expressing cells (in vitro), respectively. Total tissue protein per unit cortex weight was estimated by the BCA assay.
(1)Where [E] = Total OCT2 expression in human kidneys (in vivo) and in OCT2-expressing cells (in vitro), respectively. Total tissue protein per unit cortex weight was estimated by the BCA assay.
Values used for IVIVE of metformin CLr,sec
The OCT2 total cell expression-based predicted metformin CLr,sec was corrected as follows: 1) the percent of OCT2 expressed in the plasma membrane in OCT2-expressing cells and 2) the difference in plasma membrane potential between HEK293/MDCKII cells and human renal epithelial cells. The latter was based on the observations by Burt et al. (2016) and Koepsell and Keller (2016). The rate of OCT2-mediated metformin transport was directly proportional to the difference in electrochemical driving force across the plasma membrane of OCT2-expressing cells and kidney epithelial cells (Burt et al., 2016; Koepsell and Keller, 2016). We assumed that both HEK293 and MDCKII cells had a similar resting membrane potential difference of −35 mV (see Discussion for details). Correcting for the twofold difference between the plasma membrane potential of the OCT2-expressing cells (−35 mV) and kidney epithelial cells (−70 mV) resulted in a twofold correction of our predicted metformin CLr,sec.
Results
OCT2-Mediated [14C]-metformin Uptake Clearance in OCT2-Expressing HEK293 and MDCKII Cells
[14C]-metformin uptake clearance (mean ± S.D.) was 36.7 ± 2.8 and 2.0 ± 0.1 µl/mg cellular protein per minute (n = 3 replicates from a single experiment) in HEK293 and MDCKII cells, respectively.
Total Expression of OCT2 in the Human Kidney Cortex and OCT2-Expressing HEK293 and MDCKII Cells
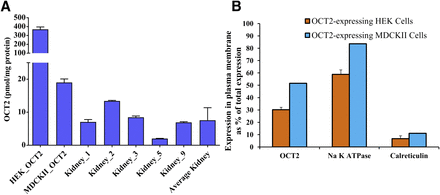
Total OCT2 expression (mean ± S.D.) in the human kidney cortex, HEK293, and MDCKII cells was 7.6 ± 3.8, 369.4 ± 26.8, and 19 ± 1.1 pmol/mg protein, respectively (Fig. 1A; Table 1). Total protein content in human kidney cortex homogenate was 30% wt/wt.
Total cellular expression of OCT2 in human kidney cortex and OCT2-expressing HEK293 and MDCKII cells and plasma membrane expression of OCT2 in OCT2-expressing HEK293 and MDCKII cells. (A) OCT2 expression in the human kidney cortex (n = 5), HEK293, and MDCKII cells was 7.6 ± 3.9, 369.4 ± 26.8, and 19 ± 1.1 pmol/mg protein (mean ± S.D. of triplicates of a single experiment). (B) The percent of total OCT2, Na+-K+-ATPase and calreticulin expressed in the plasma membrane of OCT2-expressing HEK293 (mean ± S.D, n = 3 independent experiments) was 30.2 ± 1.9, 58.8 ± 3.6, and 6.7 ± 2.3, respectively, while that in MDCKII (average of n = 2 independent experiments) was 51.6, 83.7, and 11.1 respectively. Mass balance showed that the recovery of all proteins was 94.5%–114.4%. Data are average of two and three independent experiments for OCT2-expressing MDCKII and HEK293 cells, respectively.
Plasma Membrane Expression of OCT2 in OCT2-Expressing HEK293 and MDCKII Cells
The percent of OCT2 expressed in the plasma membrane of OCT2-expressing HEK293 and MDCKII cells was only 30.2 ± 1.9 and 51.6, respectively (Fig. 1B), while that of Na+-K+-ATPase (plasma membrane marker) was 58.8 ± 3.6 and 83.7, respectively (Fig. 1B). The corresponding percent of calreticulin (endoplasmic reticulum marker) biotinylated was only 6.7 ± 2.3 and 11.1, confirming minimal intracellular penetration of the sulfosuccinimidyl-2-(biotinamido) ethyl-1, 3-dithiopropionate (sulfo-NHS-SS-biotin) reagent (Fig. 1B).
IVIVE of In Vivo CLr,sec of Metformin Based on OCT2 Expression as well as Resting Membrane Potential
A literature review of human in vivo metformin renal clearance data from multiple studies resulted in a mean (geometric mean) CLr,sec (assuming CLfiltration is 120 ml/min) of 415 ml/min (range 215–643 ml/min; Supplemental Table 2). Assuming 100% of OCT2 transporters are expressed in the plasma membrane of OCT2-expressing HEK293 and MDCKII cells, metformin’s CLr,sec was predicted (eq. 1) to be 46.2 and 50.4 ml/min, respectively. When corrected for OCT2 plasma membrane expression in HEK293 and MDCKII cells, the predicted metformin CLr,sec improved to 153.0 and 97.7 ml/min respectively (Table 1). When this predicted metformin CLr,sec (ml/min) was further corrected for the membrane potential difference between OCT2-expressing cells and the renal epithelial cells, the predicted metformin CLr,sec improved to an average value of 250.7 ml/min (Table 1), a value lower than the observed geometric mean of CLr,sec but within the observed range (215–643 ml/min).
Discussion
To predict the clearance of a drug when transporters are involved, it is important that the plasma membrane expression of the transporter(s) be used to scale in vitro transport clearance to in vivo. Therefore, we optimized a cell-surface biotinylation method to determine the percent of expression of a transporter on the plasma membrane (Kumar et al., 2017). The use of this optimized biotinylation method (Kumar et al., 2017) showed that ≤52% of OCT2 is expressed in the plasma membrane of OCT2-expressing HEK293 and MDCKII cells (Fig. 1B). We confirmed that this result was not due to sulfo-NHS-SS-biotin concentration being limiting (data not shown).
Metformin is primarily cleared (∼70%) by active renal secretion (CLr,sec) mediated exclusively by OCT2 located in the basolateral membrane of the proximal tubule epithelial cells (Burt et al., 2016). Then, it is effluxed into the renal tubule by the apical MATE1 and MATE2-K. Moreover, OCT2-mediated uptake of metformin (from the plasma) appears to be the rate-determining step in metformin’s CLr,sec. Human studies have shown that inhibition of OCT2 by dolutegravir (50 mg, twice daily for 7 days) increases metformin plasma AUC to a larger extent (145%) than inhibition of both MATE1 and MATE2-K, by a 50 mg oral dose of pyrimethamine (35%; (Song et al., 2016; Kusuhara et al., 2011). Dolutegravir does not inhibit MATE1 and MATE2-K at doses used (Song et al., 2016) and pyrimethamine is a more potent inhibitor of MATE1 and MATE2-K (IC50: 0.1 µM) than OCT2 (IC50: 4.8 µM).
Although the involvement of other transporters in metformin CLr,sec cannot be discounted, based on the above data, it is reasonable to assume that OCT2 is the rate-determining step in CLr,sec of metformin. Were this is the case, as described by us (Patilea-Vrana and Unadkat, 2016), the renal secretory clearance of metformin will be determined only by its OCT2-mediated clearance. Thus, the renal secretory clearance of metformin should be well-predicted by the uptake clearance of metformin by OCT2-expressing cells, provided this uptake clearance is scaled to that in vivo by the difference in OCT2 expression in the cells versus kidney cortex. When we assumed that 100% of OCT2 was located in the plasma membrane of nonpolarized HEK293 and polarized MDCKII cells, the in vivo CLr,sec of metformin was underpredicted [∼50 ml/min vs. 415 (range 215–643) ml/min] by about eightfold. When this IVIVE was corrected for the plasma membrane expression of OCT2, the prediction of CLr,sec of metformin improved markedly, but was still lower (153.0 and 97.7 ml/min in OCT2-HEK293 and MDCKII cells, respectively, Table 1) than its in vivo secretory CLr,sec.
For accurate IVIVE of the clearance of a drug, the cell line must replicate the mechanism of transport in vivo. Based on literature data, this is not the case here. OCT2 transport activity is driven by the negative resting membrane potential of the cell. The resting membrane potential of the human renal epithelial cells is −70 mV (Wright and Dantzler, 2004) while for HEK293 and MDCKII cells it is −20 to −35 mV (Condreay et al., 2006; Hsu et al., 2010) and −20 to −50 mV, respectively (Westphale et al., 1992; Lang and Paulmichl, 1995). Correcting for this difference in membrane potential between OCT2-expressing cells and renal epithelial cells improved our prediction of metformin CLr,sec (250.7 ml/min) and resulted in a value within the range observed in vivo (215–643 ml/min; mean 415 ml/min).
An interesting question that arises from our data is what is the percent of plasma membrane expression of OCT2 in kidney epithelial cells? Immunolocalization data suggest that OCT2 is predominately located on the plasma membrane of the kidney epithelial cells (Fisel et al., 2016). Unfortunately, this question cannot be addressed by biotinylation without first culturing the cells. Therefore, it will be interesting in the future to determine the plasma membrane expression of OCT2 in primary kidney epithelial cells. Nevertheless, if less than 100% of OCT2 were expressed in the plasma membrane of these cells, the above IVIVE would be even more discrepant. Therefore, assuming that OCT2 in kidney epithelial cells is 100% expressed in the plasma membrane is not a reason for the underprediction of the in vivo metformin CLr,sec.
Many groups have used primary cells (e.g., human/rat hepatocytes) and empirical scaling factors (Jones et al., 2012; Zou et al., 2013; Izumi et al., 2017) to predict hepatobiliary clearance of drugs. Such methods lack mechanistic insight and therefore may not necessarily apply to a new molecular entity. By incorporating mechanisms of transport and quantitative proteomics to account for the difference in expression of transporters between hepatocytes and liver tissue, such IVIVE has been shown to be successful without using an empirical scaling factor (Bosgra et al., 2014; Ishida et al., 2018). However, this approach has never been applied to the renal secretory clearance of a drug. Here we report the first such successful IVIVE where our prediction falls within the observed metformin CLr,sec values (albeit lower than the mean). Moreover, our data highlight the importance of utilizing cells that mimic the mechanism(s) of transport of drugs in vivo as well as proteomics to quantify plasma membrane expression of transporters to conduct IVIVE of transporter-mediated clearance of a drug.
Authorship Contributions
Participated in research design: Kumar, Yin, Prasad, Wang, Unadkat.
Conducted experiments: Kumar, Yin, Billington.
Contributed new reagents or analytic tools: Yin, Billington, Brown, Wang.
Performed data analysis: Kumar, Yin, Prasad, Wang, Unadkat.
Wrote or contributed to the writing of the manuscript: Kumar, Yin, Billington, Prasad, Brown, Wang, Unadkat.
Footnotes
- Received May 12, 2018.
- Accepted August 3, 2018.
V.K. was supported by the Simcyp Grant & Partnership Scheme. This study is supported in part by National Institutes of Health National Institute on Drug Abuse [Grant P01-DA032507] and National Institute of General Medical Sciences [Grant R01-GM066233].
↵
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
Abbreviations
- BCA
- bicinchoninic acid assay
- DPBS
- Dulbecco’s phosphate-buffered saline
- CLr,sec
- renal secretory clearance
- DTT
- dithiothreitol
- IVIVE
- in vitro-to-in vivo extrapolation
- KRH
- Krebs-Ringer-HEPES
- LC-MS/MS
- liquid chromatography tandem mass spectrometry
- OCT2
- organic cation transporter 2
- SIL
- stable isotope labeled
- sulfo-NHS-SS-Biotin
- sulfosuccinimidyl-2-(biotinamido) ethyl-1, 3-dithiopropionate
- Copyright © 2018 by The American Society for Pharmacology and Experimental Therapeutics