Abstract
As domestic animals such as cat, horse, and dog increasingly become the clinical targets for drug discovery programs, the need to understand how these animals metabolize xenobiotics becomes more important. In the present study, substrates and inhibitors that were reported to be selective for particular P450 isozymes were used as probes to study in vitro metabolism in horse, dog, cat, and human liver microsomes. Seven selective catalytic activity markers for cytochrome P450-mediated reactions were measured: phenacetinO-deethylase (P4501A1/2), coumarin 7-hydroxylase (P4502A6), tolbutamide hydroxylase (P4502C8/9), S-mephenytoin 4′-hydroxylase (P4502C19), dextromethorphan O-demethylase (P4502D6), chlorzoxazone 6-hydroxylase (P4502E1), and testosterone 6β-hydroxylase (P4503A4). Metabolic activity was found in every species with each substrate. Under the conditions of this study, it was observed that no one species was more active for any given substrate. However, rather large interspecies differences were observed. There was no marked sex difference in the way the various species metabolized the different substrates. The effect of selective P450 inhibitors on the various activities was tested with furafylline (P4501A2), mouse monoclonal antibody inhibitory to CYP2A6, sulfaphenazole (P4502C9), tranylcypromine (P4502C19), quinidine (P4502D6), diethyldithiocarbamate (P4502E1), and troleandomycin (P4503A4). In most cases, these inhibitors were effective to varying degrees against the activity seen in horse, dog, and cat liver microsomes. However, even at high concentrations, furafylline did not inhibit phenacetinO-deethylase activity in cat and troleandomycin did not affect testosterone 6β-hydroxylase activity in horse. Sulfaphenazole was not tested in dog and cat because of the low tolbutamide hydroxylase activity. Overall, these results show that there are also large interspecies differences in the way the selective P450 inhibitors affect the in vitro metabolism of the various substrates in horse, dog, and cat liver microsomes.
Early knowledge of the potential biotransformations of new drug candidates in the target species is of great interest. Recent focus on developing therapeutic agents for domestic animals such as horse, dog, and cat has pointed out the need to understand how these animals metabolize xenobiotics. However, the material required to test in vivometabolism in large animals such as the horse is not practical at a drug discovery stage. Therefore, the use of in vitrotechniques to determine important metabolic pathways can be useful. Phase I biotransformations, involving cytochrome P450s, are known to play a major role in the metabolism of exogenous compounds such as drugs.
Human P450s1 have been characterized over the years (1, 2), and using immunochemical methods (3) it has been shown that P450 1A2, 2A6, 2C, 2D6, 3A4, and 2E1 constitute the majority of the P450s present in the liver. It has also been shown that there are certain substrates that are metabolized specifically by one P450 isozyme or highly related forms (2, 4-6), and these can be used to phenotype microsomes obtained from human livers (2). For example, enzymatic assays using phenacetinO-deethylase activity have been reported to be specific for P4501A1/1A2, tolbutamide hydroxylase activity for P4502C8/2C9,S-mephenytoin 4′-hydroxylase activity for P4502C19, dextromethorphan O-demethylase activity for P4502D6, testosterone 6β-hydroxylase activity for P4503A4. Also, it has been widely shown that some chemicals can inhibit, in a very specific way, the in vitro activity of P450 mediated enzymatic reactions in human (2, 7-10): furafylline is a selective inhibitor for P450 1A2, sulfaphenazole for P4502C9, tranylcypromine for P4502C19, quinidine for P4502D6, diethyldithiocarbamate for P4502E1, troleandomycin for human P4503A4. To our knowledge, there is no chemical P4502A6 inhibitor reported so far, but inhibitory antibodies against CYP2A6 are available. The chemical inhibitors have a number of advantages in that they are simple to use and readily available. The major drawback is that proper experimental conditions have to be used to ensure the selectivity. For example, recent accounts have shown that ketoconazole, a very potent P4503A4 inhibitor, can inhibit the catalytic activity of other P450s when used at high concentration (11).
It is not yet known which P450 isozymes are important for the metabolism of drugs in the liver of horses, dogs, and cats, although the presence of some P450s has been reported. Drug metabolism in the dog has been studied the most because it is used as an animal model in pre-clinical studies and toxicity tests. Catalytic activities using specific probes for P4501A1/1A2, 2A6, 2C19, 2D6, 2E1, and 3A4 have been reported in the dog (12-14). Characterization of some cytochrome P450s by amino acid sequence has shown that P450s belonging to the P4501A (15, 16), P4502C (16, 17), P4503A (18), and P4502D (13, 14) subfamilies are present in the dog.
Data on cytochrome P450 enzyme activities in horse and cat are limited. A few years ago Yeary and Gerken (19) presented a short communication on the biotransformation of some substrates in hepatic horse microsomes, and Martı́nez-Zedillo et al. (20) published an in vitro metabolism study on parathion in horse. More recently, Komori et al. (21) reported the characterization of a P450 from horse liver microsomes that belongs to the P4502C subfamily. Some data on the metabolism of drugs in cats can be found in an interspecies study involving P4502A and 3A substrates (22, 23). To our knowledge, no cytochrome P450 has been isolated and fully characterized in the cat.
As the catalytic probes and chemical inhibitors are well characterized in the human systems (1, 2) and as little is known about the P450-mediated metabolism in horses, cats and to some extent, in dogs, it would be relevant to use these tools to learn more about phase I biotransformations in these animals. In this study, we report thein vitro cytochrome P450-mediated hepatic activity of several substrates (phenacetin O-deethylation, coumarin 7-hydroxylation, tolbutamide hydroxylation, S-mephenytoin 4′-hydroxylation, dextromethorphan O-demethylation, chlorzoxazone 6-hydroxylation, testosterone 6β-hydroxylation) and the effect of selective P450 inhibitors on these activities in human, horse, dog, and cat liver microsomes to evaluate species and gender variation in drug metabolism.
Materials and Methods
Chemicals.
β-Nicotinamide adenine dinucleotide phosphate, EDTA, D-glucose-6-phosphate, glucose 6-phosphate dehydrogenase, acetaminophen, chlorzoxazone, coumarin, dextromethorphan, diethyldithiocarbamic acid, phenacetin, tolbutamide, tranylcypromine, troleandomycin, quinidine, 7-hydroxycoumarin, and 6-hydroxychlorzoxazone were purchased from Sigma Chemical Co. (St. Louis, MO). 6β-Hydroxytestosterone and testosterone were obtained from Steraloids (Wilton, NH). Bovine serum albumin, Folin & Ciocalteu’s Phenol Reagent and the Modified Lowry protein assay reagent were purchased from Pierce (Rockford, IL). Dextrorphan D-tartrate and 4-hydroxytolbutamide were purchased from RBI (Natick, MA). Furafylline was synthesized at Merck & Co. (Rahway, NJ) for specific needs. (±)-4′-Hydroxymephenytoin andS-(+)-mephenytoin were obtained from Ultrafine Chemicals (Manchester, UK). Sulfaphenazole was obtained from Ciba-Geigy (Switzerland). Monoclonal antibody inhibitory to CYP2A6 was purchased from Gentest Corp. (Woburn, MA). All other reagents were of highest purity commercially available or HPLC Grade.
Tissue and Microsomes.
Human tissues (18 to 57 years old, four females, nine males) were obtained from various sources (F. Guengerich, Vanderbilt University School of Medicine, Nashville, TN; IIAM, Exton, PA; Québec Transplant, Montréal, QC, Canada). Beagle dogs (> 48 weeks, seven females and seven males) were obtained from Marshall Research Labs (North Rose, NY) and were fed with Iams Eukanuba from 3.5 weeks to 7 months, then with Purina Marshall Canine 2109 or Harlan Teklad Canine 8653. Cats (1 year old, four females and four males) were obtained from Harlan Sprague Dawley (Madison, WI) and were fed with Harlan Teklad Cat diet 7770. Horses (5–20 years, five females and five males) were obtained from Abattoir Richelieu Inc (Massueville, QC, Canada).
Microsomes were prepared from frozen (−80°C) tissue as described in the literature (24). Protein concentrations of the microsomal fractions were determined by the method of Lowry et al. (25) using BSA as a standard.
Enzymatic Assays.
The microsomal incubation conditions used to study the metabolism of the various substrates were adapted from reported procedures (6,26-32) and are specified in table 1. These experimental conditions showed reaction rates in human microsomes that were linear with incubation time and protein concentration. Each incubation was performed in a 100 mM phosphate buffer at pH 7.4 (except for coumarin incubations which were conducted in 50 mM TRIS) containing 20 mM glucose 6-phosphate, 2.0 mM NADP, 2.0 mM magnesium chloride, 2.0 units of glucose 6-phosphate dehydrogenase, and the appropriate amount of microsomal protein in a total volume of 500 μL. There was a 2 min pre-incubation step at 37°C before the reaction was started by the addition of the specific substrate. The substrate stock solutions were prepared in different solvents as specified in table 1 and the organic solvent content did not exceed 3% (v/v) and 1% (v/v) for methanol and acetonitrile, respectively, when added to the incubations. The organic solvent content in the final incubation was kept constant for each specific substrate studied. After a specific period of time, the reactions were quenched by adding the appropriate chemical to precipitate the proteins. The incubation mixtures were then centrifuged for 10 min at 13 000 rpm in an Eppendorf Centrifuge 5415C. An aliquot of the supernatant (injection volume of 50 μl) was analyzed by HPLC under various conditions as described below. The different metabolites were quantified using an external calibration curve.
Description of microsomal incubation conditions
The designs of the inhibition studies were similar to those published by Newton et al. (11). Inhibition studies were performed on a sample from each species that had shown activity similar or lower to the one determined in humans. The same incubation conditions as those reported previously were used for inhibition, except that the inhibitor (table 1) was added at the pre-incubation step which was 2 min for sulfaphenazole, and quinidine and 15 min for furafylline, tranylcypromine, diethyldithiocarbamic acid, and troleandomycin. In the case of the inhibitory antibody, the antibody and the microsomes were kept on ice for 15 min before the normal pre-incubation step conducted in presence of the cofactors. The inhibitors were dissolved in an appropriate solvent and the organic content did not exceed 3% (v/v) when added to incubations. For each set of experiments, the organic solvent content was kept constant to eliminate nonspecific inhibition caused by the solvent. The reaction was started by the addition of the substrate, incubated and quantitated as described above. In the case of coumarin, the concentration of substrate and the amount of protein used in the incubation for the inhibition study had to be reduced to 25 μM and 0.075 mg, respectively, to follow the manufacturer’s recommendation.
HPLC System.
The HPLC System consisted of a Waters 600S Controller, a Waters 717 plus Autosampler, a Waters 996 Photodiode Array Detector and a Shimadzu RF-551 Fluorescence HPLC monitor, the data was collected and processed by Millennium version 2.15 software.
Phenacetin-O-deethylase activity.
The analysis of the supernatant was performed using a Zorbax Phenyl column, 4.6 × 150 mm and a detection wavelength of 254 nm. A gradient mobile phase was used, consisting initially of 90:10, pH 3.5 phosphoric acid in water and methanol, and was brought to 15:85 in 25 min which was held for 5 min, all at a flow rate of 1.5 ml/min. Under these conditions, acetaminophen and phenacetin eluted at 5.5 and 15.8 min, respectively.
Coumarin-7-hydroxylase activity.
The supernatant was diluted 1:1 in water and analyzed by HPLC using a NovaPak C18 column (3.9 × 150 mm) and fluorescence detection at an excitation wavelength of 371 nm and an emission wavelength of 454 nm. The mobile phase consisted of 1% acetic acid and methanol (65:35) at a flow rate of 1 ml/min. Under these conditions, 7-hydroxycoumarin and coumarin eluted at 3.9 and 6.1 min, respectively.
Tolbutamide hydroxylase activity.
The analysis of the supernatant was conducted using a NovaPak C18 (3.9 × 150 mm) with UV detection at 237 nm. A gradient mobile phase was used, consisting initially of 85:15, 10 mM sodium acetate (pH 4.3) and acetonitrile, and was brought to 39:61 in 13 min which was held for 2 min, all at a flow rate of 2 ml/min. Under these conditions, 4-hydroxytolbutamide and tolbutamide eluted at 4.5 and 8.5 min, respectively.
S-Mephenytoin 4′-hydroxylase activity.
The supernatant was analyzed by HPLC using a NovaPak C18 column (3.9 × 150 mm) and UV detection at 204 nm. The mobile phase consisted of 20mM sodium perchlorate, methanol, and acetonitrile (69:25:6) at a flow rate of 1 ml/min. Under these conditions, 4-hydroxymephenytoin and S-mephenytoin eluted at 4.1 and 12.7 min, respectively. For inhibition studies, the supernatant was analyzed under HPLC conditions different from those described above because of co-elution of the inhibitor with the metabolite. Instead, a gradient mobile phase, consisting initially of 75:25 ammonium acetate buffer (20 mM, pH = 5) and methanol was brought to a composition of 50:50 in 22 min at a flow rate of 1 ml/min. Under these conditions tranylcypromine, 4-hydroxymephenytoin, and S-mephenytoin eluted at 4.7, 7.0, and 15.3 min, respectively.
Dextromethorphan-O-demethylase activity.
The supernatant was analyzed by HPLC using a Zorbax C18 column (4.6 × 150 mm) with fluorescence detection using an excitation wavelength of 270 nm and an emission wavelength of 312 nm. The mobile phase consisted of 1 mM perchloric acid and acetonitrile (75:25) at a flow rate of 1 ml/min. Under these conditions, dextrorphan and dextromethorphan eluted at 3.1 and 10.4 min, respectively.
Chlorzoxazone 6-hydroxylase.
The supernatant was diluted 1:1 in water and analyzed using a Zorbax C18 column (4.6 × 150 mm) with UV detection at 287 nm. A gradient mobile phase consisting initially of 80:20, 0.05% phosphoric acid and acetonitrile was brought to a composition of 60:40 in 20 min which was held for 10 min, all at a flow rate of 1 ml/min. Under these conditions, 6-hydroxychlorzoxazone and chlorzoxazone eluted at 5.7 and 15.7 min, respectively.
Testosterone-6β-hydroxylase activity.
The supernatant was analyzed using a Supelcosil LC18 column (4.6 × 150 mm) and UV detection at 254 nm. A gradient mobile phase consisting initially of 90:10, solvent A (methanol:water:acetonitrile, 39:60:1) and solvent B (methanol:water:acetonitrile, 80:18:2), was brought to a composition of 10:90 in 15 min which was held for 5 min, all at a flow rate of 1 ml/min. Under these conditions, 6β-hydroxytestosterone and testosterone eluted at 9.2 and 16.3 min, respectively.
Statistics.
Enzyme activities between species were compared using a single factor ANOVA with a p < 0.05 indicating a significant difference. A multiple comparison of the activities was then accomplished using the Tukey-Kramer HSD (honestly significant difference) test with a p < 0.05 indicating a significant difference (33). Enzyme activities between sexes were compared using a two-tailed Student’s t test with ap < 0.05 indicating a significant difference (33). The term range represents the ratio of highest activity obtained over the lowest.
Results and Discussion
Metabolic Activities.
The results of seven cytochrome P450-mediated activities obtained in liver microsomes from male and female human, horse, dog, and cat are reported in table 2.
P450-mediated activities in human, horse, dog and cat microsomal incubations
There was no significant statistical difference in the phenacetin-O-deethylase activity among human, dog, cat, and horse. Similarly, Sharer et al. (12) found no significant differences between the activity in dog and human microsomes when looking at ethoxyresorufin O-deethylase activity, another catalytic marker for human P450 1A1/2. The variability observed in the human activity was tremendous (73X) but is consistent with other reported values (34). The cause of this wide variation may be the result of exposure of some individuals to inducers of P4501A1/1A2 (34).
Human showed significantly higher coumarin 7-hydroxylase activity than cat (8-fold), dog (6-fold), and horse (4-fold), while no significant difference was observed between any other species. The coumarin 7-hydroxylase activities in human and dog liver microsomes in the present study are very similar to those reported by Sharer et al. (12) (0.448 and 0.075 nmol/(mg·min) respectively). Pearceet al. (22) found that the rate of coumarin 7-hydroxylase followed the rank order of human > dog > cat which is consistent with our results.
Horse was found to have significantly higher tolbutamide hydroxylase activity than cat (34-fold), dog (10-fold), and human (2-fold). Human showed significantly higher activity than cat (20-fold) and dog (6-fold), while no significant difference was observed between dog and cat. The tolbutamide hydroxylase activity found in human liver microsomes agrees with the activity reported by previous investigators (9, 12). A small or undetectable amount of tolbutamide hydroxylase activity in dog microsomal incubates was observed in our experiments; this is in line with the study by Sharer et al. (12).
No significant difference was observed among any of the species forS-mephenytoin 4′-hydroxylase activity. Yasumori et al. (35) have reported species differences in the stereoselective metabolism of mephenytoin by P450 2C: humans have a 17-fold preference for the 4′-hydroxylation of S-mephenytoin, while dogs have a 3-fold preference for 4′-hydroxylation of R-mephenytoin. In the present study, only the metabolism of S-mephenytoin was investigated and the data obtained from dog and human agree with those reported by Sharer et al. (12). There was a large amount of variability among humans, which has been observed previously (2). This could be explained by the fact that P4502C19 is polymorphic (2).
Regarding the dextromethorphan O-demethylase activity, horse had, by far, the greatest activity of all the species (22-fold, 52-fold, and 24-fold greater as compared with human, dog, and cat, respectively). As reported in the experimental part, the values obtained for the horse were from a 5 min incubation because no parent compound remained after the regular 30 min incubation. The level of activity found in human microsomes was in agreement with previously reported data (9).
Horse had significantly higher chlorzoxazone 6-hydroxylase activity than human (2-fold), cat (2-fold), and dog (2-fold). No significant difference was observed between dog, cat, and human. Sharer et al. (12) also found no significant difference between human and dog using another P4502E1 marker, N-nitrosodimethylamineN-demethylase. Peter et al. (6) found activity in 14 humans ranging from 0.58 to 2.3 nmol/(mg·min), which is higher than the average activity detected in the human samples in the present study. Their experimental conditions differed from ours in terms of solubilization of the compound and this might explain the difference obtained in the results.
Human microsomal incubations had significantly higher testosterone 6β-hydroxylase activity than horse (7-fold), cat (7-fold), and dog (7-fold) incubations. Contrarily, Sharer et al. (12) reported that dog microsomes had significantly higher midazolam 1′-hydroxylase activity and erythromycin N-demethylase activity, two catalytic markers for P4503A, than human. On the other hand, Sonderfan and Parkinson (36) also reported some P4503A4-related activities in dog as well as in cat and found a relative order of human greater than dog and cat, which is consistent with our study. Niwaet al. (37) reported that the formation rate of 6β-hydroxytestosterone in dog was ∼ 0.59 nmol/(mg·min) which is higher than our data while Komori et al. (21) reported an activity of 0.24 nmol/(mg·min) in horse microsomes which agrees with our data.
In general, no one species was more active in metabolizing any given substrate even though it was interesting to note that, very often, horse had significantly greater activities than the two other target animals. More variability is seen in the human and horse metabolic activity than the dog and cat. This was expected as the humans and horses in this study were taken from the general population, while the dogs and cats were from inbred laboratory strains. The laboratory animals have therefore many fewer genetic differences. Also their environments and diet are the same, eliminating possible factors that could contribute to differential induction of some of the P450s (1). If this study had been conducted using dogs and cats obtained from the general population, more variability in the metabolic data would have been expected.
There were no marked sex-related differences in the metabolism of the different catalytic activity markers tested in human, dog, horse, and cat. This is consistent with previous work reported in human (3) as well as in dog (36).
Inhibition Studies
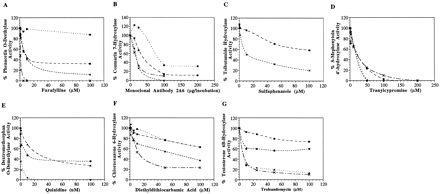
The effect of the specific P450 inhibitors on the metabolism of the corresponding probe in the various species is shown in fig.1. The initial catalytic activities (i.e. in the absence of the inhibitor) of each specimen used for the inhibition studies are reported in the legend of fig. 1.
Inhibition of P450 mediated activities in human, horse, dog and cat microsomal incubations.
Assays were performed as indicated in Materials and Methods. Inhibition of (A) phenacetinO-deethylase activity by furafylline (initial activities: human: 2.484, horse: 0.371, dog: 0.824, cat: 0.677 nmol/(mg·min)); (B) coumarin 7-hydroxylase activity by monoclonal antibody 2A6 (initial activities: human: 1.385, horse: 0.276, dog: 0.108, cat: 0.053 nmol/(mg·min)); (C) tolbutamide hydroxylase activity by sulfaphenazole (initial activities: human: 0.110, horse: 0.091 nmol/(mg·min)); (D)S-mephenytoin-4-hydroxylase activity by tranylcypromine (initial activities: human: 0.131, horse: 0.107, dog: 0.051, cat: 0.040 nmol/(mg·min)); (E) dextromethorphanO-demethylase activity by quinidine (initial activities: human: 0.302, dog: 0.044, cat: 0.131 nmol/(mg·min)); (F) chlorzoxazone 6-hydroxylase activity by diethyldithiocarbamate (initial activities: human: 0.289, horse: 0.296, dog: 0.293, cat: 0.284 nmol/(mg·min)); (G) testosterone 6β-hydroxylase activity by troleandomycin (initial activities: human: 2.740, horse: 0.224, dog: 0.446, cat: 0.171 nmol/(mg·min)). Human: X; Horse: ▪; Dog: ▴; Cat: •.
The design of the inhibition studies was similar to that published by Newton et al. (11) to facilitate making comparisons with the literature. It should be emphasized that in human the substrates and inhibitors used are specific for one P450 isozyme. Hence, one should expect effective inhibition of the metabolic activity in the human liver microsomes. In the animals, the inhibition studies were performed on a sample that had similar or lower catalytic activity than the human used in this study. Therefore, effective inhibition can be expected if an enzyme homologous to the one reported for the activity of the specific probe is responsible for the activity.
Furafylline (fig. 1A), a specific mechanism-based inhibitor of P4501A2 (8, 38), caused 80% inhibition of phenacetinO-deethylase activity in human liver microsomes at very low concentrations (1 μM). Newton et al. (11) reported the same finding. It is known that in human liver there is no significant amount of P4501A1, and therefore complete inhibition of phenacetinO-deethylase activity can be obtained in human with furafylline. At the highest concentration tested (100 μM), furafylline inhibited the activity in the dog and horse by 90% and 65%, respectively, while the activity in the cat was not inhibited. This could mean that, while an enzyme homologous to P4501A2 plays a role in the metabolism of phenacetin in dog and horse, another enzyme might also be involved. This latter statement seems to be the case for the phenacetin O-deethylase activity in cat liver microsomes.
Fig. 1B demonstrates that the monoclonal antibody that recognizes human CYP 2A6 effectively inhibited coumarin 7-hydroxylase activity in human, dog, and horse. There was nearly 70% inhibition in cat at 100 μg per incubation. The complete inhibition of the activity in human, dog, and horse liver microsomes suggests that an enzyme similar to P4502A6, or that cross-reacts with the monoclonal antibody, is responsible for the activity seen. The aforementioned could also be said for the observed inhibition of the activity in the cat, although it must be pointed out that the inhibition is not complete, and therefore another enzyme could also be involved.
The window of tolbutamide hydroxylase activity was too small in the dog and cat microsomes to have quantifiable data for an inhibition study. Therefore, sulfaphenazole could not be used as a diagnostic inhibitory probe for P4502C8/9 in these species. Sulfaphenazole (fig.1C) caused 80% and 40% inhibition in human and horse, respectively, at 100 μM. Newton et al. (11) reported that 100 μM sulfaphenazole inhibited tolbutamide hydroxylase activity in human microsomes by 80%, as was seen in the present study. The tolbutamide hydroxylase activity seen in the horse was not completely inhibited by sulfaphenazole and, as said previously, this could be explained by the involvement of other isozymes responsible for the activity.
Tranylcypromine (fig. 1D), a P4502C19 inhibitor (10), completely suppressed the S-mephenytoin 4′-hydroxylase activity in all species. Hence, an enzyme that is highly homologous to P4502C19 could be responsible for the metabolism ofS-mephenytoin in dog, cat, and horse microsomes.
The effect of quinidine, a potent P4502D6 inhibitor, is shown in fig.1E. The effect of the inhibitor on the activity in the horse is not reported. The results would be difficult to interpret since the activity in the horse was so high in comparison with the human data (experimental conditions were designed for activity similar to human). Quinidine caused 80% inhibition of dextromethorphan-O-demethylase activity in human at concentrations as low as 1 μM while, at 100 μM, it caused 60% inhibition in dog and cat liver microsomes. The interpretation of the inhibition data in animal species, in which little data on P450 are available, can be difficult. This is particularly true for P4502D6 where interspecies differences have been reported in terms of inhibition. For example, it has been reported that the rat P4502D1 shares 70% homology with the human P4502D6 (39) and that both species have similar substrate specificities (40). However, quinine is a potent inhibitor of rat P4502D1, while its stereoisomer quinidine is not potent (41). The converse is true for human P4502D6 (41).
The specific inhibitor of P4502E1, diethyldithiocarbamate (fig.1F) caused 80%, 60%, 40%, and 40% inhibition in chlorzoxazone 6-hydroxylase activity in human, dog, cat and horse liver microsomes, respectively, at 100 μM. Newton et al. (11) reported a similar extent of inhibition of chlorzoxazone 6-hydroxylase activity with diethyldithiocarbamate in human microsomes. The suppression of activity in all species to some extent indicates that an enzyme similar to human P4502E1 could play a role in the chlorzoxazone 6-hydroxylase activity in horse, dog, and cat. However, once again, because the inhibition was incomplete, other enzymes are probably involved as well.
Troleandomycin is a mechanism-based inhibitor of human P4503A4 and requires NADPH-dependent metabolism for inhibition. Newton et al. (11) reported 80% inhibition of testosterone 6β-hydroxylase activity in human microsomes by troleandomycin at concentrations ≈ 10 μM. In the present investigation, the activity in human was also inhibited by 80% as was the activity in dog, at concentrations of troleandomycin greater than 10 μM. This is consistent with the findings of Ciaccio and Halpert (42), who reported that troleandomycin can inhibit the testosterone 6β-hydroxylase activity in dog. The 6β-hydroxylation of testosterone was partially inhibited in cat (40%) and in horse (20%) at 100 μM of troleandomycin (fig.1G).
Overall, no one species behaved exactly like human regarding the efficiency of the various inhibitors. In general, the metabolic activities in the cat were less inhibited than the other species, even at high concentrations. It is important to note that nonspecific inhibition of P450s can be observed at high concentrations of specific inhibitors. That fact should be taken into consideration when inhibition is only observed at high inhibitor concentrations. For example, it has been reported that many of the specific inhibitors used in this study (furafylline, quinidine, diethyldithiocarbamate, and troleandomycin) can cause up to 20% inhibition of other P450 activities at the high range of concentrations used (11).
It can be concluded that there are rather large interspecies differences in in vitro P450-mediated metabolism in horse, dog, and cat liver microsomes. There is variation in the metabolic activity of the specific P450 catalytic markers, as well as differences in the way the specific inhibitors affect the various P450 catalytic activities. Considering the limited amount of data available on hepatic cytochromes P450 in domestic animals, including substrate and inhibition specificities, these results are best used as guidelines for future studies. However, it is clear from the data generated in this study that new chemical entities developed for domestic animals should be tested for their in vitro metabolic profile in all target species since the metabolism may be markedly different between species. This kind of study would give indications about the possible in vivo metabolic fates of potential veterinary medications, even at an early stage in drug discovery.
Acknowledgments
We would like to acknowledge Dr. Anne-Marie Taylor (Laboratory Animal Resources, Merck Frosst, Pointe-Claire-Dorval, QC, Canada) for organizing procurement of the cat and horse liver tissue and Dr. Darryl Patrick (Safety Assessment Department, Merck Research Laboratories, Westpoint, PA) for providing us with the dog liver tissue.
Footnotes
-
Send reprint requests to: Nathalie Chauret, Merck Frosst Centre for Therapeutic Research, P.O. Box 1005, Pointe-Claire-Dorval, Quebec, Canada H9R 4P8.
- Abbreviation used is::
- P450
- cytochrome P450
- Received February 12, 1997.
- Accepted July 9, 1997.
- The American Society for Pharmacology and Experimental Therapeutics