Abstract
Precision-cut human liver slices are an important tool for defining the metabolism and hepatotoxicity of drug candidates early in development. Because of the frequent use of this in vitrotool, a knowledge of the catalytic activities of the drug-metabolizing enzymes during human liver slice culture is necessary. Therefore, marker catalytic activities for various cytochrome P450 (P450 or CYP) forms, as well as phase II activities (glucuronidation and sulfation of 7-hydroxycoumarin), were measured in slices from three different human livers during 96 hr in culture. Standard viability measures were found to be stable from 8 to 24 hr and then declined to 96 hr. Catalytic activities measured for the P450s were ethoxyresorufinO-deethylase (CYP1A2), coumarin 7-hydroxylase (CYP2A6), (S)-mephenytoin N-demethylase (CYP2B6), diclofenac 4′-hydroxylase (CYP2C9), (S)-mephenytoin 4′-hydroxylase (CYP2C19), bufuralol 1′-hydroxylase (CYP2D6), chlorzoxazone 6-hydroxylase (CYP2E1), and midazolam 1′-hydroxylase (CYP3A). The P450 activities decreased by approximately 20% by 4 hr and by at least 65% by 24 hr and were not measurable by 96 hr. In contrast to the phase I activities, 7-hydroxycoumarin glucuronosyltransferase activity was increased at the 8-hr time point by approximately 100% and then decreased to approximately initial values by 96 hr. The 7-hydroxycoumarin sulfotransferase activity of the slices decreased significantly more slowly than did the P450 activities. In conclusion, using conventional methods of liver slice preparation and culture, most of the metabolic capabilities of human liver slices are rapidly lost with time. Therefore, the development of culture methods for human liver slices that can improve the preservation of the drug-metabolizing capabilities may be required.
Recently, the use of human liver slices has emerged as an important tool in the field of drug metabolism (Ekins, 1996). The availability of human liver tissue, the creation of a mechanical apparatus that can consistently produce slices of uniform thickness (Krumdieck et al., 1980), and the development of a dynamic organ culture system (Smith et al., 1985, 1986) have made liver slice cultures a practical alternative to hepatocyte cultures.
In drug development, information on the metabolic fate of a NME1 is of the utmost importance. Human liver slices can contribute to this information at a relatively early stage in the development process. In liver slices, the cellular architecture of the liver is preserved and, therefore, all of the drug-metabolizing enzymes and cofactors are present. All cell types are present in slices, and cell-cell interactions are maintained. Therefore, this model yields an integrated picture of the oxidative and conjugative pathways of hepatic metabolism of the NME.
Although the human liver slice model is useful in determining metabolic pathways for NMEs and has been used to predict the metabolic clearance of drugs, little information exists regarding the preservation of the individual catalytic activities of the drug-metabolizing enzymes with time in culture. These enzymes include specific P450 isoforms (CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4), the glucuronosyltransferases, and the sulfotransferases. Previous studies indicated that there was a decline in the drug-metabolizing capabilities of human liver slices over a 72-hr time period, but only three enzymes (CYP1A2, CYP3A, and CYP4A) were investigated (Lakeet al., 1996,1997). Those studies, therefore, did not investigate the majority of the oxidative enzymes or phase II enzymes and examined only one time point. It has been reported that >200 laboratories are using this culture system for metabolism and toxicity studies (Gandolfi et al., 1995). Undoubtedly, the number of laboratories using the slice culture method has continued to increase. Despite the large number of laboratories using this model system, characterization of the drug-metabolizing enzymes in culture has not been performed. This lack of basic knowledge regarding the activities of the drug-metabolizing enzymes in culture over time warrants attention. In particular, determination of the catalytic activities of the drug-metabolizing enzymes is a true measure of their performance, as opposed to measurements of enzyme levels, which are a function of the regulation of expression. It was the goal of this study to determine the activities of the aforementioned enzymes in human liver slice cultures over a 4-day period. With this information, an appropriate culture time for metabolism studies can be established and better interpretation of experimental results, regarding the metabolism of NMEs in human liver slice cultures, can be performed. This information may be particularly important with respect to in vitro/in vivo extrapolations.
Materials and Methods
Chemicals.
Waymouth’s 752/1 medium without phenol red and glutamine was purchased from Specialty Media (Lavalette, NJ). Fungizone and gentamicin were purchased from Gibco BRL (Gaithersburg, MD). Fetal bovine serum was obtained from Harlan Bioproducts (Indianapolis, IN). The Bacharach potassium standard for the flame photometer was purchased from Baxter (McGaw Park, IL). Chlorzoxazone, zoxazolamine, NADPH, UDP-glucuronic acid, 3′-phosphoadenosine-5′-phosphosulfate, coumarin, 7-hydroxycoumarin, 7-ethoxycoumarin, and flunitrazepam were purchased from Sigma Chemical Co. (St. Louis, MO). Resorufin and ethoxyresorufin were purchased from Aldrich (Milwaukee, WI). Racemic nirvanol and 4′-hydroxymephenytoin were gifts from Dr. Stephen Hall (Indiana University School of Medicine, Indianapolis, IN). (S)-Mephenytoin, diclofenac, 4′-hydroxydiclofenac, bufuralol, 1′-hydroxybufuralol, 7-hydroxycoumarin glucuronide, and 7-hydroxycoumarin sulfate were obtained from Gentest Corp. (Woburn, MA) or Salford Ultrafine Chemicals and Research (Manchester, UK). Meclofenamate was purchased from Cayman Chemical (Ann Arbor, MI). Midazolam and 1′-hydroxymidazolam were gifts from Hoffman-LaRoche (Nutley, NJ). Phenobarbital was synthesized at Eli Lilly and Co. (Indianapolis, IN). All other chemicals were of the highest grade commercially available.
Liver Specimens.
Three individual human liver specimens were used in this study. Human liver 1 was obtained from the Department of Pathology at the University of Pittsburgh. Donor 1 was a 44-year-old Caucasian woman with an unknown cause of death. Human livers 2 and 3 were obtained from the International Institute for the Advancement of Medicine. Donor 2 was a 26-year-old Caucasian man, for whom the cause of death was a self-inflicted gunshot wound. Donor 3 was a 53-year-old Caucasian man, for whom the cause of death was a stroke. The liver specimens were perfused with University of Wisconsin solution, shipped to the laboratory on ice, and used immediately upon receipt.
Preparation and Incubation of Slices.
All slicing equipment and glassware were autoclaved or sterilized with 70% ethanol before use. Cylindrical tissue cores were made by slowly passing a sharpened, 8-mm-diameter, stainless steel tube through the liver, with a motorized coring press. Cores and slices were kept cold in modified Sack’s cold preservation buffer (Mondon and Fortner, 1982), before, during, and after slicing. Cores were placed in a Brendel/Vitron tissue slicer, and the slice thickness was adjusted to yield a weight of 15–20 mg/slice. Individual slices were floated onto a titanium screen in a Teflon roller insert, and the roller was blotted and placed in a 20-ml scintillation vial containing 1.7 ml of filter-sterilized Waymouth’s 752/1 medium supplemented with 10% fetal calf serum, 50 μg/ml gentamicin, 2.5 μg/ml fungizone, and 350 μg/ml L-glutamine. The vial was sealed with a cap with a 2-mm-diameter hole, placed in a dynamic roller culture incubator at 37°C, and gassed with 95% O2/5% CO2, at a flow rate of 0.5 liter/min. At each time point (0, 4, 8, 24, 48, 72, and 96 hr), slices were sonicated in 1.0 ml of 50 mM Tris-HCl and stored at −80°C until they were used in the incubations. Each vial contained one slice. Each time point replicate consisted of four pooled slices from four separate vials. There were four replicates for each time point.
Potassium Retention and Protein Determination.
At each time point, slices were sonicated in 1 ml of ice-cold distilled water, and 800 μl of the homogenate was rapidly transferred to 80 μl of perchloric acid to precipitate the protein. The acidified homogenate was centrifuged, and 100 μl of the supernatant was diluted into 900 μl of water for potassium analysis by flame photometry. Protein assays were performed with the remaining 200 μl of the sample (Lowry et al., 1951).
Homogenate Incubations and HPLC Analyses.
Initial rate conditions for linearity of product formation with respect to protein concentration and time were determined for all catalytic activity assays. Initial rate conditions could not be used for the (S)-mephenytoin 4′-hydroxylase activity because of the low level of conversion of this substrate. In general, incubations consisted of homogenate (0.5–1.0 mg of protein/ml of incubation, depending on the assay), substrate, appropriate cofactor (1 mM NADPH, 5 mM UDP-glucuronic acid, or 0.4 mM 3′-phosphoadenosine-5′-phosphosulfate), and 100 mM sodium phosphate buffer (25 mM sodium phosphate buffer was used for the coumarin 7-hydroxylase assay). A 3-min preincubation was performed for all assays. All assays were initiated by the addition of substrate. Reactions were terminated by the addition of organic solvent or acidification. The rates of formation of 1′-hydroxymidazolam (Wrighton and Ring, 1994), 1′-hydroxybufuralol (Stevens and Wrighton, 1993), and 4′-hydroxymephenytoin and nirvanol (Wrighton et al., 1993) were determined using substrate concentrations of 10, 25, and 50 μM and incubation times of 5, 25, and 60 min, respectively. HPLC methods described in the indicated references were used, with slight modifications. The rates of formation of 7-hydroxycoumarin (Greenlee and Poland, 1978) and resorufin (Lubet et al., 1985) were determined using substrate concentrations of 100 and 5 μM and incubation times of 30 and 20 min, respectively. Fluorometric methods described in the indicated references were used, with slight modifications. 6-Hydroxychlorzoxazone formation was measured by HPLC using a mobile phase consisting of 70% 50 mM potassium phosphate, pH 3.0, containing 0.1% triethylamine and 30% acetonitrile. The chlorzoxazone concentration was 400 μM, and the incubation time was 1 hr. Zoxazolamine was used as the internal standard. After the reaction was stopped and protein was precipitated with 20% trichloroacetic acid, the samples were centrifuged. Then, 40 μl of the supernatant was injected onto an Inertsil C8 column (250 × 4.6 mm, 5 μm; Metachem Technologies, Torrance, CA), with monitoring at 287 nm. The flow rate was 1 ml/min, and the total run time was 16 min. Formation of 4′-hydroxydiclofenac was measured by HPLC using a linear gradient from 80% mobile phase A (50 mM sodium phosphate, pH 7.4, containing 0.03% triethylamine) to 60% mobile phase A. Mobile phase B consisted of acetonitrile. Meclofenamate was used as the internal standard. The diclofenac concentration was 10 μM, and the incubation time was 30 min. After the reaction was stopped and protein was precipitated with an equal volume of acetonitrile, the samples were centrifuged. Then, 50 μl of supernatant was injected onto a Betabasic C18column (50 × 4.6 mm, 5 μm; Keystone Scientific, Bellefonte, PA), with monitoring at 282 nm. The flow rate was 1 ml/min, and the total run time was 15 min. Glucuronidation and sulfation of 7-hydroxycoumarin were determined by HPLC using a slight modification of a method previously described (Ekins et al., 1995). The 7-hydroxycoumarin concentration for both assays was 10 μM, and 7-ethoxycoumarin was used as the internal standard for the assays. The glucuronosyltransferase assay used an incubation time of 30 min, and the sulfotransferase assay used an incubation time of 60 min.
Results
To determine the relative viability in culture of the liver slices from the three human livers, potassium levels and protein concentrations were monitored at each time point (table 1). Protein concentrations of slice homogenates from livers 2 and 3 declined at a greater rate than did those of homogenates from liver 1 (37.5 and 49.6% of time 0 values at 96 hr, compared with 75.3%, respectively). Potassium retention was measured in liver 1 and liver 3 slice homogenates (table 1). After a 4-hr equilibration period, potassium levels remained stable (>30 μmol/g) through 72 hr for liver 1 and through 24 hr for liver 3. After 72 hr for liver 1 and after 24 hr for liver 3, potassium levels dropped to <20 μmol/g. Potassium levels in liver 2 were not determined.
Effect of time in culture on potassium retention (PR) and total protein (TP) levels in human liver slices
Catalytic activities selective for eight P450 isoforms, as well as glucuronosyltransferase and sulfotransferase activities, were measured in human liver slice homogenates over the 4-day period (time points of 0, 4, 8, 24, 48, 72, and 96 hr). The P450s examined for catalytic activity were CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A. Using 7-hydroxycoumarin as a substrate, glucuronosyltransferase and sulfotransferase activities were also measured at each time point.
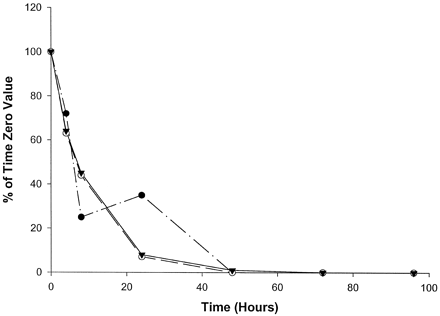
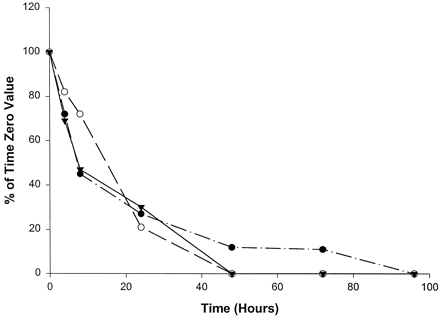
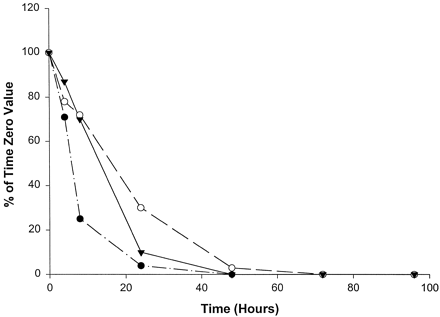
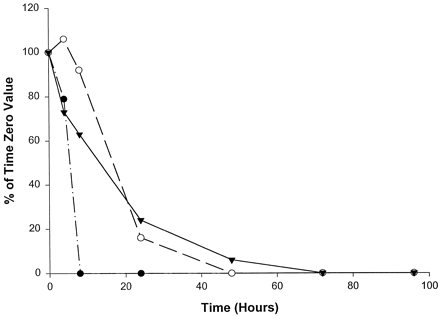
Catalytic activities selective for CYP2A6 (fig.1), CYP2D6 (fig.2), and CYP2E1 (fig.3) were the most stable of the P450 activities examined, with measurable activities in homogenates from all three livers being present through at least 24 hr. Specifically, CYP2A6-mediated coumarin 7-hydroxylase activity decreased in slices from all three livers to levels <50% of initial values by 8 hr in culture. Slices from livers 2 and 3 showed steady declines in coumarin 7-hydroxylase activity, with no measurable activity by 48 and 72 hr, respectively (fig. 1). However, liver 1 showed a slight recovery (10% increase) of activity at 24 hr; activity then declined below the limits of quantitation by 48 hr. As shown in fig. 2, CYP2D6-mediated bufuralol 1′-hydroxylase activity in slices from all three livers declined steadily through 24 hr, to levels approximately 25% of the initial values. Bufuralol 1′-hydroxylase activity continued to decline to levels nearly below the limits of quantitation within 48 hr in all three livers. Interestingly, slices from liver 1 exhibited minimal but measurable bufuralol 1′-hydroxylase activity (11% of the time 0 value) through 72 hr (fig. 2). Chlorzoxazone 6-hydroxylase activity representative of CYP2E1 also steadily declined through 24 hr in slices from all three livers, to levels 4–30% of initial rates (fig. 3). Only liver 2 showed measurable activity (3% of the time 0 value) at 48 hr in culture (fig. 3).
Effect of time in culture on coumarin 7-hydroxylase activity (CYP2A6) in human liver slices.
•, Liver 1 (time 0, 10.6 pmol/min/mg); ○, liver 2 (time 0, 57.3 pmol/min/mg); ▾, liver 3 (time 0, 406.7 pmol/min/mg).
Effect of time in culture on bufuralol 1′-hydroxylase activity (CYP2D6) in human liver slices.
•, Liver 1 (time 0, 31.8 pmol/min/mg); ○, liver 2 (time 0, 32.7 pmol/min/mg); ▾, liver 3 (time 0, 13.1 pmol/min/mg).
Effect of time in culture on chlorzoxazone 6-hydroxylase activity (CYP2E1) in human liver slices.
•, Liver 1 (time 0, 138.1 pmol/min/mg); ○, liver 2 (time 0, 89.5 pmol/min/mg); ▾, liver 3 (time 0, 112.8 pmol/min/mg).
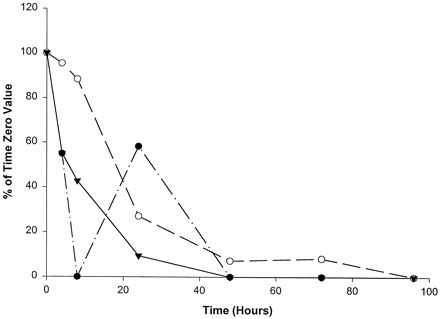
Catalytic activities selective for CYP1A2 (fig.4) and CYP2C9 (fig.5) were the most variable in their declines in homogenates of slices from the three livers examined. The time of complete loss of activity ranged from 8 to 72 hr in culture. With slices from liver 1, ethoxyresorufin O-deethylase activity (CYP1A2) declined to undetectable levels by 8 hr, recovered to approximately 50% of the time 0 value at 24 hr, and was not measurable at any later time points examined (fig. 4). It is important to note that the levels of ethoxyresorufin O-deethylase activity were near the limits of detection for slices from liver 1. Thus, small changes in catalytic activity yielded large percentage differences. Slices from livers 2 and 3 declined steadily in ethoxyresorufin activity, with measurable activity to 72 and 24 hr, respectively (fig.4). As shown in fig. 5, CYP2C9-mediated diclofenac 4′-hydroxylase activity decreased to nondetectable levels most rapidly (by 8 hr) in slices from liver 1, with measurable activities only at the 4-hr time point. Slices from liver 3 had measurable activity to 72 hr (fig. 5). Slices from liver 2 showed a time course intermediate between those of the other two livers, with measurable activity to 24 hr (fig. 5).
Effect of time in culture on ethoxyresorufinO-deethylase activity (CYP1A2) in human liver slices.
•, Liver 1 (time 0, 0.8 pmol/min/mg); ○, liver 2 (time 0, 4.1 pmol/min/mg); ▾, liver 3 (time 0, 5.8 pmol/min/mg).
Effect of time in culture on diclofenac 4′-hydroxylase activity (CYP2C9) in human liver slices.
•, Liver 1 (time 0, 25.9 pmol/min/mg); ○, liver 2 (time 0, 45.5 pmol/min/mg); ▾, liver 3 (time 0, 72.9 pmol/min/mg).
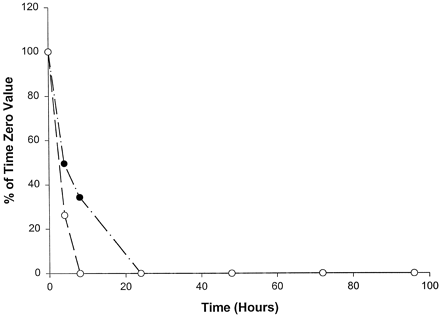
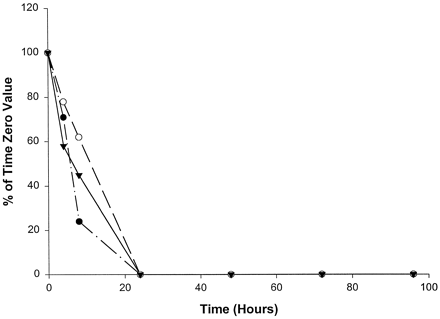
Compared with the catalytic activities of the other P450s, the catalytic activities selective for CYP2C19 (fig.6) and CYP3A4 (fig.7) decreased most rapidly in homogenates from slices from all three livers examined, with no measurable activity by 24 hr. (S)-Mephenytoin 4′-hydroxylase activity (CYP2C19) was absent in liver 1 at all time points, including the initial time point, which was most likely the result of the well-documented genetic deficiency of CYP2C19 (Wrighton et al., 1993). (S)-Mephenytoin 4′-hydroxylase activity was initially low in homogenates from slices of livers 2 and 3 and disappeared quickly with time in culture, becoming undetectable after 4 hr in liver 3 and after 8 hr in liver 2 (fig. 6). Midazolam 1′-hydroxylase is a form-selective catalytic activity for CYP3A and decreased rapidly in homogenates from slices of all three livers, with measurable activities only through 8 hr (fig. 7). Nirvanol formation by CYP2B6 (Heyn et al.,1996), from the low concentration of (S)-mephenytoin used to determine the catalytic activity of CYP2C19, was not detectable in slices from any of the three livers examined, even at the initial time point.
Effect of time in culture on (S)-mephenytoin 4′-hydroxylase activity (CYP2C19) in human liver slices.
•, Liver 2 (time 0, 3.4 pmol/min/mg); ○, liver 3 (time 0, 2.5 pmol/min/mg); liver 1 was deficient in CYP2C19 at time 0.
Effect of time in culture on midazolam 1′-hydroxylase activity (CYP3A) in human liver slices.
•, Liver 1 (time 0, 213.6 pmol/min/mg); ○, liver 2 (time 0, 127.0 pmol/min/mg); ▾, liver 3 (time 0, 336.5 pmol/min/mg).
In general, there were dramatic decreases in activity over time for all of the P450-selective catalytic activities, with no measurable activity in the homogenates from the slices by the 96-hr time point. Although the initial activities of the P450s varied substantially among slices from the three livers, the percentage decreases in activity with time were relatively consistent. Of the three livers examined, no individual liver was superior in the preservation of its drug-metabolizing capabilities in the slices, based on the form-selective catalytic activities measured.
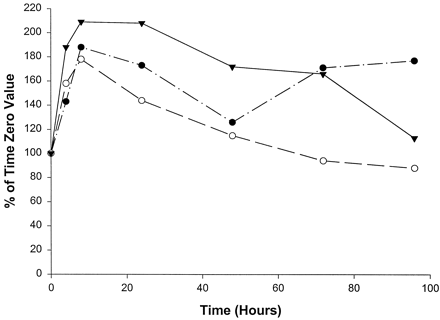
Unlike the P450 form-selective catalytic activities, 7-hydroxycoumarin glucuronosyltransferase activity (expressed per milligram of slice homogenate protein) increased in homogenates from slices from all three livers through 8 hr, to approximately twice the initial values (fig.8). By 48 hr in culture, 7-hydroxycoumarin glucuronosyltransferase activity had declined in slices from all three livers, with the greatest decreases being seen in slices from livers 1 and 2. After 48 hr, slices from liver 1 exhibited a strong recovery in activity, whereas those from livers 2 and 3 continued to decrease in activity (fig. 8). Slices from liver 1 increased in activity to 171% of the time 0 value at 72 hr and continued to increase slightly through 96 hr, to 177% of the time 0 value. Slices from liver 2 decreased steadily in activity from 48 hr (115% of the time 0 value) to 96 hr (88% of the time 0 value). Slices from liver 3 also decreased in activity, declining from 172% of the time 0 value at 48 hr to 113% of the time 0 value by 96 hr.
Effect of time in culture on 7-hydroxycoumarin glucuronosyltransferase activity in human liver slices.
•, Liver 1 (time 0, 68.7 pmol/min/mg); ○, liver 2 (time 0, 135.7 pmol/min/mg); ▾, liver 3 (time 0, 189.3 pmol/min/mg).
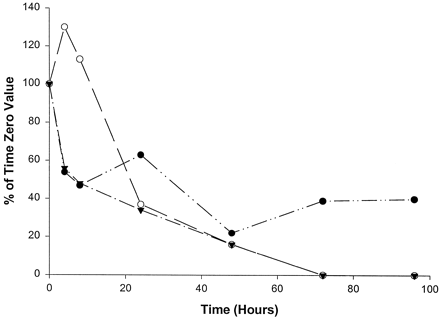
7-Hydroxycoumarin sulfotransferase activity was variable in the cultures of slices from the three human livers over the 96 hr examined (fig. 9). Sulfotransferase activity in slices from liver 1 declined to 47% of the time 0 value by 8 hr, recovered somewhat to 63% by 24 hr, decreased to 22% at 48 hr, and then recovered to 40% of the time 0 value by 96 hr. In slices from liver 2, sulfotransferase activity increased to 130% of the time 0 value by 4 hr and then steadily decreased to nondetectable levels by 72 hr. Sulfotransferase activity in slices from liver 3 decreased steadily at all time points, reaching nondetectable levels at 72 hr.
Effect of time in culture on 7-hydroxycoumarin sulfotransferase activity in human liver slices.
•, Liver 1 (time 0, 52.6 pmol/min/mg); ○, liver 2 (time 0, 27.8 pmol/min/mg); ▾, liver 3 (time 0, 83.1 pmol/min/mg).
Discussion
Precision-cut slices of human liver have proven to be invaluable as in vitro tools in the fields of drug metabolism (Ekins, 1996) and toxicology (Gandolfi et al., 1995). To date, few studies have been carried out to assess the activities of the drug-metabolizing enzymes present in human liver slice cultures over time. In this study, we have provided a comprehensive evaluation of the effect of time in dynamic organ culture on the catalytic activities of individual P450 isoforms. In addition, the catalytic activities of the glucuronosyltransferases and sulfotransferases were examined using a substrate probe that is not selective for individual isozymes.
The viability parameters monitored in this study were potassium retention and slice protein levels. The parameters measured indicate that potassium retention and protein levels are well maintained for at least 8–24 hr. Similar results with respect to protein levels in rat liver slices have been observed in other studies (Lake et al., 1993; Beamand et al., 1993). Potassium retention in the current study was maintained at a relatively constant level through at least 24 hr of culture (through 72 hr of culture in one liver). It should be noted that the potassium levels observed in the current study were somewhat lower than levels observed in previous studies performed with rat and human liver slices (Fisher et al., 1995; Vickers et al., 1995; Olinga et al., 1997).
In general, the catalytic activities of the P450s in human liver slices were substantially decreased by 4–8 hr in culture in the current study. A similar trend has been seen by others (Lake et al.,1996,1997) in human liver slice cultures. However, not all of the individual enzymes reported here were examined by Lake et al. (1996, 1997), and no slice viability data were presented. Typically, investigators used a nonselective biotransformation probe to examine the catalytic functions of their slices. The most common substrate used in the measurement of P450 activity of slices in culture is 7-ethoxycoumarin (Ekins, 1996). However, 7-ethoxycoumarin is deethylated in humans by several P450s, including CYP1A2, CYP2B6, and CYP2E1 (Yamazaki et al., 1996). To obtain a clear understanding of the loss of activity of each P450, enzyme activities selective for individual enzymes must be determined.
As described in Results, there were slight but important differences in the rates of loss of the catalytic activities of the individual P450s. One of the most important findings was that CYP3A-mediated midazolam 1′-hydroxylase activity was not measurable after the 8-hr time point. With the dominant role of CYP3A in drug metabolism, the rapid disappearance of CYP3A activity is of concern.Lake et al. (1997) reported measurable testosterone 6β-hydroxylase activity and detectable immunoreactive CYP3A protein after 72 hr in culture. The freshness of the liver tissue may be the reason for the loss of CYP3A activity and other P450 activities in such a short time in the current study. CYP2C19 activity also disappeared quickly with time. CYP2C19 is important because of its polymorphic expression; in fact, one of the livers tested appeared to be deficient in CYP2C19, based on its lack of initial (S)-mephenytoin 4′-hydroxylase activity. The levels of activity for CYP2C19 are typically low in human liver microsomes and, not surprisingly, even lower in slice homogenates (expressed per milligram of protein), with levels near the limits of detection. CYP3A activity, however, was well above the limits of detection for the initial value, so loss of CYP3A activity cannot be attributed to quantitation problems caused by low activity. The maintenance of activities selective for CYP1A2 and CYP2C9 was highly variable among the slices from the different livers. For CYP1A2 activity in slices from one of the livers, the levels were very low even at time 0, contributing to the variability when values were expressed as percentage changes. CYP1A2 levels in human liver slices were monitored for 72 hr in previous studies (Lake et al., 1996, 1997), but such a precipitous drop was not observed. As discussed for CYP3A, the freshness of the liver tissue used may be an issue. CYP2C9 activity in the homogenates of the slices was easily determined at the initial time point; therefore, the reason for the variability in the percentage decline in CYP2C9 activity is not readily apparent. Activities selective for CYP2A6, CYP2D6, and CYP2E1 proved to be the most conserved over time in human liver slice culture.
Assays of 7-ethoxycoumarin glucuronosyltransferase and sulfotransferase activities are two of the most commonly used methods for measurement of phase II activity in human liver slice cultures (Ekins, 1996). An unusual stimulation of glucuronosyltransferase activity was seen in all three livers, peaking at 8 hr of culture at approximately twice time 0 values. An increase in 7-ethoxycoumarin glucuronosyltransferase activity over time was seen previously in rat liver slices (Fisheret al., 1995). Sulfotransferase activity decreased over time in a manner similar to that of the activities of the P450s, but more gradually.
Most of the data on the characterization of drug-metabolizing enzymes over long culture periods have been generated with rat liver slices (Lake et al., 1993; Wright and Paine, 1992; Gokhaleet al., 1997). In the studies cited above, rat liver slices maintained their catalytic activities better than did the human liver slices in this study. However, there was still a significant decline in the P450 activities examined in rat liver slices. The greater stability of the activities of the P450s in rat liver slices, compared with human liver slices, may be the result of the processing and transport time involved with human liver tissue. Systematic investigations of factors that are important in the maintenance of the drug-metabolizing capabilities of human liver slices, especially those of the P450s, are needed to optimize the system. Reducing the period of time between the collection of tissue and the initiation of experiments would likely prove beneficial, but shorter times may not be possible to achieve. Whereas rat tissue is available for slicing immediately, our laboratory (and, in fact, most laboratories) currently receives human liver tissue 24–48 hr after processing begins. The culture conditions and transport buffer used in this study are similar to conditions optimized in other studies (Fisher et al., 1995; Olinga et al.,1997; Barr et al., 1991). Either organ preservation methods or culture conditions or both need to be further optimized to maintain the activity of the drug-metabolizing enzymes when longer culture times are required.
From the data presented in this study, it is obvious that the P450s, as a family of enzymes, do not survive well in human liver slice cultures, with most of the activity being lost by 24 hr. The sulfotransferase activity examined fared a little better in culture than did the P450s but also decreased dramatically by 24 hr. In contrast to these two enzyme systems, the glucuronosyltransferase activity examined not only survived in culture but increased in activity before returning to values approximating the initial values. To model in vivooxidative metabolism as accurately as possible, the optimal slice culture incubation time appears to be <4 hr. If the incubation is allowed to proceed for >4 hr, the quantitative profile of metabolism may be skewed in favor of phase II metabolism, specifically glucuronidation. It is more likely that, if the phase II conjugation step requires oxidative biotransformation, a larger component of the metabolic profile might consist of parent drug if the P450 activities are low. This problem might arise when a low-turnover substrate that requires a longer incubation time is examined.
The liver slice model also presents problems when used as a physiologically based pharmacokinetic model. When in vitroand in vivo data are compared, the rate of metabolism observed with liver slices underestimates the clearance of substrate when kinetic modeling is performed (Houston and Carlile, 1997). Of the three model systems (isolated hepatocytes, microsomes, and liver slices) examined by Houston and Carlile (1997), liver slices were the poorest predictor of clearance. It has been proposed that the diffusional limitations of the slices are responsible for this underestimation of clearance (Worboys et al., 1997; Houston and Carlile, 1997; Ekins et al., 1995). The studies cited above were performed with slices from rat liver; however, the poor diffusion of substrate into slices should pertain to all species, as shown previously (Ekins et al., 1996). To date, clearance studies have not been performed in human liver slices. Another contributing factor in the underestimation of clearance with liver slices may be the rapid loss of the catalytic activities of the P450s. Long incubation times may have been needed to determine metabolite formation rates, which may have resulted in little or no active enzyme available for the biotransformation of the substrate. Thus, until methods for human liver slice culture can ensure metabolic competence, care must be taken in interpretation of the metabolite profiles of compounds with incubation periods of >4 hr and in subsequent extrapolation of the data to the in vivo situation.
Footnotes
-
Send reprint requests to: Mark VandenBranden, Department of Drug Disposition, Mail Drop 0825, Lilly Research Laboratories, A Division of Eli Lilly and Company, Lilly Corporate Center, Indianapolis, IN 46285. E-mail: vandenbranden_mark{at}lilly.com
- Abbreviations used are::
- NME
- new molecular entity
- CYP or P450
- cytochrome P450
- Received March 31, 1998.
- Accepted June 16, 1998.
- The American Society for Pharmacology and Experimental Therapeutics