Abstract
We have recently shown that, in human intestine, glucuronidation of androsterone and testosterone was on the nanomolar level and increased from proximal to distal intestine. In the present study, we have characterized estrogen UDP-glucuronosyltransferase activity in microsomes from intestine of seven human subjects. Intestinal microsomes from all segments of intestine from both males and females (except for one male) glucuronidated estrone (0.2–2.6 nmol/mg × min) and estradiol (0.5–3.1 nmol/mg × min) at levels 2 to 15 times higher than found with human liver microsomes (0.04–0.1 and 0.16–0.25 nmol/mg × min, for estrone and estradiol, respectively). Only with estriol were there significant hepatic glucuronidation (2.2–4.5 nmol/mg × min) and intestinal glucuronidation activities (0.2–2.2 nmol/mg × min) that were lower than those in liver. All-trans-retinoic acid was glucuronidated by all segments of intestine from both sexes at levels 50 to 80% of those found with human liver but quite low compared with estrogen glucuronidation. In the two subjects for whom stomach was available, there was no measurable activity in stomach microsomes toward any of the substrates. UGT2B RNA expression was examined in mucosa from stomach to colon from two subjects. There was significant expression of UGT2B7, but not of UGT2B4 or UGT2B15, in all segments of intestine. To our knowledge, this is the first direct demonstration of glucuronidation of estrogens by human intestinal microsomes. Thus, in humans, the intestine may be considered as part of the overall mechanism of detoxification via glucuronidation.
UDP-Glucuronosyltransferases (UGTs1; EC2.4.1.17) are endoplasmic reticulum membrane-bound enzymes that glucuronidate a wide range of often structurally unrelated endogenous and xenobiotic compounds (Dutton, 1980). UGTs are active in the metabolism of important endogenous compounds such as bilirubin (Dutton, 1980), bile acids (Kirkpatrick et al., 1984; Radominska-Pyrek et al., 1986, 1987), and steroid and thyroid hormones (Beetstra et al., 1991; Mackenzie et al., 1992). The existence of multiple UGT isoforms with different, but partially overlapping, substrate specificity in rat and human liver has been clearly demonstrated (Burchell and Coughtrie, 1989; Mackenzie, 1990; Iyanagi, 1991; Lazard et al., 1991). These enzymes have been divided into two families: the UGT1A isoforms are active mainly toward bilirubin and phenolic substrates but also glucuronidate steroids (Radominska-Pandya et al., 1999), whereas UGT2B isoforms have activity directed primarily toward steroid hormones, bile acids, and other endogenous substrates (Tukey and Johnson, 1990; Burchell et al., 1991;Radominska-Pandya et al., 1999)
UGT activities for various substrates have been found in kidney, intestine, nasal mucosa, and brain; there may be tissue-specific expression (Burchell and Coughtrie, 1989), but most studies of UGTs have been carried out with liver tissue or with recombinant UGTs of hepatic origin. However, relatively little information is available regarding glucuronidation of steroid hormones by human liver microsomes. Recent studies in the literature report glucuronidation of testosterone (Pacifici et al., 1997) and both androgens and estrogens (Gall et al., 1999) by human liver microsomes. Steroid hormones have been shown to be substrates for human liver recombinant proteins UGT2B7 and UGT2B15 (Green et al., 1994; Coffman et al., 1998;Gall et al., 1999). Steroid hormone-directed UGT2B activities have been reported in the testes (Chen et al., 1993), prostate and some corresponding tumor lines (Belanger et al., 1991, 1995; Chen et al., 1993), breasts (Beaulieu et al., 1996), ovaries (Prevost et al., 1987), and brain (Beaulieu et al., 1996).
Several investigators have recently examined, primarily in small samples of tissue obtained at biopsy, the expression of intestinal UGT1A isoforms using reverse transcription-polymerase chain reaction. The abundant presence of intestinal UGT1A mRNA (McDonnell et al., 1996; Mojarrabi et al., 1996; Munzel et al., 1996;Mojarrabi and Mackenzie, 1997; Strassburg et al., 1997) and protein, as well as the unique ability of UGTs to glucuronidate a wide variety of substances, provide circumstantial evidence for a physiological role for UGTs in the detoxification of ingested and endogenously generated compounds. Sequence analysis of isolated cDNAs demonstrated the presence of UGTs from the UGT1A family previously isolated from liver (McDonnell et al., 1996; Mojarrabi et al., 1996). Novel intestinal UGTs have also been identified: transcripts for UGT1A8 and 1A10 have been found in colon but not in liver (Mojarrabi and Mackenzie, 1997;Strassburg et al., 1998). Subsequently, these two isoforms, UGT1A8 and 1A10, which are absent in liver, have been identified by reverse transcription-polymerase chain reaction in both small intestine and colon (Cheng et al., 1998, 1999). These isoforms were then expressed in HK293 cells, and catalytic activity toward catechol estrogens was identified (Cheng et al., 1999). In contrast, studies on the intestinal glucuronidation of steroids by UGTs from the 2B family are limited. To fill this gap, we first undertook studies to identify steroid glucuronidation activities in the human intestine.
Recently, we have shown that human intestinal microsomes from both males and females have UGT activity toward the male steroid hormones, androsterone (A) and testosterone (T) (Radominska-Pandya et al., 1998). In males, both activities increased from proximal to distal intestine, and intestinal activity toward testosterone was comparable with or higher than that found in human liver.
In the present study, we have examined intestinal glucuronidation of three estrogens, estrone (E1), estradiol (E2), and estriol (E3), and have found significant activity toward all three substrates. Additionally, glucuronidation of all-trans-retinoic acid (atRA) was also investigated. While there was substantial interindividual variation, overall, estrone and estradiol were both glucuronidated by microsomes from all segments of intestine, from both males and females, with activities higher than those found in human liver. In contrast, intestinal UGT activity toward estriol and all-trans-retinoic acid was lower than that in human liver. There was no activity toward any of the substrates in stomach microsomes from two subjects for whom this tissue was available. We also examined expression of three UGT2B isoforms by Northern blot analysis in two subjects and identified UGT2B7 as a major 2B isoform expressed in human intestine. These findings represent a novel discovery as the first direct demonstration of estrogen glucuronidation by human intestinal microsomes.
Materials and Methods
[3H]Estradiol and [3H]atRA were purchased from NEN Life Science (Boston, MA), and [14C]estrone was from Amersham (Arlington Heights, IL). [14C]UDP-glucuronic acid (UDP-GlcUA) was purchased from American Radiolabeled Chemicals (St. Louis, MO). Brij 58, UDP-GlcUA, saccharolactone, unlabeled estrone, estradiol, estriol, estradiol-17-O-(β-d-glucuronide) (E2-17G), estriol-3-O-(β-d-glucuronide) (E3-3G), estriol-16-O-(β-d-glucuronide) (E3-16G), estriol-17-O-(β-d-glucuronide) (E3-17G), and atRA were from Sigma (St. Louis, MO).
Human intestinal tissue was obtained from organ donors (details are summarized in Table 1) by transplant surgeons at University Hospital, Little Rock, AR, according to a protocol approved by the Human Research Advisory Committee of the University of Arkansas for Medical Sciences. The human liver samples used in these studies were obtained from Drs. R. Vonk and F. Kuipers, University of Groningen, Groningen, The Netherlands (HL15, from a 56-year-old male) and Dr. M. Relling, St. Jude Children's Research Hospital, Memphis, TN (HL174, from a 23-year-old female).
Donor information
Intestinal Microsome Preparation.
Human intestine was placed in iced saline immediately after resection and was kept on ice until used. Tissue from jejunum to rectum was included for all subjects. For subjects H9, H10, and H11, stomach and duodenum were also included. Working at 4°C, small intestine (without duodenum, which was treated separately when it was available) was divided into four segments of 80 to 100 cm in length. Cecum was discarded, and colon was treated as a single segment. Stomach, duodenum, each segment of small intestine, and colon were opened, the contents were removed, and the tissue was rinsed in cold 0.9% NaCl. Mucosa was removed from each segment by scraping with a glass slide. Mucosal samples were weighed and transferred to Dounce homogenizers kept on ice. Microsomes were prepared as previously described (Radominska-Pyrek et al., 1987) except that 2, rather than 3, volumes of buffer were used for homogenization, and trypsin inhibitor (Sigma Type III-O from chicken egg white; 2 mg/g of mucosa) was added to the buffer before homogenization.
RNA Isolation and Northern Analysis.
RNA was isolated from tissues collected post mortem and kept at 4°C for a short period before processing. Mucosa from various sections of the intestinal tract was collected and frozen in ∼0.2-ml portions in liquid nitrogen. Total RNA was isolated from ∼200 μg of tissue by the guanidinium thiocyanate-phenol-chloroform method (Chomczynski and Mackey, 1987) using Trizol Reagent (Life Technologies, Grand Island, NY). RNAs isolated from tissues and total RNA isolated from HK293 human embryonic kidney cells expressing UGT2B7, 5 μg per lane each, were resolved on 1% agarose-formaldehyde denaturing gels. RNA integrity and equality of loading were assessed by measuring optical densities of 28S rRNA bands in gels stained with ethidium bromide. RNAs were electrotransferred to ZetaProbe nylon membrane (Bio-Rad, Hercules, CA), at 1.5 mA/cm2 constant current in a Trans-Blot SD cell (Bio-Rad), and the efficiency of transfer was judged by comparing optical densities of 28S bands visualized on the membrane with UV light with those measured on the gel. RNAs were immobilized by UV cross-linking and examined with UGT2B4, UGT2B7, and UGT2B15 probes. Probes were prepared with a Random Primer DNA labeling kit (Bio-Rad), [α-32P]dATP (NEN Life Science), and UGT2B4, UGT2B7, and UGT2B15 cDNA templates, yielding a mixture of radioactive, single-stranded DNA fragments approximately 100 nucleotides long. Hybridizations were carried out under high-stringency conditions (Church and Gilbert, 1984), and membranes were exposed to X-ray film with intensifying screens for 12 to 72 h at −80°C. Bands on autoradiograms were scanned and quantitated using ScionImage, version 3.0, software (Scion Corporation, Frederick, MD).
Enzyme Assays.
The structures of the estrogen substrates used are shown in Fig.1. E1 and E2 activities were assayed in 100 mM Tris-HCl, pH 8.0, containing 5 mM MgCl2and 5 mM saccharolactone, with radioactive substrates (100 μM) and UDP-GlcUA (4 mM) as the sugar donor and 50 μg of microsomal protein, as described previously for bile acids (Radominska-Pyrek et al., 1986,1987; Radominska et al., 1993). E3 activity was assayed in the same way, but with the unlabeled steroid and [14C]UDP-GlcUA. The glucuronidated products and the unreacted substrate were separated by development (once for E1 and twice for E2 and E3) in chloroform/methanol/glacial acetic acid/water (65:25:2:4, v/v). Radioactive compounds were localized on thin-layer chromatography plates by autoradiography at −80°C. Zones corresponding to the glucuronide bands were scraped into scintillation vials, and radioactivity was measured by liquid scintillation counting (Rackbeta model 1214; Wallac, Gaithersburg, MD). To establish the identity of the different glucuronidated products resulting from E2 and E3 assays, standards of E2-17G and E3-3G, E3-16G, and E3-17G were chromatographed with E2 or E3 reaction products. The products of the assay were detected with autoradiography, and standards were detected by spraying with Krowicki's reagent (Banaszek et al., 1968) and heating.
Structures of substrates.
Intestinal glucuronidation of atRA, which is a substrate for UGT2B7 (Samokyszyn et al., 2000), was also investigated with intestinal microsomes from a somewhat different group of subjects and using alamethicin (60 μg/mg of protein) as an activator, as described previously (Little et al., 1997).
Analysis of Kinetic Parameters.
Kinetic analysis of E2 glucuronidation was carried out with S-4 microsomes from one female (H3) and one male (H5). The concentration of E2 ranged from 5 to 250 μM, whereas the concentration of UDP-GlcUA was held constant at 4 mM. Activity was assayed as described above, and the results were analyzed using EnzymeKinetics software (Trinity Software, Campton, NH).
Results
Tissue Donors.
Intestinal tissue was obtained from seven donors, and detailed information on these donors is shown in Table1. There were three males (21, 51, and 15 years) and four females (26, 40, 57, and 15 years). Stomach and duodenum were also obtained from one male (H11) and two females (H9, H10).
Expression of UGTs in Human Intestine.
The expression of UGT2B4, UGT2B7, and UGT2B15 in intestinal tissues taken from two subjects, H9 and H10, was examined with Northern analysis. Only UGT2B7 transcripts were detected at substantial levels in the examined tissues. Weak signals were observed, on overexposed films, from blots tested with UGT2B4 and UGT2B15 hybridization probes indicating either expression of these isoforms at very low levels or cross-hybridization of probes due to the high nucleotide sequence similarity among UGTs. The results presented in Fig.2 show that UGT2B7 expression in tissue samples from H9 was at a low level in the duodenum, reached a maximum in the middle of the small intestine, followed by a gradual decrease in further sections of the small intestine, and, finally, increased levels in the descending colon. Interestingly, very strong expression of UGT2B7 was found in the duodenum of H10, followed by a rapid decrease toward the colon, and, as in H9, increased expression in descending colon. Comparison of the expression profiles of H9 and H10 showed that small intestine and descending colon are major tissues expressing the UGT2B7 mRNA. Expression of UGTs in stomach was not analyzed due to the poor-quality RNA isolated from this tissue.
Expression of UGT2B7 in human intestinal tract.
A, autoradiogram (top) and total RNA (bottom) from H9 stained with ethidium bromide. B, autoradiogram (top) and total RNA (bottom) from H10 stained with ethidium bromide. Tissue samples: D, duodenum; S1–S4, small intestine (jejunum to ileum); C1–C3, colon (ascending, transverse, and descending). Lanes marked 2B7 contain total RNA isolated from HK293 cells transfected with UGT2B7. C, comparison of UGT2B7 expression, as quantitated by densitometry, in H9 and H10. Black bars represent 2B7 mRNA levels in H9; gray bars represent 2B7 mRNA in H10.
Enzymatic Glucuronidation of Estrogens and atRA.
Estrone
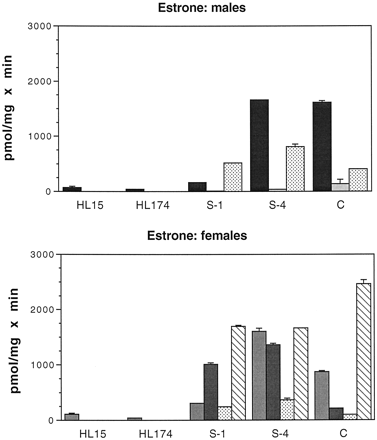
Glucuronidation of E1 by male and female intestinal microsomes is shown in Fig. 3. Glucuronidation activities of male and female human liver microsomes (HL15 and HL174) have been included for comparison in Figs. 3 to6. Both male and female human liver microsomes had low activity toward E1 (93 ± 28 and 42 ± 3 pmol/mg × min for male and female liver microsomes, respectively). With both male and female intestinal microsomes, there was a great deal of interindividual variation in glucuronidation of E1. Overall, E1 was glucuronidated by microsomes from all segments of both male and female intestine at levels higher than those found in human liver microsomes. In several subjects, intestinal activities were 10 or more times higher than in liver. Intestinal microsomes from all segments of H10 actively glucuronidated E1, but colon had an especially high activity (2400 pmol/mg × min) relative to that in colon of the other subjects and, particularly, to that of liver. Because H10 died as the result of a drug overdose, it is possible that the high activity in colon microsomes is the result of induction of UGT activity. With H10 and H11, stomach and duodenal microsomes had also been prepared. Stomach microsomes from these two subjects had essentially no activity toward E1 (19 and 6 pmol/mg × min for H10 and H11, respectively). Glucuronidation activity in duodenal microsomes from H10 (1733 pmol/mg × min) was similar to activities in the other segments of H10 intestine, while there was no significant activity in H11 duodenal microsomes.
Glucuronidation of E1 by human liver and intestinal microsomes.
Intestinal microsomes were incubated with [3H]E1 and UDP-GlcUA as described under Materials and Methods. Human liver microsomes from a 56-year-old male (HL15) and a 23-year-old female (HL174) were assayed simultaneously for comparison. S-1, proximal jejunum; S-4, terminal ileum; and C, colon. Bars represent the mean of at least two separate duplicate determinations, and the brackets give the standard deviation. Males: ▪, H5;
 , H11; ░, H12. Females:
, H11; ░, H12. Females:
 , H3; ▪, H4; ░, H6; ▧, H10.
, H3; ▪, H4; ░, H6; ▧, H10.
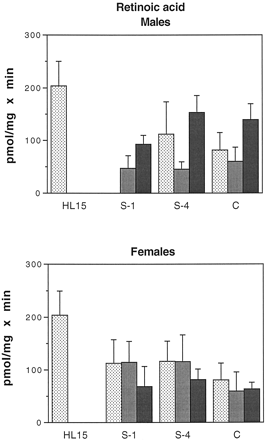
Glucuronidation of all-trans-retinoic acid by human intestinal microsomes.
Intestinal microsomes were incubated with [3H]atRA and UDP-GlcUA as described under Materials and Methods. The results shown are the sum of two products: atRAG (ca. 75%) and an unidentified retinoid glucuronide (possibly 13-cis-RAG, ca. 25%). Samples are as in the legend to Fig. 4. Bars represent the mean of at least two separate duplicate determinations, and the brackets give the standard deviation. Males: ░, H1;
 , H2; ▪, H5. Females: ░, H3;
, H2; ▪, H5. Females: ░, H3;
 , H4; ▪, H6.
, H4; ▪, H6.
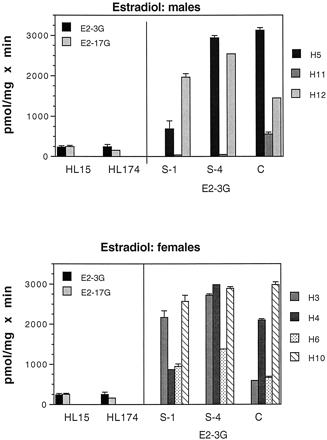
Estradiol.
As with E1, intestinal microsomes from all segments of both males (with the exception of H11) and females glucuronidated E2 at very high levels, 2 to 15 times those seen in human liver (Fig. 4). Intestinal microsomes biosynthesized exclusively the 3-O-glucuronide of E2 with activities ranging (excluding H11) from 690 to 3100 pmol/mg × min in males and from 600 to 3000 pmol/mg × min in females. In contrast, liver microsomes biosynthesized much lower levels of the E2 3-O-glucuronide (236 and 246 pmol/mg × min for male and female, respectively) and also formed the 17-O-glucuronide (252 and 159 pmol/mg × min for male and female, respectively).
Glucuronidation of E2 by human liver and intestinal microsomes.
Intestinal microsomes were incubated with [3H]E2 and UDP-GlcUA as described under Materials and Methods. Samples are as in the legend to Fig. 4. Two labeled glucuronides, E2 3-O-glucuronide (E2-3G) and E2 17-O-glucuronide (E2-17G), were formed by human liver microsomes. Intestinal microsomes formed only the E2-3G, with the exception of H12, which formed small amounts of E2-17G. Bars represent the mean of at least two separate duplicate determinations, and the brackets give the standard deviation.
E2 glucuronidation was chosen for kinetic analysis because of the high activity of intestinal microsomes toward this substrate. S-4 was chosen as the representative intestinal segment for these assays because of its high activity toward E2. Kinetic parameters were similar for a female subject (H3) and a male subject (H5). The apparentKm values were 38 and 41 μM for H3 and H5, respectively, while the correspondingVmax values were 6.4 and 7.3 nmol/mg × min for H3 and H5.
Estriol.
Only with E3 were activities in intestinal microsomes equal to or lower than those in male and female human liver (Fig. 5). Of the three possible E3 glucuronides, human liver biosynthesized only ad-ring (16- or 17-O) glucuronide. In contrast, human intestine from both male and female subjects made bothd-ring (16- and/or 17-O) and 3-O-glucuronides. In all subjects, thed-ring glucuronide was the predominant conjugate in the proximal small intestine (S-1) and tended to decrease toward the colon. In contrast, the 3-O-glucuronide tended to increase along the length of the intestine and was produced in the colon in amounts equal to or greater than those of thed-ring conjugate.
Glucuronidation of E3 by human liver and intestinal microsomes.
Intestinal microsomes were incubated with E3 and [14C]UDP-GlcUA as described under Materials and Methods. Samples are as in the legend to Fig. 4. Human liver microsomes formed only E3 16-O-glucuronide, whereas human intestinal microsomes formed both a d-ring glucuronide (16- or 17-O; upper panels) and E3 3-O-glucuronide (lower panels). Samples are as in the legend to Fig. 4. Bars represent the mean of at least two separate duplicate determinations, and the brackets give the standard deviation.
Retinoic acid.
The results of enzymatic assays using atRA as substrate are shown in Fig. 6. The results are the sum of two products: approximately 75% of the total was identified as atRA glucuronide, whereas the other 25% was the glucuronide of an unidentified retinoid, possibly 13-cis-retinoic acid. The intestinal microsomes used in these assays were from a somewhat different group of subjects; see Table 1.
As with the estrogen substrates, there was a good deal of interindividual variation in glucuronidation of atRA. There was no significant difference in activity between males and females, nor was there a distinct pattern of activity distribution along the length of the intestine. In males, activities in intestinal microsomes ranged from 47 to 153 pmol/mg × min (30–75% of values in liver), whereas in females the range was 60 to 116 pmol/mg × min (30–50% of values in liver).
Discussion
Hepatic glucuronidation reactions are extensive, covering a wide range of substrates (Burchell and Coughtrie, 1989; Tephly and Burchell, 1990). Recently, there has been increasing interest in intestinal glucuronidation in humans. Recent studies have involved identification of UGT RNA transcripts in the intestinal mucosa from surgical specimens (Strassburg et al., 1998). In earlier papers, homogenates or microsomes prepared from intestinal biopsies or tissue samples obtained from surgical resections have been used for measurement of UGT activity toward phenols, bile acids, and bilirubin (Matern et al., 1984; Parquet et al., 1985; Marschall et al., 1987; Peters and Jansen, 1988; Peters et al., 1989; McDonnell et al., 1996). Our access to intact, fresh intestine from jejunum to colon and, in some cases, from duodenum and stomach as well, has allowed us to evaluate human intestinal glucuronidation in some detail and, from the available medical history of the subjects, to investigate factors that might influence glucuronidation in the intestine. Moreover, we have been able to clone and express the unique intestinal isoforms, UGT1A8 (Cheng et al., 1998) and UGT1A10 (Cheng et al., 1999).
Previously, we have reported intestinal UGT activity toward androsterone and testosterone (Radominska-Pandya et al., 1998). In males, there was significant activity toward A and T, which increased gradually from proximal jejunum to colon, and glucuronidation of T by colon microsomes was equal to or greater than that found in hepatic microsomes. In two of three females, there was very little glucuronidation of A and T, whereas in the third subject, A and T were both glucuronidated by all segments of intestine at levels higher than those seen in males.
The major finding from these studies was unexpectedly high UGT activity toward E1 and E2: overall, glucuronidation of these two substrates by all segments of intestine from both sexes was significantly higher than that found in liver. Thus, it appears that glucuronidation of E1 and E2 takes place to a large extent in the intestine. It is interesting that while human liver microsomes biosynthesized both the 3-O- and 17-O-glucuronides of E2, the high E2 activity in intestinal microsomes was directed solely to the 3-O position.
Only with E3 was glucuronidation activity higher in liver than in intestine. As with E2, there was a distinct difference in the type of E3 glucuronide formed by the two tissues; in contrast to E2, human liver microsomes biosynthesized only a d-ring glucuronide of E3, whereas intestinal microsomes formed glucuronides at both the 3-O position and the 16-O and/or 17-O positions. The d-ring glucuronide predominated in the small intestine with the 3-O-glucuronide only becoming significant relative to thed-ring glucuronide in the colon.
atRA, another endogenous substrate for UGTs, is glucuronidated at the carboxyl function rather than a hydroxyl function, as is the case with the estrogens, but is a substrate for UGT2B7, which is discussed below. Intestinal glucuronidation of atRA approached that found in human liver, but was low compared with the steroid hormones. Activity toward atRA was fairly evenly distributed along the length of the intestine and did not show any sex difference.
There are virtually no data available on the glucuronidation of estrogens by human intestinal mucosa. The information that is available is mostly indirect, consisting of inferences and presumptions based on glucuronide conjugates recovered from bile, feces, and/or urine in in vivo studies (Hartiala, 1973; Adlercreutz et al., 1979). The only reported in vitro studies showed that E3 could be glucuronidated at all available positions by human intestinal mucosal cells (Adlercreutz et al., 1979). We have demonstrated similar results with human intestinal microsomes. Recently, an article was published in which glucuronidation of steroid hormones, phenols, and retinoic acid by human colon and liver was determined (Strassburg et al., 1999). For all comparable substrates, the activities reported for both tissues were much lower (2- to 66-fold) than those given here and in a previous study (Radominska-Pandya et al., 1998). Differences in assay conditions, including lack of detergent activation and inappropriate substrate and cosubstrate concentrations, may well account for the discrepancies.
Of the available human recombinant UGTs, UGT2B7 has been shown to glucuronidate the steroid hormones, androsterone, E2, and E3 (Gall et al., 1999) and to be expressed in liver and intestine (Radominska-Pandya et al., 1998). Therefore, expression of UGT2B7 and two other UGT2B isoforms, UGT2B4 and UGT2B15, was examined by Northern blot analysis in intestinal mucosa from two subjects. The most obvious difference between UGT2B7 expression in H10 and H9 intestine was seen in duodenum where UGT2B7 transcripts were barely detectable in H9 but were present at high levels in H10. Because H10 died of a drug overdose, we suggest that increased expression of UGT2B7 mRNA in duodenum might be a result of induction on the transcriptional level in this subject. The expression profile in H9 showed a broader range in the small intestine compared with that in H10 where a sharp decrease in expression was observed. Global expression of UGT2B7 was 25% higher in H9 than in H10; however, duodenal UGT2B7 expression in H10 represented 43% of the total amount in this subject, whereas in H9, it was only 5% of the total. We suggest that the elevated duodenal expression in H10 was a consequence of a global change in expression of UGT2B7 in H10 rather than an individual variation, and the response to increased intestinal concentrations of drugs may be localized to the proximal small intestine. Expression of UGT2B7 in distal colon was relatively proportional in both subjects. This may suggest either a different transcriptional activation mechanism specific for this tissue or simply reflect an acute physiological state in H10. Expression of two other UGT isoforms from the 2B family, UGT2B4 and UGT2B15, which are expressed in human liver (Fournel-Gigleux et al., 1989; Green et al., 1994), was also examined. Weak bands obtained from blots probed with these isoforms could be a result of nonspecific hybridization of probes with other isoforms or may represent basal transcription of these isoforms. However, significant expression of these isoforms was not detected in any segment of intestine from either subject.
Taken together, these results indicate that the human intestine has a large capacity for glucuronidation of estrogens, similar to that previously shown for androgens (Radominska-Pandya et al., 1998). However, there were striking differences in hepatic and intestinal glucuronidation of E1 and E2 as compared with E3. With E1 and E2, the ratio of intestinal to hepatic activity was very high (≫1), and only 3-O-glucuronides were formed in the intestine, whereas the liver formed both the 3-O- and 17-O-glucuronides of E2. With E3, the intestinal to hepatic activity ratio was low (<1) and, although both intestine and liver microsomes glucuronidated E3 on the d-ring, the intestine also formed low levels of the 3-O-glucuronide.
Based on these comparisons, we speculate that the intestinal activities toward E1 and E2 can be attributed to a single enzyme specific for the 3-O position and present only in intestine. This same enzyme may be responsible for the 3-O-glucuronidation of E3 in intestine. Activity toward the d-ring of E3 probably involves a distinct UGT isoform with high activity in the liver. Because UGT2B7 is expressed in liver and intestine (Radominska-Pandya et al., 1998) and has been shown to glucuronidate E3 at the 16-O and/or 17-O positions, it may be responsible for the E3-specific activity. We speculate that intestinal 3-O-specific glucuronidation activity is carried out by an as yet unidentified UGT isoform.
The exact role of intestinal glucuronidation in the overall biotransformation of steroid hormones is unclear, but the intestine represents the first metabolically active organ encountered by orally ingested compounds, providing first-pass biotransformation and a large absorptive interface. Intestinal glucuronidation of estrogens may be responsible for the low biological effectiveness of natural estrogens administered orally, as compared with transdermal delivery. It has been shown that to reach the same plasma levels, oral doses of 17β-estradiol have to be 20 times higher than transdermal doses (Helle et al., 1996). Studies on the effects of estrogen replacement therapy indicate that the oral route requires 10-fold higher doses of estrogen, as compared with transdermal application, to have the same effects on lipid metabolism and prevention of bone loss (Stevenson et al., 1993). Steroid hormones secreted in bile also encounter the intestinal interface and undergo an enterohepatic circulation (Adlercreutz et al., 1979). In in vivo situations, there is probably a dynamic equilibrium between hydrolysis of estrogen conjugates by intestinal β-glucuronidase, mainly from intestinal microflora and increasing in activity from proximal to distal intestine, and absorption and reconjugation of the aglycon by the intestinal mucosa (Adlercreutz et al., 1979). Glucuronidation would also prevent, to a large extent, further biotransformation of the aglycon (Adlercreutz et al., 1979). Regardless of the exact physiological significance of intestinal estrogen glucuronidation, the presence of UGT protein with high activity toward steroid hormones indicates that intestinal tissue is an active site of biotransformation of steroidal substrates and could be a major factor in overall steroid hormone metabolism.
Footnotes
-
Send reprint requests to: Anna Radominska-Pandya, Ph.D., University of Arkansas for Medical Sciences, Department of Biochemistry and Molecular Biology, 4301 W. Markham, Slot 516, Little Rock, AR 72205. E-mail: radominskaanna{at}exchange.uams.edu
-
This work was supported in part by National Institutes of Health Grants DK49715 and DK51971 to A.R.-P.
- Abbreviations used are::
- UGTs
- UDP-glucuronosyltransferases
- E1
- estrone
- E2
- estradiol
- E3
- estriol
- UDP-GlcUA
- UDP-glucuronic acid
- E2-3G
- estradiol-3-O(β-d-glucuronide)
- E2-17G
- estradiol-17-O(β-d-glucuronide)
- E3-3G
- estriol-3-O(β-d-glucuronide)
- E3-16G
- estriol-16-O(β-d-glucuronide)
- E3-17G
- estriol-17-O(β-d-glucuronide)
- A
- androsterone
- T
- testosterone
- atRA
- all-trans-retinoic acid
- Received October 18, 1999.
- Accepted July 6, 2000.
- The American Society for Pharmacology and Experimental Therapeutics