Abstract
We investigated whether lack of the canalicular multispecific organic anion transporter in transport-deficient (TR−) rats would result in plasma and urinary accumulation of troglitazone or its major metabolites and whether any accumulation would be associated with increased levels of bilirubin or bile acids. Administration of a single oral dose of troglitazone (200 mg/kg) to TR− rats resulted in 2- and 50-fold increases in plasma levels and 30- and 500-fold increases in urinary amounts of troglitazone sulfate and troglitazone glucuronide, respectively, compared with normal rats. No changes were found in the plasma concentrations and urinary amounts of troglitazone or troglitazone-quinone. Accumulation of troglitazone metabolites in plasma was accompanied by a 2-fold increase in the serum level of conjugated bilirubin in TR− rats, whereas no changes were observed in normal animals. Bile acids were detected in the urine of both TR− and normal rats, with an average 3-fold greater level found in the urine of TR− animals. Biliary metabolic profiles revealed a delay in the secretion of troglitazone sulfate and troglitazone glucuronide in TR− rats over the first 2- and 4-h periods, respectively. These results demonstrate the role of multidrug resistant associated protein-2 in biliary secretion of troglitazone glucuronide and troglitazone sulfate and suggest the presence of compensatory mechanisms responsible for transport of troglitazone metabolites and bilirubin-glucuronide at the basolateral and canalicular sites of hepatocytes.
Troglitazone (TRO1) was the first member of the thiazolidinedione family of drugs developed to treat type II diabetes. Metabolism of troglitazone in rats involves primarily sulfation with approximately 85% of the total administered drug excreted into the bile as TRO-Sulf (Kawai et al., 1997; Loi et al., 1999a). Approximately 10% of the administered dose is recovered in bile as TRO-Gluc and TRO-Qn. TRO-Sulf is the major metabolite in humans, with 85% of drug recovered in feces, suggesting a similar major route of excretion as shown in the rat (Rezulin package insert, Parke-Davis Pharmaceutical, Ann Arbor, MI; Loi et al., 1999b). We have previously hypothesized that transport of TRO-Sulf across the bile canalicular membrane may be deficient in certain individuals due to either competition with other substrates or a genetic polymorphism of a specific transmembrane transporter resulting in accumulation of toxic levels of TRO and/or endogenous substances that may be associated with hepatotoxicity (Kostrubsky et al., 2000). Observations made in patients with TRO-associated liver injury demonstrated signs of cholestasis characterized by a gradual increase in the serum level of conjugated bilirubin, jaundice, dark urine, and pruritus [MedWatch: the FDA medical products reporting program (http://www.fda.gov/medwatch/);Gitlin et al., 1998; Shibuya et al., 1998; Fukano et al., 2000; Menon et al., 2001]. In addition, a number of histological examinations of livers from patients who experienced liver failure indicated cholestasis as a part of a mixed type of liver damage. The possibility exists that TRO metabolites, bilirubin-glucuronide, and bile acids may share common liver transporters involved in their elimination. Competition of these substrates at a liver transporter may lead to their accumulation in the liver and systemic circulation, potentially explaining a clinical picture of cholestasis. To test this hypothesis, we used TR− rats, a Wistar strain deficient in expression of Mrp2 (canalicular multispecific organic anion transporter), a transporter responsible for the canalicular excretion of a variety of endogenous and exogenous anionic conjugates including bilirubin, leukotrienes, bile salts, diclofenac, sulfobromophthalein, and estradiol (Konig et al., 1999a; Mills et al., 1999). These rats have chronic hyperbilirubinemia, with about 80% of total circulating bilirubin present as glucuronide conjugates (Jansen et al., 1985). We investigated whether TR− rats would be exposed to greater amounts of TRO metabolites and whether any changes in metabolite distribution were associated with increased levels of bilirubin after administration of a single oral dose of 200 mg/kg TRO.
Materials and Methods
Chemicals.
TRO and metabolites were from Parke-Davis Pharmaceutical. Reagents for Vitros and Hitachi clinical analyzers were obtained from Johnson & Johnson Co. (Rochester, NY) and Sigma (St. Louis, MO), respectively.
Animals.
Male Wistar rats weighing 270 to 336 g (Charles River Laboratories, Portage, MI) and male TR−, weighing 331 to 340 g (a gift from the Academic Medical Center, Amsterdam, The Netherlands) were used throughout. Animals were acclimated to the animal room conditions (a 12-h light/dark cycle; 70–78°F; relative humidity, 30–70%; at least 10–15 fresh air changes/h) for approximately 1 week before being placed under study. While conducting the study, animals were housed individually in rodent metabolism cages under the same environmental conditions. Animals received powdered rodent chow (Purina certified chow; Purina, St. Louis, MO) and tap water by bottle ad libitum. All procedures involving animals were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and under a protocol approved by the Institutional Animal Care and Use Committee.
Surgery.
The jugular vein and bile duct cannulation procedures were conducted under isoflurane anesthesia. Cannulation was performed by isolating the jugular vein through a small skin incision and inserting medical grade silastic tubing (0.027-inch i.d.; 0.047-inch o.d.; a 25- to 28-cm length) into the jugular vein that was fixed in place with ligatures. The jugular cannulas were routed subcutaneously to the dorsal scapular area, where they were externalized for blood sample collection during the study.
The bile duct cannulas were silicone tubing of increasing diameter [medical grade silicone tubing (.012-inch i.d.; 0.025-inch o.d.) 3.0 cm in length, medical grade silicone tubing (.020-inch i.d.; 0.037-inch o.d.) 1.0 cm in length, medical grade silicone tubing (.025-inch i.d.; 0.047-inch o.d.) 17.0 cm in length] prepared by inserting the smaller diameter tubing into the next larger diameter tubing and adding silicone adhesive as suture beads for securing the catheter in position. A midline abdominal skin incision was made, and the common bile duct was exposed approximately 1.0 to 1.5 cm distal to the biliary bifurcation. The bile cannula was inserted through a small incision into the duct and fixed in position with ligatures. The cannula was then routed subcutaneously to the dorsal scapular area, where it was connected to polyethylene tubing (PE 90). The rat was then placed in a jacket, and the bile catheter was connected to a swivel assembly that was primed with 0.9% NaCl. The bile flow was maintained by gravity. To maintain electrolyte balance and normal kidney function, a balanced electrolyte solution (Normasol-R; Abbott Laboratories, Chicago, IL) and dry powdered rodent chow were supplied ad libitum for the remainder of the study. Predose urine, bile, and fecal samples were obtained for the first 18 to 22 h; the rats were housed in the metabolism cages. Rats were fasted overnight following surgery and dosed by oral gavage the morning after surgery.
Experimental Design.
Sixteen rats, eight TR− and eight normal Wistar rats (NR), were assigned to two experimental groups. The first four animals (1–4) in each group were used for plasma and urine collection. Animals 5 to 8 were cannulated and used for bile collection. A single oral dose of 200 mg/kg troglitazone was given by gavage as a suspension in 0.5% methylcellulose (prepared before the administration) at a dose volume of 10 ml/kg to animals 1 to 7. The last rat in each group acted as a sham control with no drug administered. The day before drug administration, urine and blood were collected for evaluation of pretest biochemical parameters. Blood samples (0.6 ml) for measurement of metabolites and parent drug were collected in heparin tubes at 1, 2, 3, 6, 8, 24, and 36 h after TRO administration. Serum was also collected for biochemical determinations at 2 and 36 h. Urine was collected at 0 to 8, 8 to 24, and 24 to 36 h intervals, and bile collection intervals were 0 to 2, 2 to 4, 4 to 8, 8 to 24, and 24 to 36 h. Both urine and bile samples were collected in plastic conical containers at approximately 4°C.
Metabolite Quantitation.
Sulfate, glucuronide, and quinone metabolites of TRO and parent drug were measured in plasma, urine, and bile. Analysis was performed as described previously using liquid chromatography-tandem mass spectrometry (Kostrubsky et al., 2000) with the following assay modifications. Standard calibration curves ranging from 10 to 25,000 ng/ml were prepared in either blank plasma or urine from 0.25 mg/ml stock solutions (50:50 acetonitrile/water, v/v) followed by serial dilution. The internal standard solution contained stable-label (SL)13C analogs of the glucuronide metabolite and TRO (2500 ng/ml each in 50:50 acetonitrile/water, v/v). Bile samples were diluted 1:10 in blank plasma before the assay and quantified using a plasma standard curve. Internal standard (20 μl) was added to the samples or standards (100 μl) and extracted with 300 μl of acetonitrile. Samples were vortexed thoroughly by centrifuging at 14,000 rpm for 15 min, and the supernatant (250 μl) was transferred to a 96-well polypropylene autosampler plate, which was evaporated to dryness under nitrogen at 40°C. Finally, samples were reconstituted in 100 μl of acetonitrile/water (50:50), and 7 μl was injected into the liquid chromatography/tandem mass spectrometer. The analytical column was a Supelco Discovery RP Amide C16(2.1 × 50 mm, 5 μm; Supelco, Bellefonte, PA). The mobile phase consisted of acetonitrile/10 mM ammonium acetate, pH 4 (60/40, v/v). Final chromatographic retention times for TRO, metabolites, and internal standards were between 1 and 4 min. The following ion transitions were used in electrospray negative ionization mode for analysis by the mass spectrometry: TRO (440.1 > 42.2), quinone metabolite (456.0 > 42.0), sulfate metabolite (519.7 > 439.9), glucuronide metabolite (615.8 > 439.9), SL-TRO (444.1 > 44.3), and SL-glucuronide metabolite (620.2 > 444.2). Standard and blank matrix samples were interspersed throughout the sample run.
Biochemical Determinations.
Serum was analyzed for bilirubin, alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase using a Vitros chemistry analyzer (Ortho-Clinical Diagnostic; Johnson & Johnson), and urine was analyzed for bile acids using a Hitachi 911 analyzer (Boehringer Mannheim, Indianapolis, IN). Due to limited serum availability, bile acids were not analyzed in serum.
Data Analysis.
Individual and mean concentration-time data tables were generated, and pharmacokinetic parameters were determined by noncompartmental analysis of these data using WinNonlin Professional, version 3.0A, software (Pharsight Corporation, Mountain View, CA). Maximum TRO, TRO-Sulf, TRO-Gluc, and TRO-Qn concentrations (Cmax) and time to reach Cmax(tmax) were recorded as observed. Area under the concentration-time curve was calculated from time 0 to the time of last detectable concentration using the trapezoidal rule and extrapolated to infinity. Results were analyzed by a two-factor analysis of variance with a P < 0.05 interpreted as the level of statistical significance.
Results
Effect of TRO on Excretion of Bilirubin and Bile Acids.
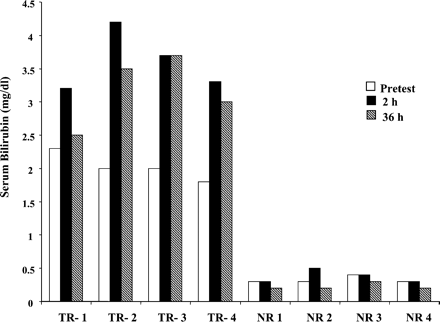
The serum bilirubin was measured in TR− and normal rats before the experiment and at 2 and 36 h after TRO administration. Bilirubin values for individual TR− and NR rats are shown in Fig.1. The average serum bilirubin was 1.95 ± 0.26 and 0.34 ± 0.05 mg/dl in four TR− and normal rats, respectively, before TRO administration. At 2 h and 36 h after TRO administration, bilirubin levels in TR− rats were significantly increased to 3.6 ± 0.5 and 3.1 ± 0.5 mg/dl, respectively (P < 0.001). At least 60% of the serum bilirubin was present as bilirubin-glucuronide. In contrast, no significant changes in bilirubin were observed in NR rats. There were no differences found in alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase between TR− and NR rats.
Serum bilirubin in individual TR− and NR rats.
Bilirubin was measured as described under Materials and Methods before TRO (pretest) and at 2 and 36 h after administration of 200 mg/kg TRO in four TR− or normal rats.
Bile acids were analyzed in urine from both normal and TR− rats. Bile acids were detected in samples from all four TR− rats (3.6 ± 3 μmol/kg) up to 24 h after TRO administration and in the urine of two of the four NR Wistar rats (0.95 ± 0.8 μmol/kg) between 0 and 8 h after dosing. No bile acids were detected in urine from any of the sham control rats.
Troglitazone Metabolism.
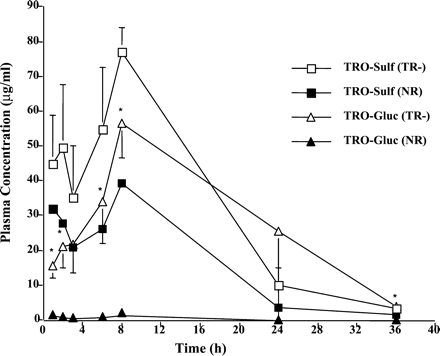
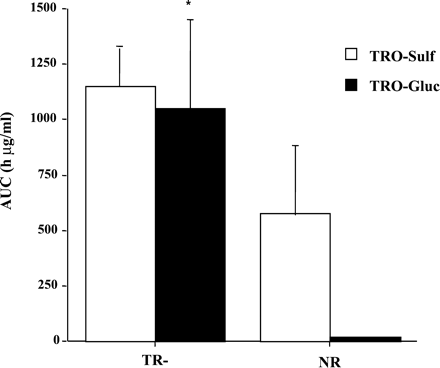
Plasma concentrations of TRO and its metabolites were determined at different time points over the 36 h, as shown in Fig.2. TRO-Sulf was the major metabolite in both TR− and normal animals. However, there was a 2-fold increase in plasma exposure to TRO-Sulf in TR− rats (Figs. 2 and3) compared with NR rats. In agreement with previously reported results (Kawai et al., 1997; Loi et al., 1999a), TRO-Gluc was a minor metabolite detected in the plasma of NR rats accounting for approximately 3% of total plasma metabolites. In contrast, TRO-Gluc was a major metabolite in TR− rats, with a 50-fold increase in plasma concentration compared with NR rats (Figs. 2 and 3). TRO-Sulf and TRO-Gluc accounted for 51 and 46% of the total plasma TRO metabolites, respectively, in TR− rats. No differences were found for parent drug or TRO-Qn in plasma between the two types of rats, which accounted for 4 to 14% of the total metabolites (Fig.4).
Plasma concentrations of TRO-Sulf and TRO-Gluc in TR− and normal rats over 36 h.
Rats were administered a single oral dose 200 mg/kg TRO, and blood was collected via jugular vein catheter at the indicated time points. Plasma was analyzed for TRO metabolites, as described underMaterials and Methods. Each point is a mean ± S.D. of four TR− or normal rats. ∗, significantly different from TRO-Gluc in normal rats with P < 0.001.
Area under concentration-time curve (AUC) for TRO-Sulf and TRO-Gluc in TR− and normal rats.
Rats were administered a single oral dose 200 mg/kg TRO, and blood was taken via jugular vein catheter at the indicated time points. Plasma was analyzed for TRO metabolites and the AUCs for TRO-Sulf and TRO-Gluc were calculated as described under Materials and Methods. Each point is a mean ± S.D. of four TR− or normal rats. ∗, significantly different from TRO-Gluc in normal rats with P < 0.01.
AUC for TRO and TRO-Qn in TR− and normal rats.
Rats were administered a single oral dose 200 mg/kg TRO, and blood was harvested via jugular vein catheter at the indicated time points. Plasma was analyzed for TRO and metabolites and AUCs for TRO and TRO-Qn were calculated as described under Materials and Methods. Each point is a mean ± S.D. of four TR− or normal rats.
Total amounts of TRO-Sulf and TRO-Gluc detected in 0 to 36 h urine are illustrated in Fig. 5. There was 30- and 570-fold more TRO-Sulf and TRO-Gluc, respectively, excreted in the urine of TR− rats compared with NR animals (946 versus 29 μg for TRO-Sulf and 313 versus 0.5 μg for TRO-Gluc withP < 0.01). No significant difference was found with TRO or TRO-Qn between TR− and NR rats (data not shown). TRO and TRO-Qn accounted for approximately 4 and 18% of total drug excreted in urine, respectively, in both types of animals.
Total amounts of TRO-Sulf and TRO-Gluc excreted into urine over 36 h.
Rats were administered a single oral dose 200 mg/kg TRO, and urine was collected from four TR− and four normal rats between 0 to 8, 8 to 24, and 24 to 36 h. Urine was analyzed for TRO metabolites, as described under Materials and Methods. Results represent a total amount of TRO-Sulf and TRO-Gluc detected in the urine of four TR− or normal rats over 36 h. Each bar is the mean ± S.D. of four TR− or normal rats.
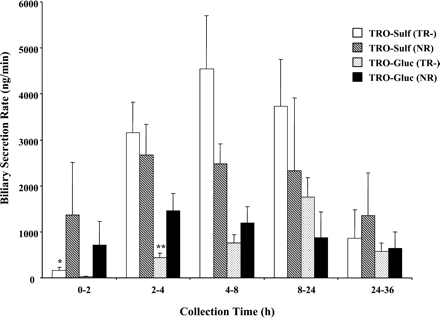
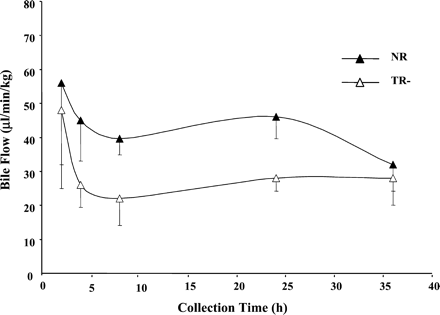
Biliary rates of TRO-Sulf and TRO-Gluc secretion are shown in Fig.6. NR rats secreted both metabolites at comparable rates over the 36 h of bile collection. In contrast, TR− rats secreted TRO-Sulf and TRO-Gluc at a 20-fold lower rate during the first 2 h of collection. Secretion of TRO-Gluc was significantly lower compared with normal rats up to 4 h. These lower rates were followed by secretion of both metabolites with rates similar to normal rats from 4 to 36 h. Although bile flow was reduced by about 40% in TR− rats (Fig.7), it remained constant over the 36 h, indicating that the difference in the rate of metabolite secretion at early times was not influenced by bile flow. In both types of rats, the total amount of TRO-Sulf (∼5 mg) and TRO-Gluc (∼2 mg) accounted for about 70 and 28% of the metabolites excreted in bile, respectively.
The rate of biliary TRO-Sulf and TRO-Gluc secretion in TR− and normal rats.
Rats were administered a single oral dose 200 mg/kg TRO, and bile was collected between 0 to 2, 2 to 4, 4 to 8, 8 to 24, and 24 to 36 h. Bile was analyzed for TRO metabolites, as described underMaterials and Methods. Results represent a rate of TRO-Sulf or TRO-Gluc secretion in bile for each time period of bile collection. Each bar is the mean ± S.D. for TRO-Sulf or TRO-Gluc of three TR− or normal rats. ∗, significantly different from TRO-Sulf in TR− rats at 2 to 4, 4 to 8, and 8 to 24 h withP < 0.01. ∗∗, significantly different from TRO-Gluc in normal rats with P < 0.05.
Bile flow in TR− and normal rats.
Rats were administered a single oral dose 200 mg/kg TRO, and bile was collected between 0 to 2, 2 to 4, 4 to 8, 8 to 24, and 24 to 36 h. Each point is a mean ± S.D. of three TR− or normal rats.
Discussion
In this study, we compared the elimination of TRO metabolites between normal and Mrp2-deficient rats to investigate whether the lack of Mrp2 would result in greater plasma exposure of TRO metabolites and bilirubin-glucuronide. Although 80% of TRO is known to be secreted in bile as the sulfate conjugate, a deficiency in Mrp2, a transporter involved in secretion of a variety of anionic substrates (Konig et al., 1999a), could result in a large increase in the plasma concentration of TRO-Sulf. Administration of 200 mg/kg TRO resulted only in a 2-fold increase in the plasma TRO-Sulf level in TR− rats, suggesting that Mrp2 is involved in the elimination of TRO-Sulf but that there are other biliary transporter(s) participating in the excretion of this metabolite as well. Recently, an inhibition of taurocholate transport by TRO-Sulf was demonstrated in canalicular liver plasma membrane vesicles prepared from normal and TR− rats, suggesting an interaction between Bsep and TRO-Sulf (Funk et al., 2001). We detected bile acids in the urine from both TR− and NR rats treated with TRO, consistent with the inhibition of Bsep by TRO-Sulf. The greater level of bile acids in TR− rats may in part be explained by an induced level a basolateral (vascular side) Mrp3. The Mrp3 has a high affinity for secretion of glucuronides and bile acids and was reported to be induced up to 10-fold in Mrp2-deficient rats (Ogawa et al., 2000). Thus, an increased level of Mrp3 may overcompensate for the lack of Mrp2 activity by secreting bile acids into blood.
A 50-fold increase in the plasma level of TRO-Gluc in TR− rats was associated with a 2-fold increase in serum-conjugated bilirubin, a known high-affinity substrate for Mrp2. This result suggests a major role of Mrp2 in the secretion of TRO-Gluc in bile and a competition for Mrp2 between TRO-Gluc and bilirubin-glucuronide. The deficiency in Mrp2 combined with an efflux from hepatocytes via up-regulated Mrp3 or a similar transporter would explain the large increase in the plasma concentration of TRO-Gluc in TR− rats.
Comparison of biliary metabolites of TRO in NR and TR− rats revealed no significant difference in the total amount of metabolites excreted over the 36-h collection period. However, there was a biphasic profile in the biliary secretion of TRO-Sulf and TRO-Gluc in TR− animals. There was a decrease in the rate of secretion of TRO-Sulf over the first 2 h and TRO-Gluc over the first 4 h collection intervals in TR− rats (Fig. 6). These findings support the hypothesis that there is a compensatory low-affinity biliary transporter for both metabolites that participates after the metabolites accumulate in hepatocytes. In agreement with these data, an alternative biliary transport mechanism was recently proposed in Bsep knockout mice (Wang et al., 2001).
In addition, a 2- and 10-fold difference in plasma concentrations of TRO-Sulf and TRO-Gluc, respectively, between TR− and NR was observed as early as 1 h after administration of TRO. This suggests the preferential secretion of metabolites into the blood at this time via a high-affinity basolateral transporter in TR− rats, thus compensating for the lack of complete biliary secretion. Finally, serum bilirubin was increased 2-fold by 2 h, the first measurement obtained. The bilirubin remained elevated for the duration of the experiment despite there being no differences in plasma metabolite concentrations between the two groups of rats by 36 h. However, excretion of metabolites into the bile was still high at 36 h (Fig. 6), suggesting that there was a sufficient quantity of TRO metabolites still present in the liver. We also found large amounts of TRO metabolites excreted in the urine of TR− rats (Fig. 5). Urinary TRO metabolites accounted for approximately 14 and 0.7% of total TRO metabolites detected in the bile and urine of TR− or NR rats, respectively. Despite a dramatic decrease in plasma concentrations of TRO-Sulf and TRO-Gluc between 8 and 24 h (Fig. 2), there were no significant differences in their rates of secretion from 0 to 8 and 8 to 24 h in TR− rats. These results may suggest saturable active transport of these metabolites. Since Mrp2 is not present at the apical side of kidney tubular cells in TR− rats, in contrast to NR rats, the transport of TRO-metabolites and bile acids into the tubular lumen may be mediated by multidrug resistance protein-1, which has been previously reported to export estradiol-17β(β-d-glucuronide) (Huang et al., 1998), and organic anion transporting polypeptide-1 or multispecific organic anion transporter K2, previously shown to transport bile acids and drug conjugates (Kullak-Ublick et al., 1995; Bergwerk et al., 1996; Kool et al., 1999; Masuda et al., 1999).
In primary cultures of human hepatocytes, we have previously shown that TRO rather than its metabolites is responsible for cytotoxicity (Kostrubsky et al., 2000). In addition, inhibition of sulfation in these hepatocytes resulted in an accumulation of parent TRO and cytotoxicity (Kostrubsky et al., 2000). One possible explanation for the toxicity observed in humans may include compromised biliary excretion of TRO metabolites, bile acids, and bilirubin. Based on the results presented in this work, it appears that there is a mutual inhibition of biliary transport of TRO-metabolites, bilirubin-glucuronide, and bile acids. Therefore, there is a potential for their liver accumulation, increase in parent TRO, and subsequent clinical signs of cholestasis. However, TR− rats have a compensating mechanism for the lack of Mrp2 by releasing metabolites, bile acids, and bilirubin into the blood via basolateral transport. One of the characterized basolateral transporters, Mrp3, has been reported to be up-regulated in cholestatic human and rat livers (Konig et al., 1999;Ogawa et al., 2000; Soroka et al., 2001). In addition, metabolites still can be secreted into bile either by a different low-affinity canalicular transporter or through nonspecific changes in membrane permeability. We do not know from this study whether the deficiency in Mrp2 was completely compensated for by secreting metabolites and bile acids into the blood and therefore protecting the liver or whether there might be a potential for their liver accumulation. The situation may be exacerbated in individuals with a dual deficiency in transporter activities, one on the canalicular and one on the basolateral site, thus partially preventing efflux of metabolites and bile acids and leading to their accumulation and resultant hepatotoxicity.
In summary, we demonstrated the role of Mrp2 in the biliary secretion of TRO-Gluc and TRO-Sulf and suggested the presence of compensatory mechanisms responsible for transport of troglitazone metabolites and bilirubin-glucuronide at the basolateral and canalicular sites of hepatocytes.
Acknowledgments
We acknowledge Betsy Nieto, Karen Gajda, and Brian Ashton for excellent animal surgery and Ann Fielder for the data analysis. We also thank Dr. Jacqueline Sinclair for help in reviewing the manuscript and Kathryn Perez for great help in preparation of manuscript.
Footnotes
- Abbreviations used are::
- TRO
- troglitazone
- TRO-Sulf
- troglitazone sulfate
- TRO-Gluc
- troglitazone glucuronide
- TRO-Qn
- troglitazone quinone
- Mrp2
- multidrug resistant associated protein-2
- Mrp3
- multidrug resistant associated protein-3
- NR
- normal rats
- TR−
- transporter-deficient rats
- SL
- stable label
- Bsep
- bile salt export pump
- AUC
- area under concentration-time curve
- Received June 11, 2001.
- Accepted September 14, 2001.
- The American Society for Pharmacology and Experimental Therapeutics