Abstract
N-Glucuronidation at an aromatic tertiary amine of 5-membered polyaza ring systems was investigated for a model series of eight 1-substituted imidazoles in liver microsomes from five species. The major objectives were to investigate substrate specificities of the series in human microsomes and interspecies variation for the prototype molecule, 1-phenylimidazole. The formed quaternary ammonium-linked metabolites were characterized by positive ion electrospray mass spectrometry. The incubation conditions for theN-glucuronidation of 1-substituted imidazoles were optimized; where for membrane disrupting agents, alamethicin was more effective than the detergents examined. The need to optimize alamethicin concentration was indicated by 4-fold interspecies variation in optimal concentration and by a change in effect from removal of glucuronidation latency to inhibition on increasing concentration. For the four species with quantifiableN-glucuronidation of 1-phenylimidazole, there were 8- and 18-fold variations in the determined apparentKm (range, 0.63 to 4.8 mM) andVmax (range, 0.08 to 1.4 nmol/min/mg of protein) values, respectively. The apparent clearance values (Vmax/Km) were in the following order: human ≅ guinea pig ≅ rabbit > rat ≅ dog (no metabolite detected). Monophasic kinetics were observed for theN-glucuronidation of seven substrates by human liver microsomes, which suggests that one enzyme is involved in each metabolic catalysis. No N-glucuronidation was observed for the substrate containing the para-phenyl substituent with the largest electron withdrawing effect, 1-(4-nitrophenyl)imidazole. Linear correlation analyses between apparent microsomal kinetics and substrate physicochemical parameters revealed significant correlations between Kmand lipophilicity (πpara or log P values) and betweenVmax/Km and both electronic properties (ςpara value) and pKa.
Numerous drugs and other xenobiotics contain aliphatic and/or aromatic tertiary amine functional groups as a part of their chemical structure. The various metabolic routes at such tertiary nitrogen atoms include glucuronidation by UDP-glucuronosyltransferases (UGTs3) that result in the formation of a polar quaternary ammonium-linked glucuronide (N+-glucuronide) metabolite. Examples of substrates in which glucuronidation occurs in human at an aliphatic tertiary amine group include various H1antihistamine and tricyclic antidepressant drugs (Hawes, 1998; Mey et al., 1999), whereas such examples at an aromatic tertiary amine group include anastrozole (McCann et al., 1997), lamotrigine (Sinz and Remmel, 1991), nicotine (Caldwell et al., 1992), and tioconazole (MacRae et al., 1990). There is a lack of fundamental knowledge regarding such N-glucuronidation, especially at an aromatic tertiary amine with respect to enzyme kinetics, structure-metabolism relationships (SMR), and species differences. Regarding in vitro enzyme kinetic parameters for aromatic tertiary amineN-glucuronidation, there are published reports only in the cases of lamotrigine catalysis by guinea pig and human liver microsomes (Remmel and Sinz, 1991; Magdalou et al., 1992) and nicotine and cotinine catalysis by human liver microsomes (Ghosheh et al., 2001;Ghosheh and Hawes, 2002). Furthermore, all reports involving quantitative techniques in the determination of the physicochemical parameters of substrates important in SMR of glucuronidation concern catalysis at an O-containing functional group (Kim, 1991;Temellini et al., 1991; Holmes et al., 1995). Moreover, there is lack of systematic investigation of species differences inN-glucuronidation at a tertiary amine (Chiu and Huskey, 1998). Some studies have indicated that suchN-glucuronidation is only significant in primates (Hucker et al., 1978; Fischer et al., 1980). However, evidence has accumulated for guinea pigs and rabbits thatN+-glucuronides are formed substantially as urinary (Beckett and Ali, 1978; Le Bigot et al., 1987;Remmel and Sinz, 1991) and hepatic in vitro (Lehman et al., 1983;Coughtrie and Sharp, 1991; Remmel and Sinz, 1991; Magdalou et al., 1992; Styczynski et al., 1992; Bruck et al., 1997; Li et al., 2001) metabolites of certain substrates. There are few reports known to the authors about the formation of such metabolites in rats, and all involve metabolism at an aromatic tertiary amine (Bieder et al., 1983;MacRae et al., 1990; Magdalou et al., 1992; Seaton et al., 1993;Singh et al., 2001). For none of the substrates undergoingN-glucuronidation at a tertiary amine have the kinetic parameters for hepatic microsomes been compared with respect to species differences.
To investigate these various aspects of N-glucuronidation at an aromatic tertiary amine, a series of 1-substituted imidazoles 1 was selected for study (Fig.1). The reasons for selecting the series 1 imidazoles as model substrates included the following. First, many recently introduced drugs and also many drugs under development contain a 5-membered aromatic polyaza ring system. Two types of N-glucuronide are documented for this type of ring system, including imidazoles;N+-glucuronides formed at an aromatic tertiary amine of N-substituted compounds (Takeuchi et al., 1989; MacRae et al., 1990; Rush et al., 1992; McCann et al., 1997) and tertiary amine N-glucuronides formed at theN-H of compounds lacking N-substitution (Stearns et al., 1991; Colletti and Krieter, 1994; Huskey et al., 1994; Perrier et al., 1994; Kuo et al., 1999; Stevens et al., 2001). Second, 1-substituted imidazoles are a model system for more complex 5-membered aza heterocycles in having only one aliphatic-like tertiary amine and one aromatic-type tertiary amine at the 1- and 3-positions, respectively, of the heterocyclic ring. Moreover, a wide range of 1-substituted substrates are commercially available with imidazoles, but not polyaza systems such as triazoles or tetrazoles, that enable investigations of SMR correlations involving electronic and lipophilic parameters. In this regard, substrates 1a-c differ with respect to lipophilicity, whereas substrates 1a and 1d-h differ in their electronic (ςpara, ς value for the substituent in the para position of the phenyl ring) and lipophilic (log P; or πpara, π value for the substituent in the para position of the phenyl ring) properties (Kubinyi, 1995). Also, study of substrates 2 and 3 that have a bulky phenyl group adjacent to an imidazole nitrogen atom at the 2- and 4-position, respectively, enables investigation of the role of steric factors in N-glucuronidation. Finally, in previous work we demonstrated that the prototype molecule 1-phenylimidazole ( 1a ) formed theN+-glucuronide at the 3-position in human liver microsomes and that of nine UGT-expressed isoforms investigated only UGT1A4 catalyzed glucuronidation of 1a-1g , whereas only 1a was conjugated by both UGT1A3 and UGT1A4 (Vashishtha et al., 2000, 2001).
Chemical structures of 1-substituted- (1), 2-phenyl- (2), and 4-phenyl- (3) imidazole substrates and the site of N-glucuronidation.
The major objectives of this study were to 1) investigate interspecies differences in the catalysis of the N-glucuronidation of the prototype substrate, 1-phenylimidazole 1a in liver microsomes of dog, guinea pig, human, rabbit, and rat, including in Michaelis-Menten kinetic constants (Kmand Vmax); 2) determine kinetic parameters for the N-glucuronidation of the series 1 compounds in the liver microsomal fraction of human; and 3) determine the relative importance of various physicochemical parameters, namely, pKa, electronic (ςpara), and lipophilic (log P; or πpara) properties in the N-glucuronidation of the series 1 compounds in human liver microsomes.
Materials and Methods
Chemicals.
1-Phenylethylimidazole ( 1c ) and 1-phenylimidazoleN+-glucuronide (Vashishtha et al., 2000) were synthesized by literature procedures. 1-Benzylimidazole ( 1b ), 4-phenylimidazole ( 3 ) (Lancaster Synthesis Inc., Windham, NH), 1-phenylimidazole ( 1a ), 1-(4-methoxyphenyl)imidazole ( 1d ), 1-(4-chlorophenyl)imidazole ( 1e ), 1-(4-bromophenyl)imidazole ( 1f ), 1-(4-trifluoromethylphenyl)imidazole ( 1g ), 1-(4-nitrophenyl)imidazole ( 1h ) (Transworld Chemicals, Rockville, MD), and perchloric acid (BDH Chemicals, Toronto, ON) were obtained from various commercial sources. 2-Phenylimidazole ( 2 ), methimazole, brij-58, CHAPS, Lubrol PX, Triton X-100, Tris base, alamethicin, ethyl 2-pyridylacetate, UDP-glucuronic acid (UDPGA) (ammonium salt), magnesium chloride,d-saccharic acid 1,4-lactone, and β-glucuronidase from Helix pomatia (Type H-2, 100,000 U/ml at pH 5.0) were purchased from Sigma-Aldrich (St. Louis, MO). Emulgen 911 was a gift from Kao Corporation (Tokyo, Japan). While all the chemicals were of reagent grade, the organic solvents (EM Science, Gibbstown, NJ) were of HPLC grade. Double distilled water (18 ± 0.05 Ω cm) deionized and purified by a Milli-QTM Water system was used (Millipore Corporation, Bedford, MA). HPLC mobile phase solvents were filtered through Millipore 0.45-μm filters prior to use.
Preparation of Liver Microsomes from Dog, Guinea Pig, Human, Rabbit, and Rat.
Livers of untreated, male dog (n = 1, 8.8 kg, beagle), guinea pig (n = 3, 350–400 g, Dunkin-Hartley), rabbit (n = 1, 2.9 kg, New Zealand white), and rat (n = 5, 220–250 g, Sprague-Dawley; Charles River Laboratories, Inc., Wilmington, MA) were removed by surgery, frozen in liquid nitrogen immediately after removal from the animals, and subsequently processed as microsomes. Human livers (white; 2 female and 4 male) were obtained from the International Institute for the Advancement of Medicine (Exton, PA). Microsomes were prepared from pooled livers of each species (equal weight taken from each liver) and the protein content of microsomal suspensions was determined as previously indicated (Vashishtha et al., 2000). Each frozen liver tissue (5 g) was cut in small pieces and homogenized in 100 mM Tris-HCl, pH 7.4, buffer and then was diluted to 4 volumes of sample weight. The samples were then centrifuged at 9,000g for 20 min. The supernatant from the first centrifugation was removed and centrifuged at 100,000g for 60 min. The microsomal pellet was suspended in potassium phosphate (100 mM, pH 7.4) containing EDTA (1 mM) and potassium chloride (0.15 M), and centrifuged again at 100,000g for 60 min. The pellet was resuspended in potassium phosphate (100 mM, pH 7.4), containing sucrose (0.25 M) and stored in aliquots at −80°C. The viability for glucuronidation of each microsomal preparation was demonstrated with the probe substrate 4-nitrophenol.
Biosynthesis of N-Glucuronides of Substituted Imidazoles Using Human Liver Microsomes.
Except for the previously reported 1-phenylimidazoleN+-glucuronide (Vashishtha et al., 2000), an attempt was made to biosynthesize theN-glucuronide of each substrate, 1b-h , 2 , and 3 , by the use of human liver microsomes. The reaction mixture (500 μl) consisting of MgCl2 (10 mM), alamethicin (25 μg/mg of protein), UDPGA (5 mM), human liver microsomes (2 mg), Tris buffer (50 mM, pH 7.4), and the substrate (1.25 mM) was incubated for 120 min at 37°C. The protein was precipitated by adding aqueous perchloric acid (1%, 500 μl), except in the cases of 2-phenylimidazole and 4-phenylimidazole, in which acetonitrile (500 μl) was added. The microsomal mixture was centrifuged at 9,000g for 10 min. The supernatant was loaded on a solid phase extraction C-18 cartridge (100 mg, SEP-PAK C18; Waters Corp., Milford, MA), which had been conditioned by passing ether (3 ml), methanol (3 ml), and water (9 ml). The loaded cartridge was washed with water (3 ml), ether (2 ml), and dried by passing air. The glucuronide metabolite was eluted using methanol (1 ml) and subsequently analyzed by both gradient HPLC-UV and electrospray ionization-mass spectrometry (ESI-MS).
HPLC Analysis.
For the quantification of the N-glucuronides of 1 substrates, the compositions of the mobile phase consisting of solvent A (10 mM perchloric acid, pH 2.5) and solvent B (acetonitrile), and the wavelength of maximal absorption were as previously described (Vashishtha et al., 2001). The amount of N-glucuronide metabolite formed (nanomoles per minute per milligram of protein) was calculated based on the ratio of the peak areas of the metabolite and an external standard. A UV absorption study of 1a and its metabolite, 1-phenylimidazoleN+-glucuronide, revealed their extinction coefficients to be very similar. Therefore, the glucuronides of substrates other than 1a (in which a pure synthetic sample of metabolite was employed) were quantified using standard curves made from standard solutions of the substrates. Note that, unlike in the previous report (Vashishtha et al., 2001), satisfactory chromatographic separation and quantification of theN-glucuronide metabolite of the phenylethyl compound 1c was achieved in the present study.
Identification of N-Glucuronide Metabolites by Mass Spectrometry.
In the first series of studies, the methanolic eluants obtained from work-up of the biosynthesis mixtures were examined by HPLC-UV analysis. For series 1 compounds the chromatography conditions were similar to those mentioned above. For compounds 2 and 3 , in vitro samples were analyzed by mass spectrometry only. Incubations were performed both with and without UDPGA, and β-glucuronidase treatment of incubated mixtures was also studied. In the latter case, to the incubated mixture (500 μl, as in biosynthesis mixtures, but using phosphate buffer, 100 mM, pH 7.4), H. pomatia (2000 U) as enzyme source was added and the mixture left for 24 h prior to the usual work-up.
In the second series of studies, the extracts of the biosynthetic mixtures (dissolved in 50% aqueous methanol) were injected into a Micromass Quattro II Triple Quadrupole mass spectrometer (Waters/Micromass Ltd., Manchester, UK) operated in the positive ion mode under ESI conditions. The instrumental conditions included electrospray needle voltage at +45 V, temperature of the heated transfer capillary at 120°C, and nitrogen as the sheath gas. Spectra were acquired over the mass range 100 to 600 in 2 s. The acquisition time was 1 min, and the recorded spectra were averaged.
Finally, in the cases of the N-glucuronide metabolites of 1-phenyl ( 1a ) and 4-phenylimidazole ( 3 ) further mass spectrometric investigations were performed with a view to making comparison of MS behavior. This work was conducted using a ThermoQuest Finnigan LCQ Deca ion-trap mass spectrometer (Thermo Finnigan, San Jose, CA). The entire system was controlled by Finnigan Xcaliber software (version 1.0), which also was used for data acquisition and processing. The instrument was operated in both positive and negative ion modes using ESI or atmospheric pressure chemical ionization (APCI). For all of the experiments involving ESI, the capillary was heated to 250°C, and the spray voltage was maintained at 5 kV. The sheath gas (N2) flow was 80 arbitrary units, and the auxiliary gas (N2) flow was 20 arbitrary units. APCI was conducted at a vaporizer temperature of 450°C, capillary temperature of 200°C, discharge voltage at 4.5 kV, and sheath and auxiliary gas (N2) flows at 60 and 0 arbitrary units, respectively. The instrument was tuned onm/z 143 ([M − H]−) or m/z 145 ([M + H]+) for negative and positive ion modes, respectively. All voltages and offsets were determined by auto-tuned software. Extracts of the biosynthesized mixtures (dissolved in 50% aqueous methanol) were infused into the source by a syringe pump at 10 μl/min flow rate.
N-Glucuronidation of 1-Substituted Imidazoles by Liver Microsomes.
Initially, the incubation conditions were optimized with respect to pH, latency disrupting agent, and time of incubation and protein concentration required to give linear rate of formation of the glucuronide in the liver microsomes of guinea pig, human, rabbit, and rat using the 1-phenyl compound 1a as a prototypic substrate. In the case of dog liver microsomes, noN-glucuronide formation was detected. The effect of pH on the rate of glucuronidation was examined in the range of 6.0 to 9.0. The time of incubation was varied from 15 to 120 min, whereas the protein concentration range examined was 0.25 to 2 mg/ml. All the latency disrupting agents, both pore-forming agent (alamethicin) and detergents (brij-58, CHAPS, Emulgen 911, Lubrol PX, and Triton X-100) were examined initially at the same two concentrations (25 and 100 μg/mg of protein). Since invariably alamethicin gave the highest turnover rate, its concentration was optimized by examining 0, 3.0, 6.25, 12.5, 25, 50, 100, and 200 μg/mg of protein. Note that the latency disrupting agent was always dissolved in the same volume of 75% aqueous methanol, namely 2 μl per 200 μl of incubation mixture.
In a similar manner, the incubation conditions (pH, alamethicin concentration, time of incubation, and protein concentration) were also optimized for the other series 1 substrates in human liver microsomes. All experiments were carried out in triplicate.
Where N-glucuronidation occurred, kinetic parameters were determined for 1a for all species but only for human in the case of the other series 1 substrates. The incubation conditions employed for human microsomes were as follows. The mixture (200 μl) consisting of MgCl2 (10 mM), saccharic acid lactone (3 mM), alamethicin (25 to 50 μg/mg of protein depending upon the substrate), UDPGA (5 mM), human liver microsomes (0.5 mg), Tris buffer (50 mM, pH 7.4), and the substrate (variable concentrations) in 2.5 μl of methanol, was incubated for 60 min at 37°C. Various control incubations were run such as to verify that the methanol used had no effect on the N-glucuronidation reaction. The reaction was stopped by incubating at 4°C with 190 μl of perchloric acid (1%). After adding external standard (10 μl; methimazole, except ethyl 2-pyridylacetate in the case of 1a and 1e in the case of 1g ), the microsomal mixture was centrifuged at 9,000g for 15 min. The supernatant (100 μl) was directly injected into the HPLC system. For kinetic studies, all analyses were performed three times, each in duplicate. The kinetic parameters were determined from the data obtained by the incubation of 8 to 10 different concentrations of the various substrates.
Calculations.
Vmax andKm values were calculated from kinetic data according to the Michaelis-Menten equations for one- and two-enzyme kinetics by nonlinear least-squares regression analysis (GraphPad Software Inc., San Diego, CA).Vmax/Kmratios were determined as a rough estimate of intrinsic clearance. Data are given as mean ± S.D. Linear correlation analyses between kinetic and physicochemical parameters were examined in which Hammett sigma values (ςpara) and pi values (πpara) for the para substituent of the phenyl ring of the 1-phenyl substituted compounds 1a and 1d-1h were from a standard text (Kubinyi, 1995), and partition coefficient (log P, n-octanol/water) and pKa were calculated with the program ACD (1995; Advanced Chemistry Development Inc., Toronto, ON).
Results
Identification of N-Glucuronide Metabolites.
Except for the 1-phenyl compound ( 1a ), the metabolite of which had been previously reported (Vashishtha et al., 2000), initially each substrate was investigated with respect to formation of aN-glucuronide metabolite by human liver microsomes. The solid phase extracts of the incubations of 1b-1h , 2 , and 3 were examined by HPLC-UV analysis. Except in the case of the 1-(4-nitrophenyl) ( 1h ) and 2-phenyl ( 2 ) compounds, an additional peak was observed in the HPLC chromatograms. That these chromatographic peaks were due to glucuronide metabolites was indicated by both the absence of the peak when UDPGA was omitted from the incubations and the large reduction in peak area when the incubate was subsequently treated withβ-glucuronidase. In the seven cases ( 1b-1g and 3 ) in which glucuronidation was indicated, positive ion ESI-MS analysis was performed on the extract. For the 1-substituted compounds 1b-1g a pseudo molecular ion peak (M+) corresponding to the quaternary ammonium-linked glucuronide metabolite of each substrate was observed at the following m/z ratios: 335 ( 1b ), 349 ( 1c ), 351( 1d ), 355 ( 1e ), 399 ( 1f ), and 389 ( 1g ). The identity of the molecular ion peak was further confirmed by the daughter ion spectrum, which gave only a peak at the molecular mass of the substrate, indicative of the occurrence of the characteristic cleavage of the glycosidic bond (M-176)+ with transfer of a proton from the glucuronic acid moiety to the aglycone. However, the positive ion ESI mass spectral characteristics of the N-glucuronide of the 4-phenyl compound ( 3 ) were indistinguishable from those of the 1-phenyl ( 1a )N+-glucuronide metabolite (Vashishtha et al., 2000) in that a molecular ion (albeit weak) was observed at m/z 321 and only a (M-176)+ ion occurred in the daughter ion spectrum, respectively coinciding with the molecular cation and daughter ion spectrum of 1a . That the metabolite of 3 was a tertiary amine N-glucuronide was established by further MS studies involving ESI and APCI. First, a major difference occurred with negative ion ESI-MS. Thus whereas, because of the inherent positive charge, a spectrum could not be obtained for the quaternary ammonium glucuronide of 1a , the spectrum of 3 glucuronide displayed a [M-1]− ion at m/z 319. Also, in turn the resulting daughter ion spectrum was essentially limited to the (M-176)− ion peak atm/z 143, indicative of the occurrence of the characteristic cleavage of the glycosidic bond. Second, the glucuronides of 1a and 3 could be distinguished by APCI-MS. In the case of the glucuronides of 1a and other 1 compounds, despite much diverse exploration of instrumental conditions, no spectrum could be obtained with the instrument set with the inherent corona discharge of the technique. In contrast, 3 glucuronide gave both [M + 1]+ (m/z 321) and [M-1]− (m/z 319) ions under APCI-MS positive and negative ion modes, respectively, which in turn gave daughter ion spectra with peaks at m/z145 and 143, respectively indicative of (M-176)+and (M-176)− glycosidic bond cleavage. However, it was not possible to unequivocally assign by MS whether glucuronidation occurred at the N-1 or N-3 position of 3 .
Optimization of Incubation Conditions.
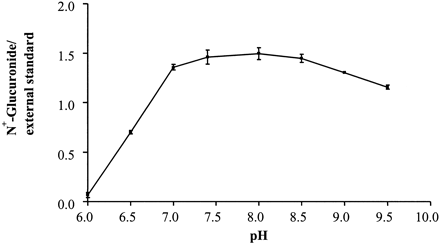
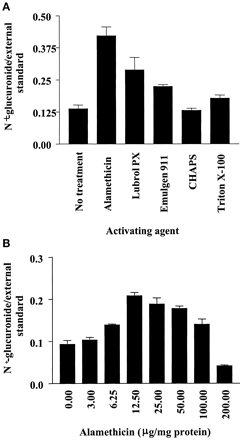
Except for dog, in which no metabolite was detected, the incubation conditions for the N-glucuronidation of the series 1 compounds by liver microsomes of various species were optimized. The optimum pH for the various species was found to either peak at pH 7.4 or to plateau in the pH 7.0 to 8.5 range, as shown for 1a and human liver microsomes (Fig.2). Initial investigation of latency disrupting agents at 25 and 100 μg/mg of human liver protein indicated that at both concentrations alamethicin was more effective than the five detergents examined (brij-58, CHAPS, Emulgen 911, Lubrol PX, and Triton X-100), as shown in Fig.3a for the 100 μg/mg concentration for all but the separately examined brij-58. In the case of brij-58 at 25 and 100 μg/mg of protein 1a glucuronide formation was 116 and 210% of control values, respectively. More detailed study of alamethicin, as shown for activation of human liver microsomes in the catalysis of 1a glucuronidation (Fig. 3b), indicated that the concentration which gave observed optimum activity was 12.5, 25, and 50 μg/mg of protein for human, guinea pig and rabbit, and rat, respectively. At this optimal concentration, the activation by alamethicin was 7-fold for the microsomes of guinea pig but only 2.5- to 3.5-fold for those of other species. The rate ofN-glucuronidation was linear up to 60 min in the cases of human and rabbit and up to 90 min in the cases of guinea pig and rat. Regarding liver protein concentration, the rate of glucuronidation was linear up to 1.5 mg/ml in the cases of guinea pig and rabbit, whereas for human and rat, this value was 2.0 and 1.0 mg/ml, respectively.
Effect of pH on the rate of N-glucuronidation of 1-phenylimidazole by human liver microsomes (n = 3).
Top, effect of various latency disrupting agents at 100 μg/mg of protein concentration on the rate of N-glucuronidation of 1-phenylimidazole by human liver microsomes (n = 3); bottom, effect of alamethicin at various concentrations on the rate of N-glucuronidation of 1-phenylimidazole by human liver microsomes (n = 3).
Species Differences in 1-PhenylimidazoleN-Glucuronidation.
The kinetic parameters for the N-glucuronidation of the 1-phenyl compound ( 1a ) were determined in the liver microsomes of the various species under the respective optimized conditions (Table 1). There were 8-, 18-, and 10-fold variations in the determined apparentKm (range, 0.63 to 4.8 mM),Vmax (range, 0.08 to 1.4 nmol/min/mg of protein), andVmax/Km(0.03 to 0.29 μl/min/mg of protein), respectively, for the four species in which metabolism was detected. When comparison is made inVmax values, the interspeciesN-glucuronidation activity is in the following order: rabbit > human > guinea pig > rat > dog (no observed activity). However, when comparison is made of apparent intrinsic clearance values (Vmax/Km), guinea pig, human, and rabbit are similar, with values 6- to 10-fold higher than in the rat.
Apparent kinetic parameters of 1-phenylimidazole N+-glucuronide formation in pooled liver microsomes from various species
Kinetic Parameters for the N-Glucuronidation of 1-Substituted Imidazoles by Human Liver Microsomes.
The kinetic parameters for the formation of theN-glucuronides of the seven series
1
compounds were determined for the same pooled human liver microsomes under optimized conditions. These parameters, obtained by nonlinear regression analysis of the rate of metabolite formation under optimized conditions versus substrate concentration are shown in Table2. The apparentKm,Vmax, andVmax/Kmparameters varied (range) 19- (0.16 to 3.1 mM), 11- (0.08 to 0.89 nmol/min/mg of protein), and 5-fold (0.11 to 0.56 μl/min/mg of protein), respectively. Linear correlation analyses between these three apparent kinetic parameters and either pKa, electronic properties (ςpara value), or lipophilicity (πpara or log P values) revealed various significant relationships, as expressed by the following equations: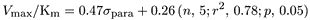
 where n is the number of substrates with available data, r2 is the correlation coefficient, and p is the statistical probability.
where n is the number of substrates with available data, r2 is the correlation coefficient, and p is the statistical probability.
Apparent kinetic parameters for the N-glucuronidation of 1-substituted imidazole substrates by pooled human liver microsomes
Discussion
The formation of a glucuronide metabolite in liver microsomal incubations of 1-substituted imidazoles 1b-1g was indicated by the dependence on the addition of UDPGA to the incubation mixture and by the susceptibility of the metabolite to β-glucuronidase treatment. Structural identification of each N-glucuronide metabolite was enabled by MS. Thus the positive ion ESI-MS spectrum of each 1b-1g N-glucuronide showed an abundant ion indicative of the preformed molecular cation, and the corresponding daughter ion spectrum showed that the protonated aglycone arose from the molecular cation. In each case, the imidazole 3N-atom was likely the site of glucuronidation as previously proven in the case of the prototype compound 1a , where a synthetic sample of the N+-glucuronide metabolite was available (Vashishtha et al., 2000). Regarding the 4-phenyl substituted compound 3 , there is complication in proving by soft ionization MS techniques whether glucuronidation occurs at a cyclic secondary or a tertiary amine group. This complication arises because under the commonly employed positive ion ESI-MS condition, the ion corresponding to the protonated tertiary amine glucuronide and the molecular cation of a N+-glucuronide appears at the same m/z value, and the subsequently derived daughter ion spectra are similar. The two types ofN-glucuronide metabolites can be distinguished by negative ion ESI-MS and APCI-MS. In the former case, whereas for aN+-glucuronide metabolite, such as 1a glucuronide, the unavoidable characteristic positive charge renders the technique impractical, a molecular negative ion is feasible for a tertiary amine glucuronide, such as 3 glucuronide, thus indicating that metabolism occurred at a cyclicN-H group. Furthermore, the use of the same MS instrument under APCI conditions can give confirmation regarding the amine functionality at which glucuronidation occurred. In the case of 1 N+-glucuronides, a molecular cation was detected only with the instrument set without the corona discharge that is inherent to the APCI-MS technique. In contrast, for the tertiary amine glucuronide of 3 , appropriate APCI-MS positive and negative ion spectra, and corresponding confirmatory daughter ion spectra were obtained. Nevertheless, it was not possible to unequivocally assign the site of glucuronidation of 3 , although it likely occurs atN1 due to the steric hindrance of the 4-phenyl group atN3. These observations regarding the site ofN-glucuronidation of the imidazoles 1 and 3 (including the structural isomers 1a and 3 ), at a tertiary and secondary amine, respectively, lend support to the generalization that glucuronidation of a 5-membered aromatic polyaza ring system occurs at a tertiary amine forN-substituted compounds (Takeuchi et al., 1989; MacRae et al., 1990; Rush et al., 1992; McCann et al., 1997), but at a secondary amine for compounds lacking a ring N-substituent (Stearns et al., 1991; Colletti and Krieter, 1994; Huskey et al., 1994; Perrier et al., 1994; Kuo et al., 1999; Stevens et al., 2001).
In optimizing the incubation conditions for glucuronidation, two important aspects are the incubation pH and diminishing the latency of enzyme activity due to the lumenal localization of the UGT active site. Investigation of 1a in liver microsomes of various species and 1a-1g in human liver microsomes indicated that, as in the previous study of the same compounds by expressed human UGT1A4 (Vashishtha et al., 2001), a pH of 7.4 was appropriate for the subsequent studies as glucuronidation was optimal either at this pH or over a pH range in which this value was at the low end of the plateau. Optimal glucuronidation of tertiary amines in human liver microsomes at pH 7 or higher is consistent with previous observations (Coughtrie and Sharp, 1991; Styczynski et al., 1992; Mey et al., 1999; Ghosheh et al., 2001). Regarding the reduction of latency to glucuronidation, the pore-forming peptide alamethicin was found to be more effective than five detergents at the two concentrations examined. In view of this observation, notwithstanding the evidence that alamethicin lacks the artifactual effects of detergents on enzyme activity (Fisher et al., 2000), alamethicin was chosen for further study. It exhibited interspecies differences with respect to both the optimal concentration and the extent of optimal effect, and on increasing its concentration, the augmentation of enzyme activity was replaced by inhibition. Therefore, it is important to optimize the alamethicin concentration according to the incubation conditions as has been shown for detergents (Styczynski et al., 1992).
Although the study of interspecies differences was limited to a microsomal sample for each species and one substrate, it is the most thorough to date with respect to the determination of hepatic microsomal kinetic parameters for glucuronidation at an aromatic tertiary amine. Large interspecies differences were observed between guinea pig, human, and rabbit in Kmand Vmax values for formation of 1a N+-glucuronide, 8- and 12-fold, respectively. However, that there was only 1.5-fold difference observed in the resultantVmax/Kmratios lends support to previous use of the guinea pig and rabbit in the study of N-glucuronidation at a tertiary amine (Beckett and Ali, 1978; Lehman et al., 1983; Le Bigot et al., 1987; Coughtrie and Sharp, 1991; Remmel and Sinz, 1991; Magdalou et al., 1992;Styczynski et al., 1992; Bruck et al., 1997; Li et al., 2001). Regarding rat, the in vitro kinetics for glucuronidation at a tertiary amine of a substrate was determined for the first time. The observed minimal formation, at most, of 1a N+-glucuronide for both rat and dog microsomes was consistent with previous studies of various substrates in which the extent ofN+-glucuronide formation was much less than in other species, including human (Beckett and Ali, 1978; Hucker et al., 1978; Le Bigot et al., 1987; Magdalou et al., 1992; Li et al., 2001; Soars et al., 2001). For the most part, the UGTs involved in the observed catalyzes in animals are not resolved. For example, in the case of rat, UGT1A3 is expressed weakly in regions of the gastrointestinal tract but not liver, whereas UGT1A4 is a pseudogene (Green and Tephly, 1998; Grams et al., 2000). Thus in view of the current incomplete knowledge of the UGTs in the various animal species examined, it is not feasible to rationalize these kinetic data in terms of species differences in UGTs (Bruck et al., 1997; Green and Tephly, 1998; Soars et al., 2001).
The monophasic kinetics observed for the N-glucuronidation by human liver microsomes of all seven series 1 substrates examined suggests that one enzyme is involved. In fact, of nine expressed human UGTs examined in a previous study, only UGT1A4 catalyzed the N-glucuronidation of each substrate 1b-1g . In the case of 1a , although both UGT1A3 and UGT1A4 catalyzed the N-glucuronidation with similar catalytic reactivity (Vashishtha et al., 2000), the low expression of UGT1A3 in liver has led to the suggestion that, in general, this isoform may play an insignificant role in hepaticN-glucuronidation (Green and Tephly, 1998). The presently observed apparent Km (range, 0.2 to 3.1 mM) and Vmax (range, 0.1 to 0.9 nmol/min/mg of protein) values for 1a-1g are comparable to those reported from various laboratories for the monophasic glucuronidation kinetics by liver microsomes of the aromatic tertiary amine of lamotrigine (Magdalou et al., 1992), nicotine, and cotinine (Ghosheh et al., 2001; Ghosheh and Hawes, 2002) and the aliphatic tertiary amine of various substrates (Styczynski et al., 1992; Soars et al., 2001). Finally, although for individual substrates there was not close agreement in all cases with respect to the corresponding value determined for expressed UGT1A4, the range of apparentKm values for 1a-1g was similar to the corresponding range (0.2 to 3.2 mM) obtained for the expressed enzyme (Vashishtha et al., 2001).
The major limitation of the present SMR study was that a relatively small number of substrates were investigated. For investigating correlations involving structural variation of the para substituent of the phenyl ring (ςpara and πpara) and more gross structural variation (log P and pKa), only six and eight substrates, respectively, were studied. Also in each case data were not available for one of these compounds, as glucuronidation was not observed. Nevertheless, significant correlations were observed between the kinetic parameters and all four physicochemical parameters examined; specifically betweenKm and both πpara and log P, and betweenVmax/Kmand both ςpara and pKa. That correlation was found between a kinetic parameter and two different measures of lipophilicity is reassuring. Also for the same series of compounds, a correlation has been previously found between log P and the kinetics for expressed human UGT1A4. However, in this case, the correlation was with Vmax and notKm (Vashishtha et al., 2001). Also previous correlation analyses of data from various studies of theO-glucuronidation of various series of phenolic substrates by rat liver microsomal preparations indicated significant correlation between reaction velocity and log P. For all but one of these series, the correlation comprised a parabolic relationship with an optimum lipophilicity of log 2 (Kim, 1991). In the present study, it was observed that the greater the lipophilicity of the substrate, as indicated by log P or πpara, the greater the enzyme affinity, but the limited number and range of values did not allow investigation of optimum values. These studies indicate that the lipophilic properties of the molecule play an important role regarding the enzyme kinetics of glucuronidation. Also, that electronic parameters play a part in these regards was indicated by the positive correlation between ςpara and apparent intrinsic clearance. Moreover, that the availability of the lone pair of electrons of the tertiary amine at the site of glucuronidation relates to the rate of metabolic reaction is indicated by the inverse correlation between pKa andVmax/Km. Also, that there is a range of pKa values in which glucuronidation occurs is indicated by the lack of detection of aN+glucuronide metabolite for the 4-nitro compound 1h , the series 1 compound with the largest electron withdrawing effect and hence lowest pKa.
In summary, the N-glucuronidation was studied for 2-phenyl-, 4-phenyl-, and a series of 1-substituted imidazoles. Unlike its structural isomers, N-glucuronidation was not detected for 2-phenylimidazole, likely due to steric factors. 4-Phenylimidazole formed a tertiary amine glucuronide, which could be distinguished from the N+-glucuronide of 1-phenylimidazole by use of ESI- and APCI-MS techniques. This MS approach to distinguish these two types of N-glucuronides is of potential general applicability. Comparison of the apparent intrinsic clearance values of 1-phenylimidazole byN-glucuronidation indicated that guinea pig and rabbit were comparable to human, but that metabolism was minimal at most for dog and rat. For the seven substrates in which anN+-glucuronide metabolite was formed by human liver microsomes, there were significant SMR correlations between lipophilicity, electronic parameters, and pKa, and the kinetic parameters.
Footnotes
-
↵1 Current address: Wyeth Research, 500 Arcola Road, Collegeville, PA 19426-3930.
-
↵2 Current address: Lilly Research Laboratories, Lilly Corporate Center, Indianapolis, IN 46285.
-
This work was supported by an AstraZeneca academic grant (to E.M.H. and D.J.M.), a Canadian Institutes of Health Research operating grant (MOP-36513 to E.M.H.), and a Health Services Utilization and Research Commission of Saskatchewan Research Fellowship (to O.G.).
- Abbreviations used are::
- UGT
- UDP-glucuronosyltransferase
- N+-glucuronide
- quaternary ammonium-linked glucuronide metabolite
- SMR
- structure-metabolism relationships
- CHAPS
- 3-[(3-cholamidopropyl)dimethylamino]-1-propanesulfonate
- UDPGA
- UDP-glucuronic acid
- HPLC
- high-performance liquid chromatography
- ESI
- electrospray ionization
- MS
- mass spectrometry
- APCI
- atmospheric pressure chemical ionization
- Received February 15, 2002.
- Accepted June 19, 2002.
- The American Society for Pharmacology and Experimental Therapeutics