Abstract
2-Amino-3,8-dimethylimidazo-[4,5-f]quinoxaline (MeIQx) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) are suspected human carcinogens generated in well done meats. After N-hydroxylation, they are O-acetylated by N-acetyltransferase 2 (NAT2) to electrophiles that form DNA adducts. dG-C8-MeIQx and dG-C8-PhIP adducts have been identified in human tissues. In the female rat, administration of PhIP leads to mammary and colon tumors, whereas MeIQx induces liver tumors. Both humans and rats exhibit NAT2 genetic polymorphism yielding rapid and slow acetylator phenotypes. Because O-acetylation is an activation pathway, we hypothesized that MeIQx- and PhIP-induced DNA damage would be greater in tumor target tissues and higher in rapid than slow NAT2 acetylators. Adult female rapid and slow acetylator rats congenic at the Nat2 locus received a single dose of 25 mg/kg MeIQx or 50 mg/kg PhIP by gavage, and tissue DNA was isolated after 24 h. Deoxyribonucleoside adducts were identified and quantified by capillary liquid chromatography-tandem mass spectrometry using isotope dilution methods with deuterated internal standards. Major adducts were those bound to the C8 position of deoxyguanosine. dG-C8-PhIP DNA adducts were highest in colon, lowest in liver and did not significantly differ between rapid and slow acetylator congenic rats in any tissue tested. In contrast, dG-C8-MeIQx adducts were highest in liver and significantly (p < 0.001) higher in rapid acetylator liver than in slow acetylator liver. Our results are consistent with the tumor target specificity of PhIP and MeIQx and with increased susceptibility to MeIQx-induced liver tumors in rapid NAT2 acetylators.
- MeIQx, 2-amino-3,8-dimethylimidazo-[4,5-f]quinoxaline
- PhIP, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- NAT2, N-acetyltransferase 2
- dG, deoxyguanosine
- SNP, single nucleotide polymorphism
- LC-MS/MS, capillary liquid chromatography-tandem mass spectrometry
- CHO, Chinese hamster ovary.
2-Amino-3,8-dimethylimidazo-[4,5-f]quinoxaline (MeIQx) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) are potent and abundant mutagens in the human diet, formed during high temperature cooking of meats (Keating and Bogen, 2004). They also have been detected in processed food flavorings, beer, wine, cigarette smoke, smoke condensate formed during frying of beef patties and bacon, and in aerosol from cooking of stir-fried fish (National Toxicology Program, 2005). PhIP has been detected in airborne particles, diesel-exhaust particles, and incineration ash from garbage-burning plants (Manabe et al., 1993).
Both MeIQx and PhIP induce tumors in the rat (Sugimura et al., 2004) and are designated as “reasonably anticipated to be a human carcinogen” (National Toxicology Program, 2005). MeIQx induces liver tumors in mice (Ohgaki et al., 1987) and up to 100% incidence of hepatic tumors in rats (Kato et al., 1988; Kushida et al., 1994). PhIP target organ specificity differs from MeIQx in the rat because it induces tumors in colon, prostate, and mammary tissue (Sugimura et al., 2004).
MeIQx- and PhIP-induced DNA adduct formation and carcinogenesis require N-hydroxylation, which occurs at relatively high rates in humans (Stillwell et al., 1999; Turesky 2002). N-Hydroxy-MeIQx and -PhIP are further O-acetylated by N-acetyltransferase 2 (NAT2) to acetoxy derivatives that are highly unstable, leading to electrophilic intermediates that form DNA adducts (Schut and Snyderwine, 1999). As reviewed previously (Turesky, 2002), glutathione S-transferases (Lin et al., 1994), sulfotransferases (Wu et al., 2000), and/or glucuronosyltransferases (Malfatti et al., 2005) also are important in the metabolic activation and deactivation of heterocyclic amine carcinogens, and differences in any of the metabolic pathways between organs and tissues could account for differences in DNA adduct formation and tumor incidence. PhIP and MeIQx are activated to electrophilic intermediates that form DNA adducts primarily at deoxyguanosine (dG) (Turesky, 2002). dG-C8-PhIP and dG-C8-MeIQx adducts have been identified in human tissues and cells (Totsuka et al., 1996; Gorlewska-Roberts et al., 2002).
Both humans and rats exhibit NAT2 genetic polymorphism yielding rapid and slow acetylator phenotypes (reviewed in Boukouvala and Fakis, 2005). Slow acetylator phenotype in both humans and rats results from nonsynonymous single nucleotide polymorphisms (SNPs) in the NAT2 coding region. Slow acetylator WKY inbred rats are homozygous for a rat Nat2 allele with four SNPs: G361A (Val121→Ile), G399A (synonymous), G522A (synonymous), and G796A (Val266→Ile), compared to the Nat2 allele in the F344 rapid acetylator inbred rat (Doll and Hein 1995). Because F344 and WKY inbred rats differ in genes other than NAT2, most likely including cytochrome P450s, glutathione S-transferases, sulfotransferases, glucuronyltransferase, and other enzymes important in the metabolism of heterocyclic amine carcinogens, we constructed congenic rat lines that are isogenic except for the Nat2 locus and very closely aligned loci (Hein et al., 2008). Thus, the congenic lines do not differ in these other metabolic pathways nor in other pathways such as DNA repair that might mask the effects of NAT2 phenotype on DNA adduct levels. Another advantage of the congenic rat lines is that the slow NAT2 acetylators are not an Nat2 knockout strain but rather mirror human NAT2 slow acetylator phenotype, wherein NAT2 enzymatic capacity is reduced by SNPs in the coding exon but is not eliminated.
We hypothesized that PhIP adduct levels would be higher in rat colon and mammary tissue, whereas MeIQx adducts levels would be higher in rat liver to reflect their tumor target organ specificity. Because O-acetylation catalyzed by NAT2 is an activation pathway, we further hypothesized that PhIP- and/or MeIQx-induced DNA adduct levels would be greater in rapid than slow NAT2 acetylators. We tested these hypotheses in a rapid and slow acetylator NAT2 congenic rat model.
Materials and Methods
Chemicals.
MeIQx, PhIP, and the dG-C8-PhIP and dG-C8-PhIP-d3 adduct standards were purchased from Toronto Research Chemicals (North York, Ontario, Canada). dG-C8-MeIQx and dG-C8-MeIQx-D3 adduct standards were kindly provided by Dr. Rob Turesky (Wadsworth Center, New York Department of Health, Albany, NY). Details of their synthesis and spectral analysis have been published previously (Paehler et al., 2002).
Animals.
Rapid and slow adult female F344/WKY rats, congenic at the Nat2 locus, were bred and maintained at the University of Louisville School of Medicine. Details on their construction were recently reported (Hein et al., 2008). Rats were housed in groups of 1 to 3 per cage on a 12-h dark/light cycle with ad libitum access to rodent diet (Lab Diet, Brentwood, MO) and tap water.
Treatment.
Each rat received a single dose by gavage (1 ml/kg) of 25 mg/kg MeIQx, 50 mg/kg PhIP, or vehicle control (dimethyl sulfoxide). Twenty-four hours after injection, the rats were euthanized by CO2 asphyxiation.
DNA Isolation.
Tissues were collected, cleaned, and snap frozen in liquid nitrogen, and then stored at −80°C. Approximately 3 g of mammary tissue was minced finely and digested with 35 mg of collagenase type III (Worthington, Lakewood, NJ) and 10 mg of neutral protease (Worthington) in 40 ml of RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with fetal bovine serum. The tissue was then digested for 3 h at 37°C. The epithelial cells were centrifuged at 400g for 10 min, and the pellet was washed three times with phosphate-buffered saline buffer.
Selected tissues and isolated mammary epithelial cells were minced and homogenized in two volumes of 20 mM sodium phosphate buffer (pH 7.4). One-tenth volumes each of proteinase K solution (20 mg/ml) and 10% SDS were added to the tissue homogenate, and the mixture was incubated at 37°C for 1 h. One volume of phenol equilibrated with 10 mM Tris HCl (pH 8.0) was added to the mixture, which was then vortexed and centrifuged at 3600g for 15 min. The aqueous layer was removed and added to 1 volume of phenol/chloroform/isoamyl alcohol (25:24:1) saturated with 10 mM Tris HCl (pH 8.0), vortexed, and centrifuged. The aqueous layer was removed and added to 1 volume of cold (−20°C) isopropanol, and the mixture was vortexed and centrifuged. The DNA pellet was washed with 70% ethanol and redissolved in 5 mM Tris (pH 7.4) containing 1 mM CaCl2, 1 mM ZnCl2, and 10 mM MgCl2. The DNA was quantified by A260. DNA quality was monitored by A260/280 and was consistently above 1.9. DNA samples (200 μg) from vehicle, MeIQx-, or PhIP- treated rats were digested and prepared for capillary liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis as described previously (Neale et al., 2008).
DNA Adduct Analyses.
Deoxyribonucleoside adducts were identified and quantified by LC-MS/MS by using isotope dilution methods with deuterated internal standards as described previously (Bendaly et al., 2007, 2009). Samples for quantitative analysis of dG-C8-MeIQx and dG-C8-PhIP were spiked with 1 ng of deuterated dG-C8-MeIQx-d3 or dG-C8-PhIP-d3 as internal standard. LC-MS/MS instrumentation and procedures have been described previously for dG-C8-MeIQx (Bendaly et al., 2007) and dG-C8-PhIP (Bendaly et al., 2009). Multiple reaction monitoring scans were used to measure the [M+H]+ to [(M-116) + H]+ (loss of deoxyribose) mass transition. For dG-C8-MeIQx, the transition from parent to fragment was measured for dG-C8-MeIQx (m/z 479 to 363) and the internal standard, dG-C8-MeIQx-d3 (m/z 482 to 366). For dG-C8-PhIP, the transition from parent to fragment was measured for dG-C8-PhIP (m/z 490 to 374) and the internal standard, dG-C8-PhIP-d3 (m/z 493 to 377).
Data Analysis.
Differences in DNA adduct levels were tested for significance by one-way analysis of variance followed by Tukey-Kramer multiple comparisons test. P values less than 0.05 were considered significant.
Results and Discussion
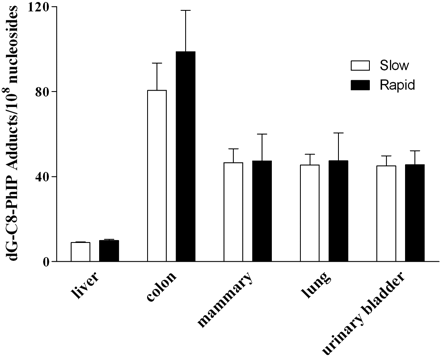
After administration of PhIP, dG-C8-PhIP DNA adduct levels were highest in colon, lower in mammary epithelial cells, and lowest in liver (Fig. 1). These results are consistent with colon and mammary gland as tumor target organs of PhIP in the rat (Sugimura et al., 2004). Previous studies have identified PhIP-DNA adducts in rat colon (Kaderlik et al., 1994; Purewal et al., 2000) and mammary epithelial cells (Ghoshal et al., 1995). PhIP-DNA adducts have also been identified in human colon (Garner et al., 1999; Lightfoot et al., 2000) and mammary cells (Gorlewska-Roberts et al., 2002; Zhu et al., 2003).
dG-C8-PhIP adducts in rapid and slow Nat2 acetylator congenic rats. Rats were dosed with 50 mg/kg. Each bar illustrates mean ± S.E. for three rats. dG-C8-PhIP adducts were significantly higher in colon than in liver for both rapid (p < 0.001) and slow (p < 0.01) acetylators. No significant (p > 0.05) differences between rapid and slow acetylators were noted in any tissue.
dG-C8-PhIP DNA adduct levels did not differ significantly between rapid and slow acetylator congenic rats in any tissue tested (Fig. 1). This result is consistent with a previous study in which PhIP-DNA adducts derived from human lymphocytes did not differ between rapid and slow NAT2 acetylators (Magagnotti et al., 2003). Previous findings in rapid and slow acetylator congenic hamsters showed both males and females had high levels in the colon, lower levels in mammary tissue, and the lowest levels in the liver (Fretland et al., 2001a,b). Furthermore, no differences in PhIP-DNA adduct levels were observed between rapid and slow acetylator congenic hamsters in colon or other tissues (Steffensen et al., 2000; Fretland et al., 2001a,b). Previous studies of PhIP-DNA adduct formation in the parental F344 and WKY parent inbred rat strains administered a diet containing 0.04% PhIP also showed higher levels of PhIP DNA adducts in the colon than the liver with significantly higher levels in F344 than WKY inbred rats (Purewal et al., 2000). Although colon PhIP DNA adducts were slightly higher in rapid than slow acetylator NAT2 congenic rats in our study, the difference was not significant. This result may be due to the dosing regimen (one single dose) versus continuous low dose, and the fact that F344 and WKY inbred rats differ in genes other than NAT2 including carcinogen-metabolizing and DNA repair pathways.
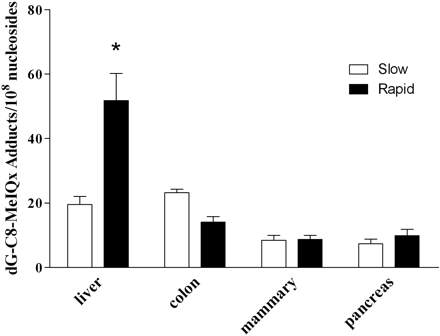
After administration of MeIQx, dG-C8-MeIQx was identified in liver, colon, mammary tissue, and pancreas, and dG-C8-MeIQx adduct levels were highest in the liver (Fig. 2). This finding is consistent with liver as the principal tumor target of MeIQx in the rat (Kato et al., 1988; Kushida et al., 1994) and mouse (Ohgaki et al., 1987). In a previous study in which MeIQx-induced DNA adducts were measured at very low doses in rodent and human tissues using accelerator mass spectrometry, MeIQx DNA adduct levels also were highest in liver and increased as a linear function of administered dose for a single-dose exposure (Turteltaub et al., 1997).
dG-C8-MeIQx adducts/108 nucleotides in tissues derived from rapid and slow Nat2 acetylator congenic rats dosed with 25 mg/kg MeIQx. Each bar illustrates mean ± S.E. for 4 to 10 rats. *, dG-C8-MeIQx adducts in rapid acetylator liver were significantly (p < 0.001) higher than in slow acetylator liver and rapid acetylator colon, mammary, and pancreas. Differences between rapid and slow acetylators were not significant (p > 0.05) in any tissue except liver.
As also shown in Fig. 2, dG-C8-MeIQx adduct levels in rapid acetylator liver were significantly (p < 0.001) higher than in slow acetylator liver, suggesting that rapid NAT2 phenotype may increase the risk of MeIQx-induced hepatocarcinoma. In contrast to most cancers, there have been very few studies that have explored the role of NAT2 acetylator genotype in hepatocarcinoma. An initial study reported that NAT2 slow acetylators had a higher incidence of hepatocellular carcinoma than rapid acetylators, but this study did not stratify by arylamine or heterocyclic amine exposure (Agúndez et al., 1996). Subsequent studies, which have stratified for these exposures (Yu et al., 2000; Huang et al., 2003), reported that NAT2 rapid acetylators have an increased susceptibility to hepatocellular carcinoma. The latter two studies are consistent with our current results in the rapid and slow NAT2 acetylator congenic rat and support our hypothesis that NAT2-catalyzed O-acetylation is an activation pathway for MeIQx DNA adducts that initiate hepatocarcinoma.
The different effects of NAT2 acetylation polymorphism on PhIP- and MeIQx-induced DNA adducts in the rat model mirror observations with human NAT2. Recent results in genetically engineered Chinese hamster ovary (CHO) cells documented a much greater role for human NAT2 polymorphism in MeIQx-DNA adduct formation and mutagenesis (Bendaly et al., 2007) than in PhIP-DNA adduct formation and mutagenesis (Metry et al., 2007; Bendaly et al., 2009). Indeed, a dose-dependent increase in dG-C8-MeIQx adducts and mutagenesis was observed in CHO cells transfected with a human rapid acetylator NAT2 allele (NAT2*4) but not in CHO cells transfected with a human slow acetylator NAT2 allele (NAT2*5B) (Bendaly et al., 2007). In contrast, CHO cells transfected with NAT2*4 or NAT2*5B did not differ significantly in PhIP-DNA adducts or mutagenesis (Metry et al., 2007; Bendaly et al., 2009).
In conclusion, our results are consistent with colon as a tumor target organ for PhIP and with liver as a tumor target organ for MeIQx. Furthermore, our results in a congenic rat model extend previous results conducted in a genetically engineered CHO cell culture model, suggesting that rapid NAT2 acetylators have higher risk to MeIQx tumors. Further investigations to test these conclusions are needed.
Acknowledgments.
We thank Dr. Rob Turesky (Wadsworth Center, New York Department of Health, Albany, NY) for generously providing dG-C8-MeIQx and dG-C8-MeIQx-D3 adduct standards.
Footnotes
-
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant R01-CA034627]; and the National Institutes of Health National Institute of Environmental Health Sciences [Grant P30-ES014443].
-
A preliminary account of this work was presented as follows: Hein DW, Metry KR, Bendaly J, Smith NB, Neale JR, and Pierce WM Jr (2007) N-Acetyltransferase 2 gentoype-dependent DNA adducts in rapid and slow acetylator congenic rats administered 2-amino-3,8-dimethylimidazo-[4,5-f]quinoxaline. Proceedings of the 21st International Symposium on Polycyclic Aromatic Compounds; 2007 Aug 5–10; Trondheim, Norway.
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.029512
-
↵1 Parts of this work were previously presented as follows: Metry KJ (2007) Role of N-Acetyltransferase 2 Polymorphism in DNA Adduct Formation and Mutagenesis by Aromatic and Heterocyclic Amine Carcinogens. Ph.D. dissertation, University of Louisville, Louisville, KY.
-
↵2 Current affiliation: WIL Research Laboratories, LLC, Ashland, Ohio.
-
↵3 Current affiliation: Array BioPharma, Boulder, Colorado.
-
↵4 Current affiliation: Alcon Laboratories, Inc., Fort Worth, Texas.
- Received July 15, 2009.
- Accepted August 6, 2009.
- Copyright © 2009 by The American Society for Pharmacology and Experimental Therapeutics