Abstract
The carboxylesterases (CESs) are a family of serine hydrolases that hydrolyze compounds containing an ester, amide, or thioester. In humans, two dominant forms, CES1 and CES2, are highly expressed in organs of first-pass metabolism and play an important role in xenobiotic metabolism. The current study was conducted to better understand species-related differences in substrate selectivity and tissue expression of these enzymes. To elucidate potential similarities and differences among these enzymes, a series of 4-nitrophenyl esters and a series of gemcitabine prodrugs were evaluated using enzyme kinetics as substrates of expressed and purified CESs from beagle dog, cynomolgus monkey, and human genes. For the substrates examined, human and monkey CES2 more efficiently catalyzed hydrolysis compared with CES1, whereas CES1 was the more efficient enzyme in dog. Quantitative real-time polymerase chain reaction and Western blot analyses indicate that the pattern of CES tissue expression in monkey is similar to that of human, but the CES expression in dog is unique, with no detectable expression of CES in the intestine. Loperamide, a selective human CES2 inhibitor, was also found to be a CES2-selective inhibitor in both dog and monkey. This is the first study to examine substrate specificity among dog, human, and monkey CESs.
Introduction
The carboxylesterases (CES) are a multigene family of enzymes found in organisms ranging from bacteria to mammals. These enzymes are members of the serine hydrolase superfamily, in which a serine residue is involved in the hydrolysis of ester, amide, or thioester bonds. A recent genomic analysis clearly defined five distinct mammalian CES subfamilies based on genetic sequence and genomic structure (Williams et al., 2010), but CES1 and CES2 subfamily proteins are the most extensively studied. In mammals, CES substrates are both endogenous (i.e., acyl-glycerols and acyl-CoA esters) and exogenous (i.e., irinotecan, cocaine, and heroin). The CESs have overlapping substrate specificity, but patterns of substrate selectivity have also been observed. For example, a comparison has been reported between the human CES1 and CES2 forms for the substrates cocaine, heroin, 4-methylumbelliferyl acetate, and 6-monoacetylmorphine (Pindel et al., 1997). In these studies, human CES1 had higher affinity for cocaine, whereas human CES2 had greater affinity for 4-methylumbelliferyl acetate and 6-monoacetylmorphine. Both enzymes displayed a similar Km value for heroin, but human CES2 was greater than 4-fold more efficient at heroin turnover. These data, along with similar findings in other laboratories, suggest that human CES1 preferentially hydrolyzes compounds that contain a larger acyl moiety, whereas human CES2 prefers compounds with a larger alcohol moiety in relation to the acyl component (Satoh et al., 2002). For some substrates, the reverse reaction, transesterification, appears to be catalyzed by human CES1 but not by human CES2 (Dean et al., 1991). Similar studies examining the substrate selectivity of the CESs from the large animal species used as preclinical models in drug discovery and development have not been reported.
Carboxylesterases are broadly expressed in vertebrate species, with species-specific patterns of tissue expression becoming apparent. In humans, CES1 and CES2 mRNA expression is highest in the liver (Satoh et al., 2002), suggesting an important role in detoxification of xenobiotics (Williams et al., 2010). Human CES1 mRNA is also expressed to a lesser extent in the heart, stomach, testis, kidney, spleen, and colon (Satoh et al., 2002). Human CES2 mRNA is highly expressed in the small intestine (Quinney et al., 2005), leading to its common description as the human intestinal carboxylesterase, and is also detected in colon and heart tissues (Satoh et al., 2002). Less is known about the expression of CES3 in humans, but it has been identified in the brain (Mori et al., 1999), liver, and colon (Quinney et al., 2005). Little is known about the expression of CES4 and CES5 in humans.
The CESs are important contributors to the metabolic pathways of xenobiotics, including drugs and prodrugs. For many prodrugs, such as prasugrel (Williams et al., 2008b) and irinotecan (Humerickhouse et al., 2000), CESs directly catalyze the bioactivation of the prodrug. However, CESs can also facilitate the clearance and inactivation of drugs and prodrugs, as in the case of clopidogrel (Tang et al., 2006). As new therapeutic agents that use these hydrolytic pathways are being developed, it is vital to understand the similarities and differences in CES activity between humans and preclinical models of drug disposition.
A comparison of CES activity in animal models has been initiated by measurement of hydrolytic activity in nondenaturing gels (Li et al., 2005; Taketani et al., 2007). Li et al. (2005) demonstrated that CES activity is detectable in rodent and feline plasma but was nonexistent in primate plasma, and this was confirmed in a recent study (Berry et al., 2009). Because of the hydrolytic capability of rodent plasma, as well as the large number of CES forms expressed in rodent liver and intestine, these species do not appear to be broadly applicable models for pharmacokinetics and metabolism of ester drugs. However, relatively little is known about the similarities or differences between human CES activity and other potentially more relevant animal models such as monkeys or dogs. Taketani et al. (2007) demonstrated that CES2 is the dominant CES in the liver of the cynomolgus monkey, whereas dog is devoid of CES activity in the small intestine as confirmed in a recent study (Berry et al., 2009). These distinctions raise questions about the broad applicability of these species for use in the development of drug candidates that are CES substrates, but further study is needed. The aim of the current study was to perform a more comprehensive comparison of substrate and inhibitor selectivity and tissue expression between the related CES1 and CES2 forms from beagle dog, cynomolgus monkey, and human. To accomplish these goals, unique reagents such as expressed and purified CESs from dogs, monkeys, and human were used.
Materials and Methods
Expression and Purification of Recombinant Dog and Cynomolgus Monkey CESs.
The cloning of the human CES1 (hCES1) and CES2 (hCES2) (Williams et al., 2008a), dog CES1 (dCES1) and CES2 (dCES2), and cynomolgus monkey CES1 (cCES1) and CES2 (cCES2) (Williams et al., 2010) was described previously. Expression and purification were conducted as described previously (Williams et al., 2008a). In brief, hCES1 was cloned from human liver cDNA and hCES2 cDNA was commercially available from Open Biosystems (Huntsville, AL). Dog CES1 and CES2 were cloned from dog liver and brain total RNA, respectively. Cynomolgus monkey CES1 and CES2 were cloned from monkey liver and small intestine total RNA, respectively. These clones were expressed in Sf9 cells using a baculovirus expression system. Infected Sf9 cells were lysed and centrifuged, and the supernatants were column-purified. Purified CES protein was N-terminal-sequenced for confirmation.
Enzyme Activity Assays with 4-Nitrophenyl Esters.
The rate of hydrolysis of a series of 4-nitrophenyl esters was determined spectrophotometrically by measuring reaction products at 402 nm−1, as described previously (Williams et al., 2008a). The substrates used were 4-nitrophenyl acetate (MP Biomedicals, Solon, OH), 4-nitrophenyl propionate (MP Biomedicals), 4-nitrophenyl butyrate (Sigma-Aldrich, St. Louis, MO), 4-nitrophenyl valerate (Sigma-Aldrich), 4-nitrophenyl dimethylacetate (Lilly Research Laboratories, Indianapolis, IN), 4-nitrophenyl trimethylacetate (Lilly Research Laboratories), 4-nitrophenyl 4-guanidinobenzoate (Sigma-Aldrich), and 6-nitrocoumarin (Lilly Research Laboratories). Table 1 gives the maximum concentration of each substrate used with each enzyme. From the maximum substrate concentration, a 1:1 serial dilution was made for a total of eight substrate concentrations for each substrate. Enzyme kinetic parameters were determined as described previously (Williams et al., 2008a). In brief, spectrophotometer data were exported to Microsoft Excel (Microsoft, Redmond, WA) to calculate the amount of 4NP formed and the rate of formation. The rate values were exported to WinNonlin (Pharsight, Mountain View, CA) to calculate Michaelis-Menten kinetic constants. The intrinsic clearance (CLint) values calculated are Vmax/Km.
Summary of maximum substrate concentration and enzyme concentration used with each combination of substrate and enzyme
Enzyme Activity Assays with Gemcitabine Prodrugs.
To develop a broader understanding of the structure-activity relationships among species, the in vitro hydrolysis of a series of ester prodrugs of gemcitabine (Lilly Research Laboratories) was assessed using the expressed enzymes. The test compounds were selected (Table 4) on the basis of two criteria: 1) a single hydrolytic site and 2) preliminary experiments showing measurable hydrolysis by the human CESs. Hydrolysis reactions were conducted at 37°C with a final dimethyl sulfoxide content of 2% in phosphate-buffered saline with a final reaction volume of 75 μl. A dimethyl sulfoxide content of up to 2% is well tolerated by CESs (Williams et al., 2008a). The final enzyme concentration was 10 μg/ml, except for cCES2, which was 1 μg/ml. Three substrate concentrations (1, 10, and 500 μM) were examined in triplicate at 0, 2, 10, 30, and 60 min time points quenched by the addition of acetonitrile with an internal standard ([2,4-13C2,15N]gemcitabine).
Study samples were analyzed by liquid chromatography-tandem mass spectrometry using a Sciex API 4000 triple quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA) equipped with a TurboIonSpray interface and operated in positive ion mode. The analytes were chromatographically separated using a Fluophase PFP high-performance liquid chromatography column (2.1 × 50 mm, 5 μm; Thermo Fisher Scientific, Waltham, MA) with a gradient liquid chromatography system composed of water-trifluoroacetic acid/1 M ammonium bicarbonate (1000:4:1, v/v) (mobile phase A), and acetonitrile-trifluoroacetic acid/1 M ammonium bicarbonate (1000:4:1, v/v) (mobile phase B). The pumps were LC-10AD units with a SCL-10A controller (Shimadzu, Kyoto, Japan) and a Gilson 215 liquid handler (Gilson, Inc., Middleton, WI) was used as the autosampler. The gradient profile changed from 3% B at 0 min to 13% B at 0.01 to 0.20 min, 35% B at 0.30 to 0.40 min, and 98% at 0.31 to 0.75 min, at a flow rate of 1.5 ml/min. Chromatography was performed at ambient temperature, with 1 ml/min directed to the mass spectrometer between 0.25 and 0.5 min (0.5 ml/min split to waste). Selected reaction monitoring (M + H)+ transitions m/z 264.0 > 112.0, 265.0 > 113.0, and 269.0 > 117.0 were monitored for gemcitabine, its 13C-isotopomer, and the internal standard, respectively. The monitored fragments were the cytosine portions of the respective molecules. The most abundant gemcitabine transition was used to quantify standards and samples with low concentrations, whereas 13C-isotopomer transition was used to quantify high concentration samples and standards. The TurboIonSpray temperature was maintained at 740°C, with collision, curtain, nebulizing, and desolvation gas (nitrogen) settings of 4, 40, 70, and 50, respectively. The ionspray voltage was set to 1500 V, whereas the respective declustering, entrance, collision, and exit potentials were 45, 10, 25, and 8 for gemcitabine transitions and 45, 10, 30, and 10 for the internal standard. The mass spectrometer quadrupoles were tuned to achieve unit resolution (0.7 Da at 50% full-width at half-maximum). Data were acquired and processed with Analyst 1.4.2 (Applied Biosystems). The Analyst data were exported to Microsoft Excel for analyses. Not all data collected indicated a linear rate of hydrolysis; thus, only the initial linear rate was calculated. Michaelis-Menten kinetic constants were calculated by GraphPad Prism (GraphPad Software Inc., San Diego, CA) using nonlinear regression. When possible, the CLint values calculated are Vmax/Km.
CES Inhibition by Loperamide.
The inhibition of CES activity by loperamide was determined using a procedure similar to the enzyme activity assays. The substrate was 4-nitrophenyl butyrate at a concentration near the Km or Ks value for the hydrolysis of 4NPB by each CES (20 μM for dCES1, 40 μM for cCES1 and dCES2, and 90 μM for cCES2, hCES1, hCES2, and hCES3). The concentrations of loperamide ranged from 0 to 500 μM, with a tailored dilution scheme (starting at 500 μM) used with each enzyme to best elucidate the inhibition curve. In particular, a 3:1 serial dilution of buffer with loperamide-buffer was used for cCES1, dCES1, and hCES1. Serial dilutions of 1:2, 1:1, 1:3, and 4:1 were used with cCES2, dCES2, hCES2, and hCES3, respectively. The collected data were exported to Microsoft Excel to compute hydrolysis rates based on the standard curves. Then the data were exported to GraphPad Prism and fit to a model using the Hill slope equation with four parameters.
Tissue Samples.
Tissues were collected from euthanized animals in accordance with local animal care and use protocols. Tissues samples were collected from the liver, duodenum, jejunum, ileum, colon, stomach, kidney, lung, and plasma of two beagle dogs and one cynomolgus monkey. A sample from the heart of one of the dogs was also obtained. These samples were used for both mRNA quantitation and Western blot analysis.
mRNA Quantitation.
All tissues except plasma were processed using an RNeasy Mini Kit (QIAGEN, Valencia, CA) to obtain total RNA. The RNA concentration was determined using absorbance at a wavelength of 260 nm−1.
Quantitative real-time polymerase chain reaction (PCR) was conducted as described previously (Williams et al., 2004) with the following exceptions. The instrument used for analysis was an ABI Prism 7900HT (Applied Biosystems, Foster City, CA), and the primer and probe sets were designed for multiplexing. Table 2 lists the primers, probes, and standards synthesized by Applied Biosystems and/or Integrated DNA Technologies, Inc. (Coralville, IA). The universal primers were used unless a dog-specific primer is listed for a particular assay. The assay reagents used were provided in the SuperScript III Platinum One-Step Quantitative RT-PCR System with ROX (Invitrogen, Carlsbad, CA). The final concentration of the primers and probes was 400 nM, and samples with RNA contained 380 ng of RNA.
Synthetic oligonucleotide sequences used for quantitative real-time PCR
Western Blot Analysis.
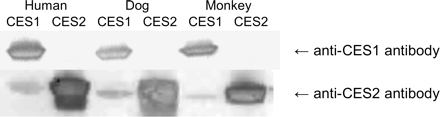
All tissues except plasma were processed using an RNeasy Mini Kit to obtain protein lysates. In these analyses, 50 μg of tissue homogenates or plasma samples or 25 (cCES2), 50 (dCES1 and cCES1), or 100 ng (dCES2) of purified proteins were loaded on 4 to 12% SDS-polyacrylamide bis-Tris gels (Bio-Rad Laboratories, Hercules, CA), separated by electrophoresis, and transferred to polyvinylidene difluoride membranes (Invitrogen). Membranes were blocked with Dulbecco's phosphate-buffered saline containing 0.1% Tween 20 (PBST) and 5% nonfat dry milk (Bio-Rad Laboratories) for 2 h at room temperature. The membranes were washed with PBST and then incubated overnight at 4°C with either a rabbit anti-human CES1 or CES2 antibody. The hCES1 antibody (Abcam, Inc., Cambridge, MA) was diluted 1:500 in 1.5% nonfat dry milk-PBST and the hCES2 antibody (graciously provided by Philip Potter at St. Jude Children's Research Hospital, Memphis, TN) was diluted 1:100,000 in 1.5% nonfat dry milk-PBST. The difference in dilutions was due to the detection against a known amount of protein, the anti-hCES1 antibody had weaker detection compared with the anti-hCES2 antibody. Membranes were washed three times for 10 min each and then were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) diluted 1:10,000 in PBST. Proteins were detected using the ECL system (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK), and the membranes were visualized on a Storm 860 system (Molecular Dynamics, Sunnyvale, CA). Figure 1 shows the cross-reactivity of the antibodies with the purified proteins.
Western blot demonstrating the cross-reactivity of the polyclonal rabbit anti-human CES1 and CES2 antibodies probed against the cynomolgus monkey, dog, and human CES1 and CES2 enzymes. The gel was loaded with 2 μg for dog CESs and 1 μg for monkey and human CESs.
Results
Enzyme Activity Assays with 4-Nitrophenyl Esters.
A limited evaluation of the substrate-activity relationship for eight compounds was undertaken using 4NP esters. Although the hydrolysis of the lactone in 6NC will not form 4NP directly, the hydrolysis product has spectral properties similar to those of 4NP. The enzyme kinetic parameters obtained are presented in Table 3.
Summary of CES activity for 4-nitrophenyl esters
Values listed are the average ± S.E. When the kinetic constants Km and Vmax were determined for a substrate and enzyme combination, the intrinsic clearance (CLint) is Vmax/Km; otherwise, the initial clearance (CLini) was calculated. Units for Km/Ks are micromolar concentration, for Vmax are micromoles of product per minute per milligram of protein, and for CLint/CLmax/CLini are microliters per minute per milligram of protein, respectively.
Typical Michaelis-Menten kinetics were observed with the majority of the CES-substrate combinations, with a few exceptions. Nonsaturable kinetics were observed for the hydrolysis of 6NC by all enzymes studied, and the hydrolysis of 4NPTMA by dCES2 was best fit with the Hill equation. Furthermore, substrate inhibition at high concentrations was observed for the hydrolysis of 4NPTMA by hCES2 and of 4NPGB by cCES2. The hydrolysis of 4NPDMA by hCES1, hCES2, cCES2, and dCES2 was best fit to biphasic or two-site kinetics.
For all substrates tested, the clearance values obtained with hCES2 and cCES2 were equal to or greater than those for the respective CES1 enzyme (Table 3). The dog enzymes displayed the inverse relationship with higher clearance values determined with dCES1 rather than dCES2 (Table 3). The one exception was the hydrolysis of 4NPGB, which had higher clearance values with CES2 versus CES1 in the monkey (15.4 and 8.02 × 10−3 ml per s/mg, respectively) and dog (0.0409 and 0.0148 ml per s/mg, respectively), but a higher clearance value with CES1 versus CES2 in humans (0.128 and 0.0425 ml per s/mg, respectively).
In general, as the carbon chain length of the substrate increased from 4NPA to 4NPP to 4NPB to 4NPV, so also did the CLint values (Table 3). Although the trend was present in all of the CESs tested, it was most pronounced with cCES2. A notable exception was observed with hCES1, for which enzyme affinity (Km) and CLint were similar for all four substrates. In general, the increasing CLint values appear to be driven by the decreasing Km values as the carbon chain length increases. The CLint values with cCES2 increased approximately 20 times between 4NPA and 4NPV because of the Km value decreasing more than 7-fold and the Vmax value increasing less than 3-fold. For some CESs, there were also increasing Vmax values as the alkyl chain increased, but the greatest increase was only 5-fold. The highest clearance values for all the CESs were achieved with the hydrolysis of either 4NPB or 4NPV. Although many of the clearance values were similar between the human and monkey orthologs, cCES2 had substantially higher clearance values relative to hCES2 for the hydrolysis of 4NPB, 4NPV, 4NPDMA, and 4NPGB.
Human CES3 was assessed using 4NPB and demonstrated a substantially lower clearance value than either hCES1 or hCES2 (Table 3). In the pilot studies for hCES3, the other substrates were tested (data not shown) but yielded substantially lower hydrolysis rates than hCES1 and hCES2. As a result of the lower clearance values by hCES3 in the pilot studies, only enzyme kinetic studies for the hydrolysis of 4NPB by hCES3 (0.150 ml per s/mg) were completed and shown for comparison with hCES1 (13.9 ml per s/mg) and hCES2 (36.2 ml per s/mg). Compared with hCES1 and hCES2, hCES3 had a similar Km value (105, 97.4, and 81.7 μM, respectively), but the difference in CLint values was the result of a substantially lower Vmax value for hCES3 (1.36, 3.38, and 0.0115 μmol per s/mg, respectively). Therefore, the binding of 4NPB as a substrate appears to be similar among the three forms, but the catalytic turnover was substantially different.
Enzyme Activity Assays with Gemcitabine Prodrugs.
Kinetic parameters were determined for the hydrolysis of gemcitabine ester prodrugs by human, monkey, and dog CESs (Table 4). A trend similar to that seen with the 4-nitrophenyl esters was observed for these substrates, in that hCES2, cCES2, and dCES1 preferentially hydrolyzed most compounds. Examples illustrating this trend include prodrugs 16, 02, and 03 for the human, monkey, and dog CESs, respectively. For prodrug 16, hCES1 and hCES2 CLint values were 1.00 and 315 μl per s/mg, respectively. For prodrug 02, cCES1 and cCES2 CLint values were 146 and 8813 μl per s/mg, respectively. For prodrug 03, dCES1 and dCES2 CLint values were 228 and 1.71 μl per s/mg, respectively. There were a few exceptions for which the CES1 and CES2 enzymes had similar CLint values. The human CES1 and CES2 had CLint values for prodrug 10 of 0.351 and 0.327 μl per s/mg, respectively. The dog CES1 and CES2 had CLint values for prodrug 05 of 4.24 and 4.63 μl per s/mg, respectively, and for prodrug 13 of 1.17 and 1.02 μl per s/mg, respectively. In addition, the maximum activity among species was dramatically different. The greatest hydrolytic clearance was observed in monkey (cCES2), followed by human (hCES2) and then dog (dCES1) with CLint values of 8813, 918, and 259 μl per s/mg, respectively, for prodrug 02.
Summary of CES activity for gemcitabine esters
Values listed are the mean ± S.E. When the kinetic constants Km and Vmax were determined for a substrate and enzyme combination, the intrinsic clearance (CLint) is Vmax/Km; otherwise, the initial clearance (CLini) was calculated. Units for Km are micromolar concentration, for Vmax are nanomoles of product per minute per milligram of protein, and for CLint/CLini are microliters per second per milligram of protein, respectively. 
CES Inhibition by Loperamide.
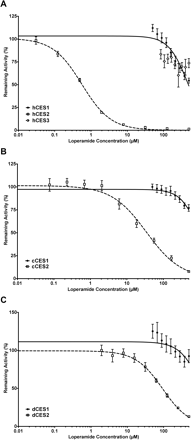
The inhibition of human, monkey, and dog CESs by loperamide was found to be selective for the CES2 subfamily (Fig. 2). A comparison of IC50 values (Table 5) indicates that loperamide is selective for CES2 inhibition versus CES1 by at least 5- to 1000-fold, depending on species. If an enzyme did not reach approximately 50% inhibition of 4NPB hydrolysis by 500 μM loperamide (maximum apparent soluble concentration), then the IC50 value was recorded as greater than 500 μM. Whereas hCES1 had an IC50 value near 500 μM, that for cCES1, dCES1, and hCES3 exceeded 500 μM. The IC50 values suggested that loperamide is most potent as an inhibitor for hCES2 followed by cCES2 and then dCES2 (0.562, 34.4, and 93.6 μM, respectively). Of interest, the loperamide IC50 values obtained in this study are similar to those using a different probe substrate (4-methylumbelliferyl acetate) (Quinney et al., 2005) for hCES1 and hCES2.
Loperamide inhibition of the carboxylesterases in human (A), cynomolgus monkey (B), and dog (C). The IC50 values are listed in Table 5.
IC50 values for the inhibition by loperamide of 4NPB hydrolysis by each CES studied
mRNA Quantitation.
The results of mRNA quantitation are listed in Table 6. In the cynomolgus monkey, a trend of decreasing CES mRNA content was noticed progressing through the intestinal tract from stomach (244 CES2 transcripts per 1000 β-actin transcripts) to colon (48.5 CES2 transcripts per 1000 β-actin transcripts). In addition, throughout the length of the intestine, cCES2 mRNA was expressed at substantially higher levels than cCES1 mRNA (48.5 and 0.0790 CES transcripts per 1000 β-actin transcripts, respectively, in the colon). In cynomolgus monkey kidney and lung, cCES1 (95.2 and 20.1 CES1 transcripts per 1000 β-actin transcripts, respectively) had higher mRNA expression than cCES2 (54.4 and 12.8 CES2 transcripts per 1000 β-actin transcripts, respectively). The hepatic mRNA expression levels were similar between cCES1 and cCES2 and are the highest of all tissues assayed (841 and 878 CES transcripts per 1000 β-actin transcripts, respectively). In the two beagle dogs, only dCES1 mRNA was detected in the kidney, liver, and lung (19.2, 251, and 14.4 CES1 transcripts per 1000 β-actin transcripts, respectively, as averaged between the two beagle dogs assessed) with the highest expression in liver. Unlike monkey, dCES1 mRNA was not detected in the gastrointestinal tract. Of importance, dCES2 transcripts were not detected in any of the tissues examined, despite detection of synthetic standards of the dCES2 sequence to be amplified.
Results of mRNA quantitation by quantitative real-time PCR
Western Blot Analysis.
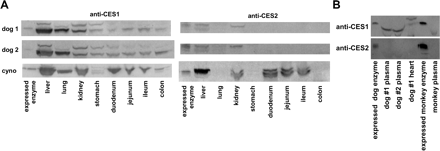
Figure 3 provides a survey of the protein expression of CES1 and CES2 in various tissues with the use of anti-CES1 and anti-CES2 antibodies. Whereas the anti-CES1 antibody demonstrated high specificity, the anti-CES2 antibody has some cross-reactivity with CES1 proteins (Fig. 1). In the cynomolgus monkey, the liver demonstrated the highest protein expression with both anti-CES1 and anti-CES2 antibodies. In addition, the apparent expression of cCES1 and cCES2 protein in monkey decreases progressing through the intestinal tract from the stomach to the colon. Of interest, a doublet was often observed with the anti-CES2 antibody and not necessarily with the anti-CES1 antibody. In beagle dogs, dCES1 is the major CES expressed. A doublet was also observed with the anti-CES1 antibody in dog. Similar to the monkey, the dog liver showed the greatest immunodetectable protein expression. Examination of plasma from the beagle dog and cynomolgus monkey for proteins immunoreactive with the anti-CES1 and anti-CES2 antibodies suggested that dog plasma contains a protein related to dCES1 but monkey plasma does not. On the other hand, neither the dog nor monkey plasma sample appeared to contain a protein related to CES2.
Western blot analysis of CES1 and CES2 expression in two beagle dogs and one cynomolgus monkey. Gels were loaded with 50 μg of tissue homogenates (A) and 50 μg of plasma (B) with expressed and purified CESs for comparison. There was 50 and 100 ng of purified protein for dCES1 and dCES2, respectively, and 50 and 25 ng for cCES1 and cCES2, respectively.
Discussion
Enzyme-Substrate Recognition.
A series of 8 nitrophenyl compounds and 16 gemcitabine prodrugs were selected to provide an initial comparison of the relative SAR of CES1 and CES2 enzymes. The ring-constrained lactone in 6NC was resistant to hydrolysis by all enzymes tested, demonstrating nonsaturable kinetics and low substrate turnover. In general, the rates of hydrolysis of the alkyl esters of 4NP were faster with human and monkey CES2 than with CES1, but this theory was not assessed using statistical analyses. However, within these species, enzyme affinity (as estimated by Km values) for a given substrate was generally similar between CES1 and CES2. This finding is consistent with the hypothesis that lipophilicity is a major determinant of enzyme affinity because of the need to access the active site through a long hydrophobic gorge (Potter and Wadkins, 2006). Previous studies have illustrated that hCES2 displays a general preference for ester substrates with a larger alcohol group. This result has been hypothesized to be related to greater conformational flexibility and a larger entrance to the active site (Redinbo and Potter, 2005). The current data are consistent with this trend, because the alcohol components of all the alkyl esters tested have acyl groups of lower molecular weight than 4NP or gemcitabine. However, the magnitude of selectivity for the 4-nitrophenyl esters by hCES2 is relatively small, probably related to the small molecular size of these compounds compared with that of more selective substrates, such as irinotecan and heroin. For the gemcitabine esters, hydrolytic rates were again faster with hCES2 and cCES2 than with CES1 with more substrate selectivity observed than with the 4-nitrophenyl esters. Many of the prodrugs had greater than 10-fold higher CLint values for hCES2 than for hCES1, with the greatest being more than 300-fold for prodrug 16. The largest differences in substrate selectivity were observed for the non-amino acid prodrugs. For these examples, the CLint value for hCES2 was 15- to 300-fold higher than that for hCES1. For the amino acid prodrugs, however, rates of hydrolysis were similar for hCES1 and hCES2, and selectivity between the two decreased to only 2- to 5-fold, which may not be biologically relevant. Within the amino acid class, compounds in which the amino group was substituted showed the greatest selectivity. These results suggest that hCES2 has much greater affinity for lipophilic substrates than does hCES1. This observation could have important implications for the design of prodrugs of gemcitabine or other similar molecules, in that lipophilic, non-amino acid ester prodrugs would be expected to be hydrolyzed very rapidly in the intestine where expression of hCES2 is high. A similar trend was observed in the monkey, in which selectivity was smallest for amino acid-containing prodrugs. For the more lipophilic non-amino acid substrates, the magnitude of cCES2 selectivity was even greater than in the human, with an almost 20,000-fold higher CLint value for prodrug 13 compared with that for cCES1. Of interest, the dCESs displayed a distinctly different trend than did those of the monkey and human, with dCES2 having lower CLint for all alkyl esters tested. Although the affinity of dCES2 for alkyl chains longer than 4NPA was similar to that for other forms, the rate of hydrolysis was markedly lower than that of hCES2 and cCES2. The same trend was observed for the gemcitabine prodrugs and rates of hydrolysis by dCES2 were lower than those for the other species for all examples. In terms of selectivity, hydrolysis by dCES1 had a CLint value that was consistently equal to or greater than that of dCES2. The cause of the low activity of dCES2 is unknown.
Intrinsic clearance generally increased as the carbon chain length increased, but this finding was not assessed using statistical analyses. In all three species, 4NPA had the lowest clearance values, and either 4NPB or 4NPV had the highest clearance values for both CES1 and CES2 forms, similar to hCES1 and hCES2 with propranolol derivatives (Imai et al., 2006). Whereas substrate affinity for the branched chain compounds was often similar to or higher than that for the linear analogs, the Vmax value was consistently lower. This result suggests that steric bulk near the site of hydrolysis reduces the rate of cleavage despite good affinity for the enzyme. For the gemcitabine esters, there did not appear to be a strong correlation between the steric bulk of the prodrug moiety and clearance for either hCESs or cCESs. For example, the CLint value for substrates having relatively small esters (prodrugs 1–3) was similar to that observed for much larger esters (prodrugs 12 and 13). Hydrolysis of the gemcitabine esters in these species was more dependent on the electronic nature of the ester substituent, with substrates containing polar atoms, such as nitrogen or oxygen, being much less susceptible to hydrolysis than those composed of simple alkyl substituents. For the dog, no clear trend was observed between the rate of hydrolysis and either steric bulk or electronics. Other distinctions between CES1 and CES2 were observed in this study, including the occurrence of non-Michaelis-Menten kinetics by CES2 for some substrates, an observation previously shown with the prodrug prasugrel (Williams et al., 2008b). Williams et al. (2008b) proposed that the observed inhibition of hCES2 could be due to excess substrate inhibition or multiple binding sites for hCES2, but the exact mechanism is unknown and warrants additional study.
Loperamide is a relatively selective inhibitor of hCES2, and as such it provides a useful tool for investigating the involvement of specific CES forms in tissue fractions and in vivo (Quinney et al., 2005). In the current study, the pattern of selectivity observed with hCES2 versus hCES1 appears to hold true in the other species, but there was a distinct difference in potency of inhibition among species. The inhibition of hCES2 by loperamide was 60 times more potent than that for cCES2, and the inhibition of cCES2 was 2 times more potent than that for dCES2. Therefore, loperamide might have utility as a probe inhibitor in all three species, but the concentrations required for full inhibition of CES2 vary considerably among species.
Although the focus of this study was an interspecies comparison of CES1 and CES2 forms, hydrolysis of these compounds was also examined with the poorly characterized human CES3. The extent of hydrolysis by hCES3 was substantially lower than that of hCES1 or hCES2 in this study, similar to a previous report (Quinney et al., 2005). These results suggest that hCES3 is of minor concern for xenobiotic metabolism but may have a specific endogenous role that has yet to be characterized.
CES Expression Patterns in Various Tissues.
A better understanding of organ-level expression is also critical to translating substrate disposition in nonhuman species to that in the clinical setting. Expression patterns of hCES1 and hCES2 were found to be similar for activity-based assays (Taketani et al., 2007) and mRNA expression patterns (Satoh et al., 2002; Quinney et al., 2005). Monkey and dog CES activity in various tissues has also been explored (Taketani et al., 2007). The current study is consistent with previous observations. Whereas the patterns of CES mRNA and protein expression in various tissues are similar between the human and monkey, these patterns differ in the dog. The most significant discrepancies between primates and canines are the lack of dog intestinal CES activity and detection of dCES1 in dog plasma. Because the anti-CES2 antibody demonstrated a weak cross-reactivity with dCES1 and the mRNA quantification studies indicated no detectable dCES2 mRNA, it is possible that the protein detected in dog tissue is actually dCES1. Another interesting observation is the doublet observed related to cCES2 (Taketani et al., 2007), which suggests that monkeys appear to have two related CES2 forms (Williams et al., 2010). The absence of intestinal dCES activity has interesting functional and evolutionary implications. Based on these data, dogs would probably have a reduced capacity to detoxify esters in the intestine. However, this ability is potentially of minor importance in carnivores, which would have infrequent exposure to toxic alkaloids. The loss of dCES2 activity and expression in dogs might reflect a lack of selective pressure to maintain esterase capacity in the gastrointestinal tract. Genomic and functional analyses of other carnivorous species could provide additional insight into the evolutionary and dietary importance of CES2.
In conclusion, the similarities in substrate recognition and tissue expression patterns suggest that the cynomolgus monkey is a promising large-animal model for human CES1 and CES2 metabolism. The beagle dog appears to be a less appropriate animal model for human CESs, because of the difference in its pattern of tissue expression and the dominance of dCES1 efficiency for the compounds tested. Although the cynomolgus monkey is most like humans among the species examined to date, caution should be exercised when using the cynomolgus monkey as a model for human CES1 and CES2 hydrolysis. Depending on the substrate, the hydrolysis rate in the cynomolgus monkey may be significantly higher than that in humans and the sensitivity of monkey CESs to inhibition appears to differ from that of humans. Therefore, it is important to examine comparative in vitro hydrolysis kinetics before conducting in vivo disposition studies.
Authorship Contributions
Participated in research design: Williams, Bacon, Bender, Lowinger, Ehsani, X. Wang, H. Wang, Qian, Ruterbories, Wrighton, and Perkins.
Conducted experiments: Williams, Bacon, Lowinger, Ehsani, X. Wang, and H. Wang.
Contributed new reagents or analytic tools: Bender, Guo, and Ruterbories.
Performed data analysis: Williams, Bacon, Bender, Lowinger, Guo, Ehsani, X. Wang, H. Wang, and Perkins.
Wrote or contributed to the writing of the manuscript: Williams, Bacon, Bender, Lowinger, Guo, Ehsani, X. Wang, H. Wang, Qian, Ruterbories, Wrighton, and Perkins.
Acknowledgments
We appreciate the gracious donation of CES2-selective antibodies by Philip Potter (St. Jude Children's Research Hospital). In addition, we thank Jingqi Bao (Eli Lilly and Company) for his thoughtful discussion of this study and Douglas O'Bannon (Eli Lilly and Company) for graciously providing the 4-nitrophenyl dimethylacetate.
Footnotes
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.111.041335.
-
ABBREVIATIONS:
- CES
- carboxylesterase
- hCES
- human carboxylesterase
- dCES
- dog carboxylesterase
- cCES
- cynomolgus monkey carboxylesterase
- 4NP
- 4-nitrophenol
- 4NPB
- 4-nitrophenyl butyrate
- bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- PBST
- Dulbecco's phosphate-buffered saline containing 1% Tween 20
- 6NC
- 6-nitrocoumarin
- 4NPTMA
- 4-nitrophenyl trimethylacetate
- 4NPGB
- 4-nitrophenyl 4-guanidinobenzoate
- 4NPDMA
- 4-nitrophenyl dimethylacetate
- 4NPA
- 4-nitrophenyl acetate
- 4NPP
- 4-nitrophenyl propionate
- 4NPV
- 4-nitrophenyl valerate
- PCR
- polymerase chain reaction.
- Received July 1, 2011.
- Accepted September 14, 2011.
- Copyright © 2011 by The American Society for Pharmacology and Experimental Therapeutics