Activation of Rat Liver Microsomal UDP-Glucuronosyltransferase Activity by Alamethicin
Abstract
The effects of detergent, alamethicin (a channel-forming peptide), and the inducers phenobarbital and 3-methylcholanthrene on glucuronidation of all-trans-retinoic acid (atRA) and 5,6-epoxy-atRA have been investigated using liver microsomes from Sprague-Dawley and Fischer 344 rats. Conditions for enzymatic glucuronidation were optimized for substrate concentration, protein, and time by using atRA and Sprague-Dawley microsomes. With detergent-activated Sprague-Dawley microsomes, 5,6-epoxy-atRA was shown to be a significantly better substrate than atRA for microsomal glucuronidation (263 vs. 116 pmol/mg/min for 5,6-epoxy-atRA and atRA, respectively). The product of incubation of microsomes with atRA and UDP-glucuronic acid was identified as a glucuronide by β-glucuronidase hydrolysis and by HPLC analysis. Alamethicin was shown to be a highly effective activator of glucuronidation activity; atRA and 5,6-epoxy-atRA glucuronidation rates were increased 2- and 3-fold, respectively, compared with detergent activation. Alamethicin (but not detergent) significantly increased retinoid glucuronidation by microsomes from Fischer 344 rats treated with phenobarbital and 3-methylcholanthrene, compared with untreated controls. The two compounds were equally effective inducers of activity, although 5,6-epoxy-atRA was again the better substrate. The same control and induced Fischer rat microsomes were photolabeled with [32P]5-azido-UDP-glucuronic acid in the absence or presence of detergent, two concentrations of alamethicin, and a 10-fold molar excess of unlabeled UDP-glucuronic acid. Photoincorporation into microsomal proteins from detergent-disrupted induced microsomes was 2–3 times greater than that of controls. Alamethicin increased photoincorporation of the probe into UDP-glucuronosyltransferase proteins an additional 1.5–2-fold in control and induced microsomes, compared with the respective detergent-activated samples.
Glucuronidation constitutes, from a general point of view, a reaction of detoxification and elimination. By conjugating a hydrophilic glucuronic acid from the high-energy donor molecule UDP-GlcUA1 to a large variety of structurally unrelated compounds, UGTs (EC 2.4.1.17) play an important role not only in the detoxification of drugs and other xenobiotics but also in the metabolism of endogenous substances. UGTs have been implicated in the metabolism and clearance of endogenous compounds like bilirubin (1), short-chain fatty acids and bile acids (2-5), and steroid and thyroid hormones (6, 7). More recently, it has become recognized that glucuronidation must also be considered as a mechanism of toxification or activation. The acyl (or carboxyl) glucuronide of lithocholic acid and steroid D-ring glucuronides have been shown to be more potent cholestatic agents than their parent compounds (8-10), whereas morphine-6-glucuronide is significantly more potent an analgesic than morphine itself and may well be responsible for the analgesic effects attributed to the parent compound (11).
Compounds that contain a carboxylic acid functional group are often metabolized by conjugation with glucuronic acid to yield acyl, or ester, glucuronides. The retinoids represent an example of this class of compounds. Rats being dosed orally with atRA secrete significant amounts of atRAG in the bile (12-16). atRAG is also found in plasma of monkeys orally dosed with atRA (17). In humans, glucuronidation of endogenous, as well as exogenous, atRA occurs, because atRAG has been identified as a normal constituent of human plasma (18). When 13-cis-RA was administered to monkeys, its glucuronide conjugate was found in plasma, albeit at much lower levels than when atRA was given (17). The same authors reported measurable amounts of atRAG and 13-cis-RAG in the plasma and urine of a woman treated for 27 days with 13-cis-RA (17). In addition, the glucuronides of 4-oxo-13-cis-RA have been detected as biliary metabolites in rats (19).
Glucuronidation of retinoids may be physiologically significant for the organism for several reasons. The active agent actually responsible for some of the pharmacological and physiological effects resulting from administration of retinoids may be a glucuronide. In support of this, atRAG has been shown to be at least as effective as atRA in stimulating rat growth, inducing differentiation of HL-60 cells or vaginal epithelium, and inhibiting DNA synthesis in mouse mammary gland, while at the same time being less cytotoxic (20). Also, because atRAG purportedly is less teratogenic than the parent compound (21), glucuronidation may serve as a mechanism to protect sensitive fetal tissues from possible damage by retinoids.
The present study was prompted by the fact that, although retinoid glucuronides have been identified as products of retinoid metabolism in several species, little attention has been paid to the enzymes responsible for the conjugation process, the UGTs. We have combined several techniques that been effective in characterizing rat and human liver microsomal UGTs as a group (22-26) with analytical techniques developed for the identification of glucuronidated products of in vivo and in vitro metabolic processes (2, 3, 27-29) for the study of retinoid glucuronidation in rat liver microsomes. Alamethicin, a channel-forming antibiotic, has been reported to be significantly more effective than detergent in overcoming the latency of 4-nitrophenol UGT activity in isolated mouse hepatocytes (30) and, in our hands, produced a 2-fold increase in microsomal hyodeoxycholic acid glucuronidation, compared with detergent (A. Radominska, unpublished observations). Therefore, we have used both detergent (Brij-58) and alamethicin as activators of microsomal retinoid UGT activity. atRAG and 5,6-epoxy-atRAG have been identified as significant products of in vitro incubations of atRA and 5,6-epoxy-atRA with detergent-disrupted rat liver microsomes. To our knowledge, this is the first report of the in vitro glucuronidation of 5,6-epoxy-atRA by rat liver microsomes. Treatment of microsomes with alamethicin rather than detergent significantly increased glucuronidation of both substrates, and only in the presence of alamethicin was it possible to detect any induction of retinoid glucuronidation by PB or 3-MC.
Materials and Methods
Chemicals.
[11,12-3H]atRA was purchased from DuPont-New England Nuclear (Boston, MA). Brij-58, UDP-GlcUA, saccharolactone, and alamethicin were purchased from Sigma Chemical Co. (St. Louis, MO). Unlabeled atRA and 13-cis-RA [(13Z)-RA] were obtained from Aldrich (Milwaukee, WI). The atRAG used as a standard for HPLC analyses was chemically synthesized in our laboratory using the method developed for the synthesis of the acyl glucuronide of lithocholic acid (31). 5,6-Epoxy-atRA was synthesized from atRA by oxidation with monoperoxyphthalic acid, as described by Wertz et al. (32), and its identity and purity were established by NMR and MS analysis.
Animals and Microsome Preparations.
Male Sprague-Dawley and Fischer 344 rats (200–250 g) were used for all studies. For UGT induction studies, rats were treated with PB (ip injection of 100 mg/kg body weight PB in saline, followed by 0.1% PB in drinking water for 4 days) or 3-MC (ip injection of 40 mg/kg in olive oil 3–4 days before sacrifice). Comparable rats were left untreated to serve as controls. Rats were fasted overnight before sacrifice, livers were excised, and microsomes were prepared as described in (33), except that they were not further subfractionated into rough and smooth endoplasmic reticulum fractions.
Enzymatic Assays.
Enzymatic retinoid glucuronidation activity in rat and human liver microsomes was assessed using a modification of TLC techniques developed for the assay of bile acid glucuronidation (2, 3). Initially, the detergent Brij-58 (final concentration, 0.05%) was used both to solubilize the hydrophobic substrate into micellar form and to activate the enzymatic activity of the microsomal UGTs. In later experiments where alamethicin (60 or 120 μg/mg of protein) was the activating agent, both it and the retinoid substrate were dissolved in 100% ethanol (alamethicin, 6 mg/ml; retinoid, 6 mM) and added directly to the incubation, keeping the total ethanol concentration below 5%. The incubation mixture routinely contained 30 μg of microsomal proteins, 5 mM MgCl2, 5 mM saccharolactone, 100 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer, pH 7.4, and 100 μM retinoid substrate, in a final volume of 60 μl. UDP-GlcUA (4 mM) was provided in either unlabeled or 14C-labeled form. Control incubations lacking either UDP-GlcUA or retinoid substrate were run with each experiment. Reactions were incubated for 30 min at 37°C and were stopped by addition of 20 μl of ethanol. Aliquots (60 μl) of the samples were applied to the preabsorbent layer of channeled silica gel TLC plates [Baker 250Si-PA (19C); VWR Scientific], dried, and developed in chloroform/methanol/glacial acetic acid/water (65:25:2:4, v/v). All manipulations to this point were carried out under yellow light. After development, plates were dried and subjected to autoradiography for 3–7 days at −80°C. Plates from experiments using [3H]atRA were sprayed with En3Hance (DuPont-New England Nuclear, Boston, MA) before autoradiography. Silica gel containing labeled metabolites (localized using the autoradiographs) and that from corresponding areas in control lanes were scraped into vials, and radioactivity was determined by scintillation counting (LKB RackBeta 1214; Wallac Inc., Gaithersburg, MD). Approximate RF values were as follows: atRAG and (13Z)-RAG, 0.25–0.30; atRA and (13Z)-RA, 0.91; 5,6-epoxy-atRAG, 0.26. For experiments using β-glucuronidase, [3H]atRA/Brij-58 micelles were incubated under standard conditions with UDP-GlcUA and Sprague-Dawley microsomes, followed by an additional 2-hr incubation at 37°C with 100 units ofEscherichia coli β-glucuronidase (Sigma) in the absence or presence of 12.5 mM saccharolactone.
HPLC Analysis.
For HPLC analysis, multiple incubations were carried out under the condition described above; control incubations contained no UDP-GlcUA. Incubations were stopped by addition of and extraction with 2-propanol; extracts were pooled, concentrated, and partially purified by passage through a solid-phase extraction column. HPLC analysis was carried out with a Hewlett-Packard 1090 M Series II fully automated HPLC system with a diode-array detector. The solvent system consisted of 0.1 M ammonium acetate, pH 5.0, mixed with acetonitrile in a gradient initialized at 80% aqueous buffer/20% acetonitrile and ramped to 5% aqueous buffer/95% acetonitrile. Samples were injected onto two reverse-phase Waters Nova-pack columns mounted in series (3.9 mm × 150 mm and 3.9 × 75 mm), at a flow rate of 0.5 ml/min. Eluting peaks were monitored at selected wavelengths (320–360 nm) to ensure detection of all relevant RA metabolites. Effluent from the UV detector was supplemented with OptiFluor scintillation cocktail (Packard Instruments, Downer’s Grove, IL) and directed through a Packard Flow-One radiochromatography detector.
Photoaffinity Labeling of Microsomes with [32P]5N3UDP-GlcUA.
The standard protocol for photolabeling of rat liver microsomes with [32P]5N3UDP-GlcUA was described in detail previously (23, 24, 34, 35). The procedure has been modified as follows: rat liver microsomes (50 μg of protein) were incubated for 10 min, on ice, in the presence or absence of 0.05% Triton and 60 or 120 μg of alamethicin/mg of protein, before addition of [32P]5N3UDP-GlcUA and subsequent UV irradiation. Because the alamethicin was added as an ethanol solution, the ethanol concentration of all samples was equalized (final concentration, 2%). Reactions were stopped with 10% trichloroacetic acid (150 μl) and processed for sodium dodecyl sulfate-polyacrylamide gel electrophoresis as previously described (34). Proteins were separated on 10% gels (36), followed by autoradiography for 1–3 days.
Results and Discussion
Enzymatic Glucuronidation of atRA.
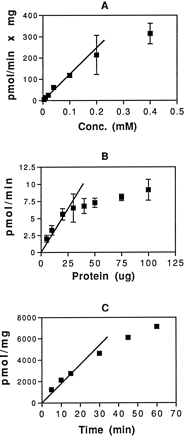
Conditions for microsomal glucuronidation of atRA were established using detergent-activated Sprague-Dawley rat liver microsomes. Fig.1 shows the dependence of atRA glucuronidation on substrate concentration (fig. 1A), protein concentration (fig. 1B), and time of incubation (fig. 1C). The following standard conditions were chosen: 0.1 mM atRA, 30 μg of microsomal protein, and 30-min incubation time. For the alternative alamethicin protocol, atRA solubility in ethanol limited us to a final concentration of 0.1 mM atRA. This was not a saturating concentration for the detergent-activated system but it was decided, for the sake of comparison, to use the 0.1 mM concentration for both systems. With this protocol, atRA glucuronidation activity was 69.2 ± 16.5 pmol/mg/min in intact (not detergent-activated) microsomes and 116.3 ± 50.8 pmol/mg/min in detergent-activated microsomes. These activities are significantly higher than the previously reported values of 4–14 pmol/mg/min (37, 38). Among the possible explanations for the discrepancy are incomplete activation of the enzyme by the 0.04% Tween 40 used in the earlier work, differences in microsome preparation, or variations in activity between different rat strains [the Wistar rats used by Miller and DeLuca (38) are known to have at least one mutation, resulting in reduced activity of the 3-hydroxysteroid UGT]. It should be noted that these values are the sum of the activities in two incompletely resolved labeled bands on the TLC plate. HPLC analysis of the reaction products, presented in full below, provided evidence that the minor polar product was the glucuronide of 13-cis-RA, which is a product of naturally occurring isomerization of atRA. Although the TLC assay used here does not provide the product resolution obtained in the HPLC analysis used in previous reports of microsomal glucuronidation of atRA, it does offer several advantages; access to HPLC equipment is not necessary, incubation mixtures need not be extracted before analysis, and multiple reactions can be processed in a relatively short period of time. Also, it is possible that the chromatographic conditions can be adjusted to provide separation of the glucuronides of various derivatives of atRA.
Establishment of conditions for enzymatic glucuronidation of RA.
A, Detergent-activated Sprague-Dawley microsomes (100 μg) were incubated with 4 mM UDP-GlcUA and varying concentrations of [3H]atRA for 30 min at 37°C. Separation and quantitation of product were as described in the text.B, Increasing amounts of detergent-activated Sprague-Dawley microsomal protein were incubated with 4 mM UDP-GlcUA and 100 μM [3H]atRA for 30 min at 37°C. Separation and quantitation of product were as described in the text.C, Detergent-activated Sprague-Dawley microsomes (30 μg) were incubated with 4 mM UDP-GlcUA and 100 μM [3H]atRA at 37°C for varying periods of time. Separation and quantitation of product were as described in the text. Results are expressed as the mean of duplicate samples from at least two experiments. The brackets give the standard deviation.
Identification of Reaction Products as Glucuronide Conjugates.
Initial confirmation that the polar reaction products were indeed glucuronide conjugates came from the complete UDP-GlcUA dependence of the reaction; no polar labeled bands were found in samples from which UDP-GlcUA was omitted. Additional proof was gained from experiments in which incubation of microsomes with [3H]atRA and UDP-GlcUA was followed by an additional incubation with β-glucuronidase in the absence or presence of saccharolactone, an inhibitor of β-glucuronidase activity (table 1). Control incubations were stopped after the standard 30-min incubation time and had an activity of 114.9 ± 6.8 pmol/mg/min. Further incubation of identical samples with β-glucuronidase in the absence of saccharolactone decreased the recovered activity to 33.3 ± 0.4 pmol/mg/min (29% of control activity), whereas incubation in the presence of saccharolactone resulted in the recovery of 92% (105.2 ± 6.3 pmol/mg/min) of the control activity.
Hydrolysis of biosynthetic atRAG by β-glucuronidase
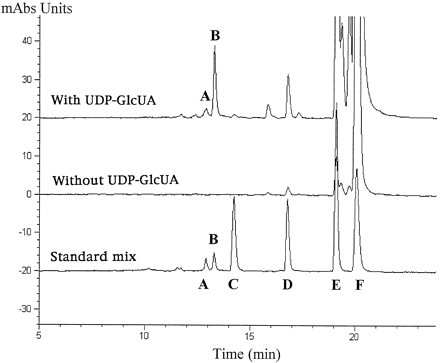
Final verification of the identify of the metabolites as glucuronide conjugates was obtained from HPLC analysis of a semipreparative biosynthetic preparation. The HPLC elution profiles of the reaction products recovered from incubations done in the presence and absence of UDP-GlcUA were compared with those of authentic standards of atRAG, atRA, (13Z)-RAG, (13Z)-RA, and 5,6-epoxy-atRA. The results shown in fig. 2 clearly demonstrate that glucuronide conjugation occurred only in incubation mixtures containing UDP-GlcUA, with atRAG being the major product. A small amount (<10%) of (13Z)-RAG was also detected, most likely the result ofcis/trans-isomerization in the course of microsomal incubations (38). The biosynthetic products were identical to the standard glucuronides, when compared on the basis of retention time and UV spectral characteristics. Radiochromatographic analysis of the effluent detected a peak of radioactivity corresponding to the atRAG peak. The (13Z)-RAG present in the standard was a byproduct of the chemical synthesis of atRAG, formed either in the course of the reaction or from (13Z)-RA present in the starting material. Only unconjugated retinoids were detected in control samples incubated in the absence of UDP-GlcUA.
HPLC chromatogram of biosynthetic atRA metabolites.
Detergent-activated Sprague-Dawley microsomes were incubated with [3H]atRA in the absence or presence of UDP-GlcUA. Details of sample preparation and HPLC conditions are given in the text. Peaks are identified as follows: A, 13-cis-RAG; B, atRAG; C, 4-oxo-atRA; D, 5,6-epoxy-atRA; E, 13-cis-RA; F, atRA.
Effect of Alamethicin on Enzymatic Glucuronidation of atRA and 5,6-Epoxy-atRA.
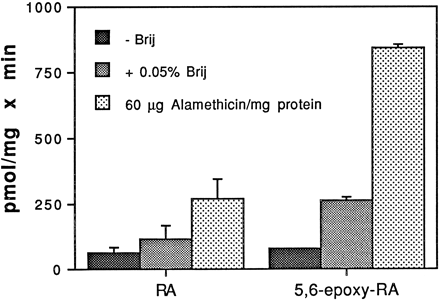
Fig. 3 shows the results of experiments in which Sprague-Dawley microsomes were incubated with [3H]atRA and UDP-GlcUA or 5,6-epoxy-atRA and [14C]UDP-GlcUA, in the absence or presence of Brij-58 and alamethicin. With atRA as substrate, preincubation of microsomes with detergent and alamethicin resulted in 2-fold and 4-fold increases, respectively, in glucuronidation activity (63.6 ± 19.7, 116.3 ± 50.8, and 270.9 ± 73.8 pmol/mg/min for control, detergent-treated, and alamethicin-treated microsomes, respectively). In control microsomes, glucuronidation of 5,6-epoxy-atRA (78.7 ± 2.7 pmol/mg/min) was not significantly different from that of atRA; however, in both detergent- and alamethicin-activated microsomes, 5,6-epoxy-atRA was glucuronidated at rates 2–3 times greater than those for atRA (262.9 ± 13.6 and 842.5 ± 13.6 pmol/mg/min for detergent and alamethicin, respectively).
Glucuronidation of [3H]atRA and 5,6-epoxy-atRA by Sprague-Dawley microsomes.
Microsomes (30 μg) were incubated with 100 μM [3H]atRA and 4 mM UDP-GlcUA or 100 μM 5,6-epoxy-atRA and 4 mM [14C]UDP-GlcUA for 30 min at 37°C, in the absence or presence of 0.05% Brij-58 and 60 μg of alamethicin/mg of protein. Separation and quantitation of product were as described in the text. Results are expressed as the mean of duplicate samples from at least two experiments. The brackets give the standard deviation.
Alamethicin is a channel-forming peptide (39) that has proven to be a very effective activator of UGT activity toward 4-nitrophenol (30) and hyodeoxycholic acid (A. Radominska, unpublished data) and, thus, it is not surprising that microsomal glucuronidation of atRA is significantly increased by preincubation with alamethicin. However, the real significance of these results is that the use of alamethicin, in conjunction with solubilization of atRA in ethanol, rather than in detergent micelles, allows atRA glucuronidation activity to be measured in the complete absence of detergent. Latency has always been a problem in determining maximal UGT activity in microsomes, and the standard method for overcoming this problem has been to use detergent to disrupt the membranes. In many cases, however, detergent can inhibit UGT activity even as it permeabilizes the membrane. Indeed, preliminary experiments demonstrated that there was no stimulation of atRA glucuronidation activity by alamethicin in the presence of detergent (data not shown).
Effect of UGT Inducers on Glucuronidation of atRA and 5,6-Epoxy-atRA.
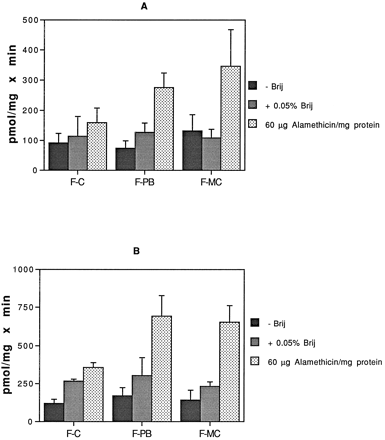
Liver microsomes prepared from Fischer 344 rats treated with PB and 3-MC, known inducers of UGTs, were assayed for glucuronidation activity. Fischer rats were chosen because they are a mutant strain lacking 3-hydroxysteroid UGT (UGT2B4) activity (40) (A. Radominska, unpublished observations); thus, using this strain of rat allows us to eliminate the involvement of this isoform in atRA glucuronidation. Fig.4 shows the effect of Brij-58 and alamethicin on the enzymatic glucuronidation of [3H]atRA (fig.4A) and 5,6-epoxy-atRA (fig. 4B) by control, PB-induced, and 3-MC induced Fischer rat liver microsomes. With both substrates, Brij-58 and alamethicin produced similar patterns of activation of glucuronidation by PB- and 3-MC-induced Fischer microsomes; however, alamethicin treatment of control Fischer microsomes did not result in a significant additional activation, compared with that produced by Brij-58. Glucuronidation of both substrates was increased almost 2-fold by PB and 3-MC treatment, compared with control microsomes, when alamethicin was used for activation, whereas little or no induction was seen when Brij-58 was used. Although we have no definitive explanation for this difference, it is possible that the protein synthesis induced by PB and 3-MC treatment makes the endoplasmic reticulum membrane more sensitive to alamethicin. Sass et al. (41) reported that, in 3-MC-treated Wistar rat liver microsomes incubated with Brij-58, glucuronidation of atRA and 13-cis-RA was reduced by one-third, compared with unactivated microsomes. Those authors suggested that lysophosphatidylcholines, found to be generated in increased amounts during incubation of 3-MC-treated microsomes, act as endogenous detergents to fully activate the UGTs. It is unclear from that report whether lysophosphatidylcholine levels in PB-treated microsomes were measured as well (reference was made to unpublished results of one of the authors), but the study does indicate the differential effects that activators may have on control and induced microsomes.
Glucuronidation of [3H]atRA and 5,6-epoxy-atRA by microsomes from untreated (F-C), PB-treated (F-PB), and 3-MC-treated (F-MC) Fischer 344 rats.
Microsomes (30 μg) were incubated with 100 μM [3H]atRA and 4 mM UDP-GlcUA (A) or 100 μM 5,6-epoxy-atRA and 4 mM [14C]UDP-GlcUA (B) for 30 min at 37°C, in the absence or presence of 0.05% Brij-58 and 60 μg of alamethicin/mg of protein. Separation and quantitation of product were as described in the text. Results are expressed as the mean of duplicate samples from at least two experiments. The brackets give the standard deviation.
Photoaffinity Labeling of Fischer Rat Liver Microsomes with [32P]5N3UDP-GlcUA.
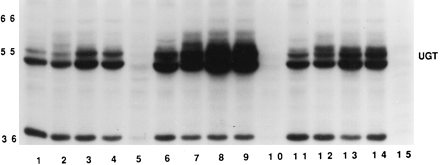
In photoaffinity labeling, the highly radioactive probe [32P]5N3UDP-GlcUA becomes covalently bound, upon UV irradiation, to proteins that utilize UDP-GlcUA as a substrate, such as the UGTs. This technique has proven to be very useful for characterizing rat and human microsomal proteins in their native membrane environment (22, 26, 42) and has been used to demonstrate induction of total UGT protein in microsomes from PB-treated Fischer rats (35). We used the technique here to assess the effect of alamethicin on photoincorporation of [32P]5N3UDP-GlcUA into microsomes from control and PB- and 3-MC-induced Fischer rats. The autoradiograph in fig. 5 and the densitometric quantitation in table2 are from a representative experiment in which microsomes from control and PB- and 3-MC-induced Fischer rats were photolabeled with [32P]5N3UDP-GlcUA in the absence or presence of detergent, two concentrations of alamethicin, or a 10-fold molar excess of unlabeled UDP-GlcUA. From fig. 5 it is obvious that, in comparison with control microsomes, photoincorporation of [32P]5N3UDP-GlcUA into the 50–56-kDa UGT proteins was increased dramatically by PB and to a lesser extent by 3-MC. The 37-kDa labeled band was identified previously as the cytoplasmically oriented UDP-glucose/dolichylphosphate-glucosyltransferase (22, 23, 43). The intensity of photolabeling was quantitated by densitometry of the autoradiograph, and the results were compared either within each treatment group, by assigning a value of 100% to the no-detergent sample, or between groups, by assigning a value of 100% to each control sample. The results are tabulated in table 2. Photoincorporation of [32P]5N3UDP-GlcUA into control, PB-induced, and 3-MC-induced microsomes was affected to similar extents by each of the experimental manipulations; addition of detergent resulted in a slight increase in photolabeling (10–20%), alamethicin at 60 μg/mg of protein produced significantly greater photoincorporation (60–100%), increasing the concentration of alamethicin to 120 μg/mg resulted in a slight decrease in photolabeling, compared with the lower concentration, and unlabeled UDP-GlcUA very effectively protected against photoincorporation of the probe. Thus, with photolabeling, as with the enzymatic assays, alamethicin was shown to be a highly effective agent for activation of UGTs.
Photoaffinity labeling of Fischer rat liver microsomes by [32P]5N3UDP-GlcUA.
Microsomes (100 μg of protein) from control (lanes 1-5), PB-induced (lanes 6-10), and 3-MC-induced (lanes 11-15) Fischer rats were photolabeled with 40 μM [32P]5N3UDP-GlcUA (see text).Lanes 1, 6, and 11, no additions; lanes 2, 7, and12, 0.05% Triton-X100; lanes 3,8, and 13, 60 μg of alamethicin/mg of protein; lanes 4, 9, and14, 120 μg of alamethicin/mg of protein; lanes 5, 10, and 15, 0.4 mM UDP-GlcUA. The 37-kDa labeled band is UDP-glucose:dolichyl phosphate glucosyltransferase. The autoradiograph shown is representative of three identical experiments.
Quantitation of the intensity of photolabeling of 50–56-kDa proteins by [32P]5N3UDP-GlcUA
On the other hand, PB was roughly twice as effective as 3-MC in terms of photoincorporation of [32P]5N3UDP-GlcUA, whereas there was little or no difference between the two compounds in terms of enzymatic glucuronidation of atRA. The reason for this discrepancy can be found in the basic difference between the experimental techniques. Enzymatic assays are specific for the aglycone substrate, in this case atRA, and involve one or, at most, a limited number of UGT isoforms. In contrast, photoaffinity labeling with [32P]5N3UDP-GlcUA is based on binding of UDP-GlcUA; because this is the obligatory co-substrate for all UGTs, all isoforms (and other UDP-GlcUA-binding proteins) photoincorporate [32P]5N3UDP-GlcUA, not just the isoform(s) specific for atRA glucuronidation. Preliminary results from studies in which the same microsomes were photolabeled with [3H]atRA indicated that alamethicin had no effect on the photoincorporation of this aglycone photoprobe. The results suggest that alamethicin stimulates enzymatic activity and [32P]5N3UDP-GlcUA photoincorporation by increasing the availability of UDP-GlcUA to the UGT active site on the luminal side of the microsomal membrane.
In summary, these studies have demonstrated the effectiveness of alamethicin for activation of rat liver microsomal UGTs specific for retinoids, without the use of detergents, which may inhibit UGT activity. The enzymatic activities for atRA determined in these studies with alamethicin are higher than most of the activities found in the literature. Additionally, to our knowledge, this is the first report of the in vitro glucuronidation of 5,6-epoxy-atRA by rat liver microsomes, although intestinal glucuronidation of 5,6-epoxy-atRA has been reported (44). PB and 3-MC have been recognized for years as inducers of hepatic UGTs in general, and these results confirm at least one published report of their induction of retinoid (atRA and 13-cis-RA) glucuronidation (41). That previous study, which was carried out with Wistar rats, showed that 3-MC pretreatment increased microsomal atRA glucuronidation, whereas PB treatment did not. In contrast, the data presented here for Fischer 344 rats indicate that the two compounds are equally effective inducing agents for enzymatic glucuronidation of atRA and 5,6-epoxy-atRA. Further study will be needed to establish the effect of strain differences on rat liver microsomal retinoid UGT induction by PB and 3-MC.
Footnotes
-
Send reprint requests to: Joanna M. Little, Division of Gastroenterology, University of Arkansas for Medical Sciences, 4301 W. Markham, Slot 567, Little Rock, AR 72205.
-
This work was supported by National Institutes of Health Grant DK45123 (A.R.).
- Abbreviations used are::
- UDP-GlcUA
- UDP-glucuronic acid
- UGT
- UDP-glucuronosyltransferase
- atRA
- all-trans-retinoic acid
- atRAG
- all-trans-retinoyl-β-glucuronide
- RA
- retinoic acid
- RAG
- retinoyl-β-glucuronide
- 3-MC
- 3-methylcholanthrene
- PB
- phenobarbital
- Received April 10, 1996.
- Accepted October 1, 1996.
- The American Society for Pharmacology and Experimental Therapeutics