Comparison with Human Liver Samples
Abstract
Cytochrome P450 (CYP) of the 3A family (CYP3A) has been detected in minipig liver microsomes by immunochemical screening (Western blotting), revealing bands that co-migrate with human CYP3A4 and 3A5. The nifedipine oxidase activity and testosterone 6β-hydroxylating activity (specific markers for CYP3A enzymes) of the human liver microsomal and minipig liver microsomal samples were comparable, as were the results of specific inhibition of this activity by triacetyloleandomycin. The presence of CYP1A, 2A, 2C, 2D, and 2E1 marker activities in minipig liver microsomes was found by testing with the respective specific substrates (7-ethoxyresorufin, coumarin, tolbutamide, bufuralol, and chlorzoxazone). 7-PentoxyresorufinO-depentylase activity (indicative of CYP2B) was absent from minipig as well as human liver microsomal samples. The results indicate that minipigs might be, in many cases, the most suitable experimental animals to predict biotransformation pathways in humans, because the activity of the most important CYP isoform in humans (CYP3A, metabolizing the majority of known drug substrates) is present in minipigs, with comparable levels and activities. Moreover, there is no need to induce CYP enzyme levels.
Although alternative methods for routine testing of drugs and other xenobiotics are able to replace the use of laboratory animals in many cases, the use of experimental animals is indispensable. To choose an appropriate experimental model relevant to human metabolism, it is highly desirable to understand species differences in the metabolism of xenobiotics. One of the most important sources of interspecies variation is the difference in the content and activity of hepatic CYP1 enzymes (Smith, 1991). Recent progress in the characterization of CYP isoforms in rats, mice, rabbits, and humans revealed variations in the content of the principal CYP isoforms. For example, the most important form of this enzyme involved in drug biotransformation in humans, i.e. CYP3A4 [CYP3A4 can comprise as much as 60% of total CYP in human liver and, moreover, this form has been shown to be responsible for metabolism of the majority of drugs tested (Guengerich, 1995a,b)], does not have a direct counterpart in uninduced rats (Souc̆ek and Gut, 1992). This is reflected in the fact that a typical substrate of human CYP3A4, nifedipine, is metabolized in rats by different isoforms from the CYP2C subfamily (Pelkonen and Breimer, 1994). In most experimental animals, the most well-known forms are members of the CYP2B subfamily, which are only minor in human samples.
Pigs and minipigs have been used as experimental animals for a relatively long time, because many of their physiological characteristics are close to those of humans (Green, 1979). Although there are reports in the literature indicating similarities in drug metabolism between humans and pigs (Namara, 1991), pigs are used only rarely, apparently because of problems with handling and because of their relatively high cost. Because minipigs have been used as experimental models in this laboratory, we have been interested in answering the question of whether there is a significant amount of an active form of the CYP3A type present in animal liver. During the course of this study, two reports have been published that indicate the presence of a CYP3A4-like form in pig liver, using cross-hybridization between pig mRNA and a cDNA probe for human CYP3A4 (Monshouwer et al., 1995) and showing inhibition of the CYP3A activities in pigs by the veterinary antibiotic tiamulin (Witkamp et al., 1995). Also, the presence of a CYP3A protein in pig intestinal microsomes has been indicated in the recent work on the metabolism of the immunosuppressant tacrolimus in humans, rats, and pigs (Lampenet al., 1995). This study presents proof of the presence of CYP3A4-like form in minipig liver microsomes and shows that the amount and activity of minipig liver microsomal CYP3A are comparable to those of the human enzyme.
Materials and Methods
Chemicals.
All chemicals were of reagent grade, obtained from either Sigma Chemical Co. (St. Louis, MO) (NADPH, NADP, glucose-6-phosphate, glucose-6-phosphate dehydrogenase, nifedipine, TAO, anti-rabbit IgG coupled to horseradish peroxidase, and chemicals for activity testing), Bio-Rad (Hercules, CA) (reagents for electrophoresis and immunoblotting), or Lachema (Brno, Czech Republic) (reagents for the preparation of microsomes). Polyclonal rabbit anti-CYP3A4 IgG was prepared at the laboratory of Prof. F. P. Guengerich (Center for Molecular Toxicology, Vanderbilt University, Nashville, TN). Samples of purified recombinant human CYP3A4 and 3A5, as well as an oxidized nifedipine standard, were generous gifts from Prof. Guengerich.
Preparation of Microsomes.
Minipig liver microsomes were prepared from experimental animals (Brno White variety of Goettingen minipig; Research Institute of Veterinary Medicine, Brno, Czech Republic) weighing 22–31 kg (male; age, 6 months). The animals (N = 7) were fed standard diet for pigs (A1) mixed with bread (0.5 kg each day), and essentially no CYP induction was performed during this experiment. The animals were killed by a single shot and exsanguinated; liver samples were taken from the medial lobes. Microsomal fractions of liver homogenates were prepared as described by van der Hoeven and Coon (1974). Rats (Wistar, male, 200–250 g; Velaz, Prague, Czech Republic) were pretreated with pregnenolone-16α-carbonitrile ip (25 mg/kg, in corn oil, five times within 3 days before sacrifice) to induce the CYP3A enzymes in rat liver. The microsomes were prepared according to the aforementioned method (van der Hoeven and Coon, 1974). Human liver samples were obtained from the Institute for Clinical and Experimental Medicine (Prague, Czech Republic), from organ donors. The microsomal fraction was prepared by the aforementioned method (van der Hoeven and Coon, 1974). All experiments were approved by the respective ethical committees. Microsomal fractions were kept frozen at −70°C until used.
Electrophoresis.
Electrophoresis was performed according to the method of Laemmli (1970), using the modification described by O’Farrell (1975). Polyacrylamide (10%, w/v) gels were used. Immunoblotting of proteins was performed according to the method of Towbin et al.(1979). Blots were incubated with primary antibody for 2 hr at 37°C. Polyclonal anti-human CYP3A4 IgG was used at 50 μg/10 ml of buffer (20 mM potassium phosphate, pH 7.4, with 150 mM NaCl and 0.05%, w/v, Tween 20). After extensive washing, the blots were incubated for 1 hr at room temperature with anti-rabbit IgG coupled to horseradish peroxidase. The blots were washed again and color was developed by treatment with a mixture of 2.5 mM 4-chloro-1-naphthol in 10 ml of methanol and 15 mM hydrogen peroxide in the aforementioned buffer without Tween 20.
Activity Assays and Chemical Inhibition.
All incubations were carried out in glass tubes (amber vials for nifedipine), in a shaking water bath, at 37°C for 15 min. The incubation mixtures contained 100 pmol of total CYP, an NADPH-generating system (10 mM MgCl2, 5 mM glucose-6-phosphate, 0.5 mM NADP, and 0.5 unit of glucose-6-phosphate dehydrogenase, EC 1.1.1.49), and 0.2 mM nifedipine substrate. Buffer (50 mM Tris-HCl, pH 7.4, with 150 mM KCl) was added to bring the final volume to 0.5 ml. In experiments with the chemical inhibitor TAO, 50, 100, and 500 μM concentrations of TAO were used. TAO was dissolved in methanol and added first, and then the methanol was evaporated under a gentle stream of nitrogen. Microsomes were preincubated with TAO in the presence of 10 μl of 2.5 mM NADPH, in the same buffer, for 20 min at 37°C. The substrate (nifedipine) was then added, and the incubation was performed as described above. The main metabolite, i.e.oxidized nifedipine, was assayed using HPLC according to the method ofGuengerich et al. (1986). Activities typical of individual CYP isoforms were tested according to established protocols, as follows: 7-ethoxyresorufin O-deethylase and 7-pentoxyresorufin O-depentylase activities for CYP1A and CYP2B, respectively (Lubet et al., 1985), tolbutamide methyl-hydroxylation for CYP2C (Knodell et al., 1987), bufuralol 1′-hydroxylation for CYP2D (Yamazaki et al., 1994), chlorzoxazone 6-hydroxylation for CYP2E1 (Peter et al., 1990), testosterone 6β-hydroxylation for CYP3A (Brianet al., 1990), and coumarin 7-hydroxylation for CYP2A (Yunet al., 1991).
Other Assays.
Total protein content was estimated by the bicinchoninic acid method, as described by the producer (Pierce, Rockford, IL). CYP concentrations in microsomes were estimated using the method of Omura and Sato (1964). The presence of CYP3A was determined by measuring a type III spectral complex with TAO, according to the method of Franklin (1991).
Results and Discussion
Identification of CYP3A in Minipigs.
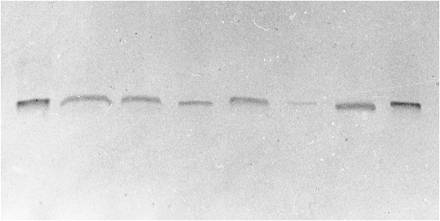
Minipig liver microsomes were screened by immunoblotting using anti-human CYP3A4 antibodies. Human liver microsomes and microsomes from pregnenolone-16α-carbonitrile-treated rats were used to compare relative amounts of CYP3A enzyme in these species. Purified recombinant human liver CYP3A4 and 3A5 forms were also present in the blots. A positive band co-migrating with purified human CYP3A4 was identified in minipig liver microsomes (fig. 1), indicating that there is a reasonable amount of CYP3A enzyme. The relative intensities of this band were comparable in the two preparations of minipig microsomes analyzed (data not shown). On the other hand, these antibodies do not cross-react with other human CYP proteins (1A1, 1A2, 2C10, or 2E1) (data not shown). Thus, it seems that minipigs have protein immunochemically similar to human CYP3A4 and/or 3A5. The presence of the CYP3A form was also verified by formation of a typical difference absorption spectrum of the TAO metabolite complex at 456 nm, as seen in fig. 2 (Franklin, 1991; Watkins et al., 1985).
Immunochemical detection of minipig CYP3A.
Lanes (from left toright): 1, purified human liver CYP3A4 (0.5 pmol); 2, liver microsomes from pregnenolone-16α-carbonitrile-treated rats (2.5 μg of protein);3, purified human liver CYP3A5 (1 pmol);4, human liver microsomes (5 μg); 5, purified human liver CYP3A5 (2 pmol); 6, minipig liver microsomes (10 μg); 7, minipig liver microsomes (20 μg); 8, purified human liver CYP3A4 (1 pmol). Electrophoresis and immunoblotting were performed as described inMaterials and Methods.
Formation of a CYP3A-TAO metabolite complex.
A, Time course of formation of a CYP3A-TAO complex, measured at 456 nm. B, Difference spectrum after 10 min, with a wavelength maximum of 456.2 nm.
Catalytic Activity of the Minipig CYP3A Enzyme.
The similarity in the apoprotein structure of minipig and human 3A enzymes (as shown by immunochemical screening, i.e. Western blotting) needed to be confirmed by similar or comparable activities of minipig and human microsomes in oxidizing a typical substrate for the human CYP3A4 enzyme (nifedipine), as well as hydroxylating another diagnostic substrate for CYP enzymes (testosterone) in the 6β-position (Pelkonen and Breimer, 1994; Guengerich, 1995b). Experiments showed that the rate of nifedipine oxidation in minipig liver microsomes (N = 7) was similar to the rate observed with human microsomes (table 1). Typical results for human microsomes in this work ranged between 1 and 3 nmol of oxidized nifedipine/nmol of CYP/min [similar results were also seen for 18 randomly selected, human liver microsomal samples (Guengerich, 1995b)]. The testosterone 6β-hydroxylating activity of minipig microsomes ranged between 1 and 2 nmol of hydroxylated product/nmol of CYP/min, corresponding to values for human liver microsomal activity (ranging between 1 and 5 nmol of product/nmol of CYP/min). Testosterone 6β-hydroxylation was highly correlated with the oxidation of nifedipine in human liver microsomes (r = 0.90), whereas in minipig microsomes the correlation was poorer (r = 0.75). The results demonstrate that the minipig CYP3A enzyme has activities toward the CYP3A4 marker nifedipine and another diagnostic substrate, testosterone, comparable to those of the human enzyme.
Enzyme activities characteristic of various CYP isoforms in microsomes of minipig and human origin
Catalytic Activities of Other Microsomal CYP Isoforms.
As the next step, other CYP enzyme activities, which are characteristic of five more CYP subfamilies found in human liver microsomal samples (Guengerich, 1995b), were determined in both minipig and human samples (table 1). The results obtained again demonstrate the similarity between minipig and human liver microsomal samples, because all of the typical activities found in human microsomes were also found in minipig microsomes; moreover, the similarity is confirmed by the fact that 7-pentoxyresorufin depentylase activity, which is characteristic of enzymes of the 2B subfamily, was not detectable in human or minipig samples. The enzyme activities specific for the CYP1A, CYP2A, CYP2C, and CYP2E1 enzymes were lower in minipig microsomes; on the other hand, the bufuralol 1′-hydroxylating activity (characteristic of the CYP2D enzymes) was higher in minipig microsomes than in those of human origin (table 1). The results seem to indicate that the variability in the minipig samples may be lower; however, before attempts are made to explain this phenomenon by other factors, it should be noted that the experimental animals were (as is usual) of the same breed, which certainly lowers the probability of large interindividual differences.
The fact that the activities specific for human liver microsomal CYPs are present in minipig samples does not necessarily mean that the same isoforms are also present there. On the other hand, it is the presence of the major CYP orthologs, in appropriate concentrations and with similar substrate specificities, that would provide the greatest confidence for investigators wishing to use a particular animal model for human CYP-dependent hepatic drug metabolism. Comparison of the results obtained here with data for pigs (Monshouwer et al., 1995) indicates that in both cases the activities typical of the CYP3A and CYP1A isoforms are comparable; the CYP2B activity was low in the pig samples and undetectable in the minipig samples.
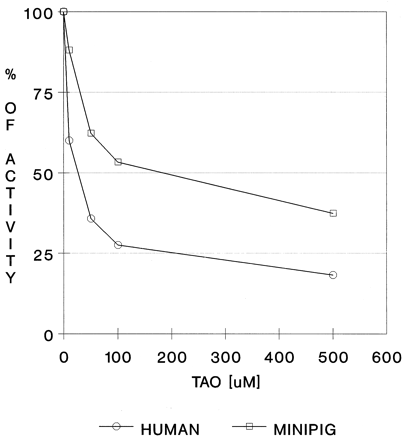
Chemical Inhibition of Nifedipine Oxidation in Minipig Liver Microsomes.
TAO, a frequently used chemical inhibitor of CYP3A4 activity, was used to demonstrate similar patterns of inhibition in human and minipig liver microsomes (fig. 3). The lower potency of TAO to inhibit nifedipine oxidation in minipig microsomes than in human microsomes may be the result of possible structural variations in the CYP active site of minipig and human 3A enzymes. The active site of CYP3A4 is thought to be quite large and flexible, in comparison with the other CYP enzymes (Guengerich, 1995b). Thus, slight changes in the primary structure of the CYP3A protein may not affect basic characteristics such as activity toward the marker substrate or affinity for a chemical inhibitor but may influence specificities or rates of microsomal oxidations.
Inhibition of nifedipine oxidation with TAO.
For experimental conditions, see Materials and Methods. The experiment was repeated twice, with duplicate samples.
In conclusion, the results justify the use of minipigs as experimental animals to predict biotransformation pathways in humans. As opposed to other routinely used experimental animals, minipigs possess the main human enzyme of drug biotransformation (CYP3A), in comparable amounts and with comparable activity. Moreover, there is no need to increase the CYP levels by enzyme induction.
Acknowledgments
The authors wish to express thanks to Prof. F. Peter Guengerich for supporting their work.
Footnotes
-
Send reprint requests to: Dr. Pavel Anzenbacher, Institute of Experimental Biopharmaceutics, Heyrovský str. 1207, 500 02 Hradec Králové, Czech Republic.
-
This project was supported by the Grant Agency of the Czech Republic (grant 203/96/0177) and by the Grant Agency of the Ministry of Health (grants IGA 3505–3 and 2763–4).
- Abbreviations used are::
- CYP
- cytochrome P450
- TAO
- triacetyloleandomycin
- Received April 9, 1997.
- Accepted September 15, 1997.
- The American Society for Pharmacology and Experimental Therapeutics