Abstract
The purpose of this study was to develop an in vivo screening method for rapid preclinical characterization of absorption and bioavailability of large numbers of compounds. This effort involved several steps. First, a pharmacokinetic characterization of a reference compound was conducted in the monkey. These data were used to verify theoretical calculations of a maximal portal dose-normalized area under the concentration-time curve. Next, a monkey screen was implemented using mixtures of up to five compounds each (i.e., cassettes) to estimate the bioavailability of approximately 200 compounds. Cassettes were administered as a single intraduodenal dose to a single monkey followed by simultaneous portal and systemic blood sampling. Definitive studies were then conducted to determine absolute bioavailability of 14 of these compounds. The studies with the reference compound demonstrated that the theoretical methodology based on a single intraduodenal dose with portal and systemic sampling provided consistent estimates of bioavailability. In the screen studies, approximately 75% of the test compounds were excluded from further evaluation due to poor absorption. Of the 14 compounds selected for follow-up evaluation from both well and poorly absorbed compounds, the absolute bioavailability of 10 of them were correctly classified from the screening data. The remaining 4 compounds were false positives, which showed low bioavailability; no false negatives were encountered. This approach allows for a rapid and reliable screen to evaluate absorption and bioavailability using a single dose in a preclinical model.
The iterative process of characterizing the preclinical pharmacokinetic properties of new chemical entities is an important component of lead compound selection and optimization in drug discovery. In recent years, the deployment of combinatorial and array chemistries has resulted in the synthesis of escalating numbers of potential drug candidates that require preclinical pharmacokinetic evaluation (Rodrigues, 1997). Accordingly, reliable techniques are needed that can rapidly generate requisite pharmacokinetic parameters for increased numbers of compounds. The use of “N-in-one” or “cassette” dosing as a means to improve throughput has been described in the literature (Frick et al., 1998; Shaffer et al., 1999), as has the use of analytical sample “pooling” (Hop et al., 1998; Kuo et al., 1998). While these techniques have proven useful and reliable, they may not completely meet the need for increased-throughput pharmacokinetic evaluation. Thus, further advances in methodology to address the needs of higher-throughput pharmacokinetic characterization would be advantageous in driving the demands of drug discovery.
Many drug candidates are targeted for oral delivery. Therefore, early estimation of the absorption properties of discovery compounds is often desired to provide guidance to the iterative chemistry effort. Furthermore, since oral bioavailability is primarily limited by either high first-pass hepatic extraction or low delivery to the portal circulation (due to low solubility, poor absorption, and/or intestinal extraction), it is often of interest to determine the relative importance of these two factors for a given compound or structural series (Kwan, 1997). Even with cassette dosing techniques, this process can excessively consume time and resources, as well as compound supply, and traditionally involves administration of test compounds by three different routes of administration (oral, intraportal, and intravenous, ideally in a crossover design) to fully characterize the factors limiting bioavailability. Thus, improvements in preclinical strategies for estimating absorption and/or hepatic extraction would increase efficiency and improve iterative cycle times used in drug discovery.
Over the past several years, we have refined an increased-throughput in vivo screen that allows estimation of the extent of absorption and first-pass hepatic extraction. This screening method has proven generally applicable to a wide variety of compound classes in several preclinical species, although a description of this screen has not been presented to date. The objective of the present study was to describe the screening method developed in this laboratory and to demonstrate the general utility of this methodology in the rapid preclinical classification of absorption and bioavailability.
Experimental Procedures
Theoretical.
The rate of drug delivery from the gut lumen to the portal circulation may be described by the following equation (Kwan, 1997):
Given this relationship, a theoretical corrected portal DNAUCmax can be estimated from literature data describing hepatic portal blood flow in various species. Table1 delineates the theoretical corrected portal DNAUCmax for several preclinical species and for human. Furthermore, since many xenobiotics are associated primarily with plasma due to plasma protein binding (Tillement and Lindenlaub, 1986), and since concentrations are more often measured in plasma than in whole blood (Hinderling, 1997), the hematocrit for each species may be used to estimate hepatic portal plasma flow and to generate an appropriate corrected portal plasma DNAUCmax (Table 1).
Estimation of theoretical corrected portal DNAUCmax values for various preclinical species and for human (see text for details)
For any given compound, a corrected portal DNAUC (i.e., DNAUCpv-DNAUCs) in blood or plasma can be measured following oral or intraduodenal administration. From this corrected portal DNAUC, an estimate can be generated for the absorbed fraction, Fa, as follows:
Materials.
All compounds were synthesized by the Department of Medicinal Chemistry at SmithKline Beecham Pharmaceuticals (King of Prussia, PA) and were ≥90% pure (typical purity >95%). All other materials were purchased from standard vendors and were of the highest available purity. All dosages were prepared as aqueous solutions with ≤20% hydroxypropyl-β-cyclodextrin (Cerestar, Hammond, IN) and ≤3% dimethyl sulfoxide; typical dimethyl sulfoxide content was ≤1%. All dosages were administered at a final volume of 4 ml/kg. Previous experience has indicated minimal impact on the pharmacokinetics of compounds by this dose volume and excipients.
Animals.
Adult male Cynomolgus monkeys (Macaca fascicularis; Charles River Primate Labs, Houston, TX) were housed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals in individual cages in unidirectional airflow rooms with controlled temperature (22 ± 2°C) and relative humidity (50 ± 10%) and 12-h light/dark cycles. Filtered tap water was available ad libitum. Animals were fed a standard animal diet (Purina Mills, St. Louis, MO) except for overnight periods before dosing. Whenever overnight fasting was used, food was provided after the 240-min blood sample was obtained on the following study day. All animal use was conducted according to protocols approved by the Institutional Animal Care and Use Committee before the study. Surgeries were conducted under aseptic conditions, and a postsurgical recovery of at least 1 month was allowed before commencement of any studies. A complete blood chemistry panel was performed before each study; studies were not conducted unless the blood chemistry values were within normal ranges. Monkeys also were conditioned for the restraint system used to collect blood samples before the study.
Pharmacokinetics of SB-250231.
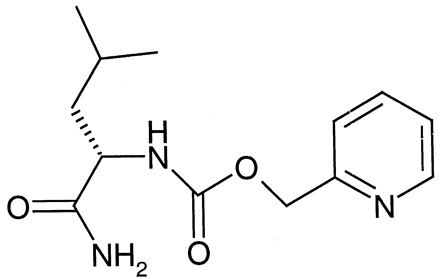
Previous preliminary observations suggested that SB-250231 (2-pyridyl-Z-leucine amide; Fig. 1) demonstrated essentially complete absorption with moderate to high first-pass hepatic extraction in the monkey (data not shown). In the present study, a complete pharmacokinetic investigation of SB-250231 was performed to verify the corrected portal DNAUCmax described above. The study was conducted using a crossover design on 3 separate study days at least 7 days apart. The monkeys (n = 3) had permanently implanted femoral and hepatic portal vein catheters and an indwelling duodenal access port. On study day 1, monkeys received SB-250231 (5 μmol/kg) as a 60-min intravenous infusion into a contralateral saphenous vein. On study day 2, the monkeys received SB-250231 (5 μmol/kg) as a 60-min intraportal vein infusion. On study day 3, the monkeys received SB-250231 (10 μmol/kg) as an intraduodenal bolus. Blood was collected from the femoral catheter on study days 1 and 2 and from both the hepatic portal vein and femoral vein simultaneously on study day 3. Plasma was prepared by centrifugation and analyzed for SB-250231.
Chemical structure of SB-250231 (2-pyridyl-Z-leucine amide).
Screening Studies.
The screening method consisted of administering a test mixture containing up to five compounds at a time at dosages of 4 μmol/kg for each component to a single monkey. Studies were conducted on 1 study day per week, with 6 to 7 days between studies, and a 2-week cycle interval (i.e., 2 weeks on, 2 weeks off). Each monkey received only one cassette per study day; however, monkeys were used on more than 1 study day. Each monkey had received permanently implanted femoral and hepatic portal vein catheters and an indwelling duodenal access port before the study. In each screening study, a monkey received the test mixture as an intraduodenal bolus, with serial plasma samples collected from the femoral and hepatic portal veins for up to 4 h after administration. Compounds in each mixture were selected for compatibility with the analytical methodology to maximize the molecular weight difference between compounds in each compound set. Concentrations of each compound in the dose solutions and plasma samples were determined as described below. Definitive pharmacokinetic parameters were obtained for compounds of interest using conventional study designs similar to that described for SB-250231 above.
Analytical Procedures.
All samples were analyzed for drug concentration by the Drug Analysis group within the Department of Drug Metabolism and Pharmacokinetics at SmithKline Beecham Pharmaceuticals. All plasma samples were subjected to protein precipitation by acetonitrile and quantified using a Sciex API 365 tandem mass spectrometer with a turbo-ionspray interface (Perkin Elmer Sciex Instruments, Ontario, Canada). Specific assay conditions were dependent upon the structural characteristics of the compounds constituting each cassette. For the definitive pharmacokinetic study with SB-250231, the mobile phase was 70% acetonitrile and 30% 10 mM ammonium acetate (pH 4.0); positive ion multiple reaction monitoring was used for detection. The lower limit of quantification for SB-250231 and for all compounds in the screening studies was 10.0 ng/ml for 50 μl of plasma. All parameters were derived from plasma concentrations within the validated range of the analytical method for each compound.
Data Analysis.
For the definitive studies with SB-250231 and other compounds of interest, concentration versus time profiles were obtained for each animal. Pharmacokinetic parameters were derived using the noncompartmental module of WinNonlin Professional version 2.1 (Pharsight, Mountain View, CA). Bioavailability (F) was estimated by dividing the intraduodenal systemic DNAUC by the intravenous DNAUC. Similarly, Fh of SB-250231 was estimated by dividing the systemic DNAUC after hepatic portal vein administration by the intravenous DNAUC. Using these values for F and Fh, absorption (Fa) of SB-250231 in the monkey was estimated as
Results
SB-250231 displayed low clearance in the monkey (13 ± 5 ml/min/kg) with a distributional volume of 0.86 ± 0.83 l/kg and a half-life of 61 ± 34 min (Table2). First-pass hepatic extraction was approximately 35% after intraportal vein administration. Due to technical difficulties during the intraduodenal leg of the experiment, no data were obtained for one monkey. Bioavailability was 32 and 70% in the two monkeys successfully completing the experiment. In these monkeys, hepatic extraction (from the intraportal infusion) was 46 and 35%. Comparison of the portal and systemic DNAUC after intraduodenal dosing according to eq. 4 suggested a hepatic extraction of 60 and 58% in these two monkeys. Considering only the intraduodenal data, and applying the corrected portal DNAUCmax concept, predicted bioavailability of SB-250231 in the monkey would be approximately 41%, based on 100% absorption and 59% hepatic extraction.
Pharmacokinetic parameters defining systemic disposition of SB-250231 in the monkey following intravenous, intraportal, and intraduodenal administration
Next, a screening study was initiated in the monkey using the single-dose cassette design. Over approximately 12 months, the absorption and hepatic extraction characteristics of 218 new chemical entities were evaluated. During this period, none of the monkeys displayed any apparent compound- or dose vehicle-related toxicity, and blood chemistry values consistently returned to normal values before each study leg using the 2-week on/2-week off dosing cycle.
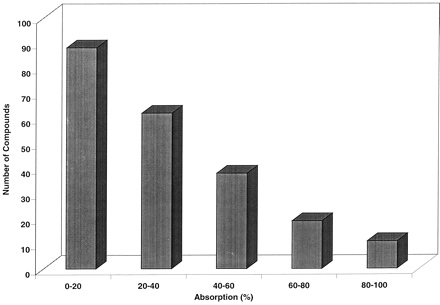
The 218 compounds tested in the screen model were structurally diverse, representing several different research efforts and chemical classes. The average molecular weight was 505 ± 72 (range = 322–888), with a calculated octanol:water partition coefficient of 3.41 ± 1.17 (range = 0.23–7.10). No clear correlation between molecular weight or lipophilicity and the measured DNAUC values was observed. A wide range of portal and systemic DNAUC values was achieved; the distribution of corrected portal DNAUC values is displayed in Fig. 2. Of the 218 compounds, predicted bioavailability (based on the portal and systemic DNAUC values) was 0% for 147 of the analogs, and ranged from 0 to 10% for an additional 50 compounds. Only 21 of the 218 compounds screened demonstrated predicted bioavailability in excess of 10%. Of the initial set of screening compounds, 14 were selected, including representatives across the range of absorption and bioavailability values, for follow-up evaluation in the monkey using a conventional study design. The average molecular weight of the follow-up compounds was 497 ± 48 (range = 442–565), with an octanol:water partition coefficient of 3.26 ± 0.75 (range = 2.27–4.66). These values were not significantly different from the initial 218 compounds. The bioavailabilities achieved in the follow-up studies were then compared with those predicted from the single-dose, single-animal screen studies. The data for these 14 compounds for the screen and follow-up studies are displayed in Table3. The follow-up compounds demonstrated a wide range of pharmacokinetic parameters, including clearance (7–40 ml/min/kg), distributional volume (0.2–3.9 l/kg), and oral bioavailability (0–31%), a range that encompasses parameter values typically seen in discovery.
Distribution of absorption estimates for the 218 compounds screened in the monkey in the current study.
Absorption was estimated based on eq. 3.
Screening and follow-up pharmacokinetic data for the 14 compounds selected for further evaluation
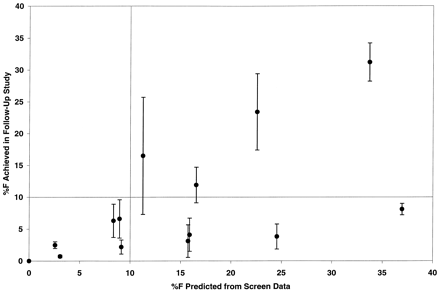
The relationship between the bioavailability predicted by the screen and that determined in follow-up evaluation is displayed in Fig.3. To convert this relationship into nominal data for the purpose of “grading” the screen performance, a 10% bioavailability estimate was selected as a cutoff point. That is, if the screen predicted oral bioavailability to be above 10%, the compound was considered to offer promise as a candidate molecule, and could be further investigated, whereas those compounds demonstrating predicted bioavailability of below 10% would be abandoned or assigned a lower follow-up priority. Of the 14 follow-up compounds, all 6 (compounds 1–4, 8, and 9) that were identified in the screen as having low bioavailability were correctly classified. Furthermore, of the remaining eight compounds that were identified in the screen as having promising oral bioavailability, four (compounds 11–17) were correctly classified. The remaining four compounds tested (compounds 5–7 and 10) were “false positives”, that is, the bioavailability predicted from the screen exceeded that determined in the follow-up work. Of these four incorrectly classified compounds, no major differences in clearance (7–20 ml/min/kg), volume of distribution (0.4–1.1 l/kg), or bioavailability (3–8%) were observed compared with the larger follow-up data set (Table 3). Finally, none of the compounds tested in the follow-up studies were identified as “false negatives”, having apparently poor bioavailability when tested in the screen, but demonstrating favorable bioavailability in follow-up studies. Overall, the screen over-predicted bioavailability in the follow-up studies by about 2.5-fold (an average 6% overprediction of F); all of the compounds demonstrated higher predicted bioavailability in the screen than in the follow-up study, with the exception of compound 12 (11 versus 17% for the screen and follow-up, respectively). When the four false positives were set aside, the overprediction averaged only 1.6-fold (a 1.5% overprediction of F); the average overprediction for compounds 5 through 7 and 10 was 5-fold (18% overprediction).
Relationship between predicted oral bioavailability resulting from the screening studies in the monkey and actual oral bioavailability measured in a conventional single-component follow-up monkey study.
Symbols represent a single measurement from the screen, and a mean ± S.D. for three monkeys in the follow-up studies.
Discussion
This investigation presents the development of an in vivo pharmacokinetic screen that allows rapid and accurate identification of promising oral drug candidates. The experiments using SB-250231 suggested that the maximum corrected portal DNAUC values derived here were in general agreement with the experimental parameters, using literature values for hepatic blood flow and hematocrit. Furthermore, predicted bioavailability of SB-250231 in the monkey was similar to that observed experimentally. Refinement of a corrected portal DNAUCmax value in any given experimental subject, as well as further exploration of the various assumptions required by this model, will likely yield more accurate values (seeDiscussion below). However, the present data suggest that the proposed model is useful as an in vivo screen in drug discovery that can provide evidence of favorable absorption and eliminate compounds with poor performance.
Using this model and the corrected portal DNAUCmax approach, a screen was conducted to evaluate over 200 new chemical entities. Obviously, a complete pharmacokinetic characterization of this number of molecules in a nonhuman primate is beyond the reasonable scope of drug discovery. Therefore, this screen was devised to provide maximum information about absorption and systemic exposure while deploying minimal resources. The present data were generated from studies using a single monkey per experiment, over only a 4-h time course that generated a minimal number of samples for analysis, and used only ∼25 μmol (generally ∼15 mg) of each compound. This screen has largely proven successful, for several reasons. First, the screen effectively identified and excluded compounds with poor absorption. Nearly 70% of the 218 compounds screened demonstrated predicted absorption of less than 40%. Even moderate first-pass extraction for these compounds would result in bioavailability below that acceptable for development candidates. This screen thus allows follow-up resources to be focused on those compounds most likely to succeed. Second, this screen also provided reasonable estimates of bioavailability in follow-up studies. Of the compounds evaluated in follow-up studies, the screen correctly classified 71%. More importantly, none of the incorrectly classified compounds were “false negatives”, in which a promising analog is incorrectly predicted to have poor performance, and would thus not be retested. As described by Frick et al. (1998), false positives may be tolerated in screening, since such errors will be discovered upon retesting, while false negatives are more deleterious, since such compounds would be assigned lower priority for follow-up studies or might be excluded from further evaluation altogether.
Regarding the four compounds that were false positives, numerous potential reasons exist for this observation. As in most cassette dosing studies, drug interactions may occur. For example, inclusion of a P-glycoprotein inhibitor in a cassette could saturate an intestinal absorption barrier and produce enhanced absorption (Yu, 1999). Similarly, inclusion of a cytochrome P-450 inhibitor could reduce the rate of hepatic or intestinal metabolism of one or more constituent and result in higher exposure than when the components are tested as discrete entities (Thummel et al., 1997). However, the present data suggest that inclusion of such an inhibitor does not automatically yield drug interactions that invalidate the results of the screen. Of the 14 follow-up compounds in this study, 12 of the cassettes from which the compounds originated, including each cassette containing the 4 outliers, contained at least 1 compound that was a potent inhibitor (IC50 < 10 μM) of human cytochrome P-450 1A2, 2C9, 2C19, 2D6, or 3A4 (data not shown). Thus, although a different P-450 inhibitor was present in each cassette from which the outliers were obtained, the presence of a P-450 inhibitor in the eight other cassettes did not yield a false positive result.
For the present effort, an apparent false positive rate of approximately 30% did not hinder the usefulness of the screen. Relatively few compounds were sufficiently well absorbed to merit follow-up evaluation; this set was further limited by factors such as unacceptable cytochrome P-450 inhibition, inadequate pharmacological potency, or insufficient compound supply to allow follow-up studies. For this compound series, therefore, follow-up investigation of each of the compounds of interest was possible. However, for a structural series with more favorable dispositional properties, larger numbers of compounds could be screened that appear to have acceptable absorption, and, consequently, the number of false positives could prove an obstacle to follow-up evaluation. In such instances, modifications to the screening method may be used that decrease throughput but improve data quality. For example, the current screen used mixtures of up to five compounds, in accordance with internal (Potts et al., 1995) and external (Berman et al., 1997; Cox et al., 1999; Shaffer et al., 1999) data describing success with this cassette size. Obviously, decreasing the cassette size would decrease the risk of false positives if such errors are due to drug interactions. Also, to minimize variability and optimize absorption, this screen used intraduodenal administration. In some instances, intraduodenal and oral administration produce similar absorption, such as with flumequine (Ruiz-Garcia et al., 1999) or CGS-20625 (Hirschberg et al., 1995); a few compounds may demonstrate better oral than intraduodenal absorption, including selegiline (Barrett et al., 1996). However, in most cases, intraduodenal absorption is superior to oral absorption, consistent with gastric metabolism, acid-mediated compound degradation, insolubility at low pH, or other factors. Examples include 2-(allylthio)pyrazine (Han and Lee, 1999), nitrofurantoin (Watari et al., 1983), and omeprazole (Larsson et al., 1983). The present data are consistent with these observations; the intraduodenal screen consistently gave higher bioavailability than the oral follow-up studies, with the exception of compound 12. Thus, in designing a screen, oral administration may result in fewer false positive results. Finally, all of the estimates generated from the screen were based on literature values for hepatic blood flow and hematocrit. It is well recognized that these parameters are subject to extensive variability within animal strain and also are dependent upon factors such as vendor, housing conditions, disease, posture, and exercise (Brown et al., 1997). Furthermore, the indwelling hepatic portal vein catheter may also have altered hepatic blood flow in this screening study (Heberer et al., 1985; Hadengue et al., 1988). To derive more accurate parameter estimates, the hematocrit and hepatic portal blood flow of each animal used in a screen could be determined. Since this may be impractical in a screening initiative, one alternative could be to determine reference values for animals within the larger colony from which test subjects are drawn, thereby reaching a more accurate value than relying on literature references.
Apart from modifications to improve accuracy, additional refinements could be incorporated to customize this screen for a particular structural series. If the number of compounds to be screened is small, or if a series proves problematic in cytochrome P-450 inhibition, decreasing the cassette size may be appropriate. Also, the absorption of many compounds is dissolution rate-limited. By administering solutions of such compounds, the likelihood of false positives upon retesting in a solid dose formulation is increased. In this case, more useful information may be obtained by administering suspensions rather than solutions. Finally, another permutation would be to administer cassettes of compounds in the absence and presence of an inhibitor of cytochrome P-450 and/or P-glycoprotein (Zhang et al., 1998). This approach would allow a direct assessment of the effect of these potential barriers on a structural series and could help guide the chemistry effort in a constructive direction.
In summary, the screening method described here provides a rapid and accurate means for evaluating the drug properties of new chemical entities following a single dose in a nonhuman primate model. Although some false positives were observed, and although the predicted bioavailability estimates did not precisely match those generated in follow-up studies, the present data clearly demonstrate that this screening approach is useful for characterizing large numbers of compounds in a relatively rapid fashion. Additional refinements and more robust validation of this model may further advance the utility of this screening method in advancing drug discovery.
Acknowledgments
We acknowledge the expert technical assistance of members of the Preclinical Pharmacokinetics group, including Leonard Azzarano, Jayme Mumaw, Theresa Roethke, Gary Stelman, Mike Walsh, and Kelli Zeigler, and the Laboratory Animal Sciences group, including Earl Jenkins and Guy Vaden, in the conduct of the in-life portions of the experiments described here. Also, this work would have been impossible without the invaluable efforts of all the members of the Drug Analysis group, particularly Jeanelle McSurdy-Freed and Yanwen Qian for the development of analytical methodology that allowed cassette dosing and rapid sample turnaround. Particular gratitude is also extended to Yanwen Qian, Amanda Price, and Lorrie Day for the analysis of the monkey samples for the definitive studies with SB-250231.
Footnotes
-
Send reprint requests to: Keith W. Ward, Ph.D., Drug Metabolism and Pharmacokinetics, SmithKline Beecham Pharmaceuticals R&D, UW 2720, 709 Swedeland Road, King of Prussia, PA 19406. E-mail:keith_w_ward{at}sbphrd.com
-
↵1 This work was presented in part at the 2000 Annual Meeting of the American Association of Pharmaceutical Scientists, October 29–November 2, Indianapolis, Indiana.
- Abbreviations used are::
- AUC
- area under the concentration-time curve
- C
- concentration
- DNAUC
- dose-normalized AUC
- DNAUCmax
- maximum theoretical DNAUC
- Eh
- hepatic extraction
- F
- bioavailability
- Fa
- absorbed fraction
- pv
- portal vein
- s
- systemic circulation
- Received June 12, 2000.
- Accepted August 21, 2000.
- The American Society for Pharmacology and Experimental Therapeutics