Abstract
We tested the ability of human liver microsomes (HLMs) and recombinant human cytochrome P450 (CYP or P450) isoforms to catalyze the N-demethylation of nirvanol-free (S)-mephenytoin [(S)-MP] in vitro. In mixed HLMs, the kinetics of (S)-MPN-demethylation suggested two contributing activities. A high-affinity/low-capacity component exhibited aKM of 174.1 μM and aVmax of 170.5 pmol/mg protein/min, whereas a low-affinity/high-capacity component exhibited aKM of 1911 μM and aVmax of 3984 pmol/mg protein/min. The activity of the high-affinity component was completely abolished by sulfaphenazole, with little effect on the low-affinity component. Of the recombinant P450 isoforms tested, only CYP2B6 and CYP2C9 formed nirvanol from (S)-MP. The KM value (150 ± 42 μM) derived for recombinant CYP2C9 was close to that obtained for the high-affinity/low-capacity component in mixed HLMs (KM = 174.1 μM). The predicted contribution of this activity at concentrations (1–25 μM) achieved after a single 100-mg dose of racemic MP is approximately 30% of the rate of nirvanol formation. At concentrations of >1000 μM, we estimate that >90% of the rate can be explained by the low-affinity activity (CYP2B6). Therefore, the N-demethylation of (S)-MP to nirvanol may be a useful means of probing the activity of CYP2B6 in vitro when concentrations of >1000 μM are used, but it is unlikely to be a suitable phenotyping tool for this isoform in vivo, where concentrations of >1000 μM are rarely encountered.
Mephenytoin (MP1;3-methyl-5-phenyl-5-ethylhydantoin) is an antiepileptic agent that is also a well-recognized probe drug for the CYP2C19 metabolic polymorphism (Küpfer et al., 1984). This chiral drug undergoes stereoselective metabolism (Küpferet al., 1981) in which the S-(+)-enantiomer is hydroxylated at the 4′-position but also undergoesN-demethylation to an active metabolite (Troupin et al., 1976), i.e. nirvanol (5-phenyl-5-ethylhydantoin). The R-(−) enantiomer is primarily N-demethylated (Küpfer et al., 1981).
In humans, the primary route of (S)-MP metabolism after a single 100-mg dose of the racemate is to (S)-4′-OH-MP (Küpfer et al., 1984). This reaction is catalyzed by a genetically polymorphic P450 isoform, CYP2C19 (Wrighton et al., 1993; Goldstein et al., 1994), and forms the basis for the most common tests used to determine the CYP2C19 phenotype (Wedlund et al., 1984). During chronic therapeutic use of the drug at doses of 100–600 mg/day, N-demethylation of (S)-MP to nirvanol is predominant (Küpfer et al., 1984; Troupin et al., 1979). The enzymes that catalyze N-demethylation of (S)-MP to nirvanolin vivo have not been identified. Using HLMs in vitro, this reaction has been shown to be catalyzed by CYP2B6 at substrate concentrations (0.2–3 mM) (Heyn et al., 1996) that may be relevant to total serum MP concentrations (40 μg/ml or 183 μM) observed after chronic clinical dosing (Küpfer et al., 1981).
A growing list of substrates for recombinant CYP2B6 have been identified in vitro, using several indirect methods of characterization (literature cited in Ekins et al., 1997). There is evidence that CYP2B6 is expressed in HLMs, with wide interindividual variability that could be the result of genetic polymorphism or environmental exposure (Code et al., 1997). However, investigations of hepatic microsomal CYP2B6 have generally been limited because of the lack of specific substrate probes and specific chemical inhibitors or immunoinhibitors (Ekins et al., 1997; Guo et al., 1997). Consequently, little is presently known about its actual role in xenobiotic oxidative metabolism. Recently, (S)-MP N-demethylation to nirvanol was recommended and used as a probe for CYP2B6 in vitro (Heyn et al., 1996), but the concentrations used were 20–150 times the peak concentrations (13 μM) of (S)-MP achieved after the standard dose of 100 mg used to determine the metabolic phenotype of CYP2C19, even in poor metabolizers with respect to this isoform (Küpfer et al., 1984). It follows that, if CYP2B6 is the only enzyme catalyzing this reactionin vivo, then this substrate could be used to simultaneously probe CYP2C19 and CYP2B6 in vitro and probably also in vivo. In this study, we determined the kinetics of N-demethylation of (S)-MP across a wide range of substrate concentrations and identified the specific P450 isoforms involved in this reaction, paying particular attention to the concentrations that appear relevant after the single 100-mg dose used to determine metabolic phenotypes.
Materials and Methods
Chemicals and Reagents.
Chlorzoxazone, dextromethorphan hydrobromide, phenacetin, glucose-6-phosphate, glucose-6-phosphate dehydrogenase, NADP, EDTA, and tolbutamide were purchased from Sigma Chemical Co. (St. Louis, MO). (S)-MP (99.3% pure), nirvanol, and sulfaphenazole were purchased from Ultrafine Chemicals (Manchester, UK).
HLMs.
The preparation and metabolic characteristics of the HLMs were as described previously (Harris et al., 1994). The microsomes were resuspended to a protein concentration of 5–12 mg/ml in reaction buffer (0.1 M sodium/potassium phosphate, 1.0 mM EDTA, 5.0 mM MgCl2, pH 7.4) and were stored at −80°C until used. Protein concentrations were determined using the method described by Pollard et al. (1978).
Preparation of Nirvanol-Free (S)-MP.
Contaminant nirvanol in (S)-MP was eliminated by differential collection of fractions from HPLC. (S)-MP was dissolved in 50% methanol in water to a concentration of 10 mg/ml, and 100 μl were injected directly onto a Microsorb C18 (5 μm, 150 mm × 4.6 mm) analytical column (Rainin Instrument Co., Woburn, MA). The flow rate of the mobile phase (30% methanol in water) was 1.0 ml/min, and the eluant was monitored using a Waters (Milford, MA) model 490 UV detector, at a wavelength of 211 nm. Collected solutions were evaporated to dryness by speed vacuum, and the samples were weighed. Extraction efficiency was 80–90%, and reinjection of the purified (S)-MP (10 mg/ml, 100 μl) showed that the amount of nirvanol remaining was below the lower limit of quantification of the assay (50 ng/ml, <0.0005%). Stock solutions of nirvanol-free (S)-MP were prepared by dissolution in 100% methanol (10 mg/ml for assays with HLMs and 9 mg/ml for assays with recombinant P450 isoforms) and were then sequentially diluted with water to prepare the concentrations used. The highest final concentrations of methanol in incubations were 0.44% and 0.46%, respectively.
Incubation Conditions and Enzyme Assays.
The formation of nirvanol from (S)-MP was tested by incubating appropriate concentrations of nirvanol-free (S)-MP with HLMs, using incubation conditions that were linear for time and protein concentration (tested ranges of time and protein concentration were 15-150 min and 0.15–0.75 mg/ml, respectively). Incubation conditions for the formation of nirvanol from (S)-MP were modified from the method used to study (S)-MP hydroxylation, as described previously (Meier et al., 1985). In short, a mixture of 25 μl of (S)-MP (final concentration, 0.5–2000 μM), 5 mM potassium phosphate buffer (pH 7.4), and an NADPH-regenerating system (0.5 mM NADP, 2.0 mM glucose-6-phosphate, 0.4 unit/ml glucose-6-phosphate dehydrogenase, 0.1 mM EDTA, and 4.0 mM MgCl2) was preincubated for 5 min. Reactions were started by the addition of pooled HLMs (HL2, HL8, and HL9 or HL10, HL16, and HL17) (final concentration, 0.75 mg of protein/ml) and were incubated at 37°C for 60 min (final incubation volume, 250 μl). Inhibition of (S)-MP N-demethylation in pooled HLMs (HL2, HL8, and HL9) was tested with sulfaphenazole (25 μM) and tolbutamide (100 μM). Reactions were stopped by the addition of 100 μl of cold acetonitrile. After addition of the internal standard (50 μl of 20 μg/ml phenytoin), the aqueous layer was extracted by the addition of 3 ml of methylene chloride, and the organic layer was removed, centrifuged at 2000 rpm for 5 min in a Beckman J-6M centrifuge (JS4.0 rotor), and then dried in a speed vacuum. Dried pellets were reconstituted with 250 μl of mobile phase, and 100 μl were injected into the HPLC apparatus. Rates of production of metabolite were quantified by using the ratio of the AUC of the metabolite to the AUC of the internal standard phenytoin. Instruments used for HPLC were controlled by a Waters Millennium 2010 chromatography manager and included a Waters model 600 HPLC pump, Waters model 717 autosampler, and Waters model 490 UV detector. Standard curves for nirvanol were linear in two concentration ranges (0.1–20 μg/ml and 6.3–200 ng/ml).
cDNA-Expressed Human P450 Isoforms.
cDNA-expressed human P450 isoforms (CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4) were purchased from Gentest Corp. (Woburn, MA). Protein concentrations and P450 contents were as supplied by the manufacturer. The suspensions of cDNA-expressed HLMs were stored at −80°C and thawed at 37°C before incubation. Screening for nirvanol formation by these isoforms was performed using a (S)-MP concentration of 200 μM and a protein concentration of 0.76 mg/ml, except in the assay using CYP3A4 (1.4 mg/ml). The experiments using recombinant CYP2C9 to examine the kinetics of nirvanol formation were performed using 2 mg/ml cDNA-expressed material and a (S)-MP concentration range of 0.5–100 μM. After preincubation (5 min) of a mixture containing the substrate (with or without inhibitor) and an NADPH-generating system, reactions were initiated by the addition of cDNA-expressed P450 isoforms and were incubated for 120 min. The procedures for extraction and measurement of metabolite were the same as described for the assay with HLMs. For the recombinant CYP1A2 and CYP2E1 assays, any alcohol component in the substrate was eliminated by evaporation to dryness and reconstitution of the substrate in 0.1 M phosphate buffer before the start of the incubation. Each assay included a positive control with a documented substrate for each pathway, i.e. phenacetin O-deethylation for CYP1A2, tolbutamide 4-methylhydroxylation for CYP2C9, (S)-MP 4-hydroxylation for CYP2C19, dextromethorphanO-demethylation and N-demethylation for CYP2D6 and CYP3A, respectively, and chlorzoxazone 6-hydroxylation for CYP2E1. Each positive control was analyzed using a method developed for the detection of the relevant metabolite (Ko et al.,1997).
Data Analysis.
Graphical analyses were performed using the Excel (Microsoft Corp., Redmond, WA) graphics package. Initial estimates acquired by linear regression of unweighted data in Eadie-Hofstee plots were used to determine the apparent KM
andVmax values through a nonlinear regression analysis (WINNONLIN version 1.5; Scientific Consulting Inc., Lexington, KY), using the following two-site binding equation:
Results and Discussion
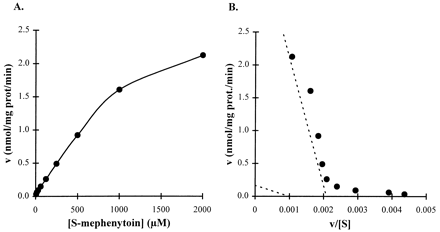
We characterized the kinetics of nirvanol formation from (S)-MP by incubating nirvanol-free (S)-MP (0.5–200 μM) with pooled HLMs (HL2, HL8, and HL9). The results depicted as a Michaelis-Menten plot in fig.1A demonstrate thatN-demethylation of (S)-MP to nirvanol is saturable. Our results do not concur with earlier reports that suggested that this pathway is nonsaturable at the substrate concentrations used (Meier et al., 1985; Jurima et al., 1985; Hall et al., 1987). In contrast to reports that the N-demethylation of (S)-MP is catalyzed by a single enzyme, visual inspection of Eadie-Hofstee plots of our data showed biphasic kinetic behavior (fig. 1B), suggesting the involvement of at least two enzymatic activities. These activities were best described by a two-site model with high-affinity/low-capacity (KM,1 andVmax,1) and low-affinity/high-capacity (KM,2 andVmax,2) components. The kinetic parameters derived using a nonlinear regression analysis for two sites are shown in table 1. Given the wide interindividual variability of the nirvanol formation rate (Heynet al., 1996), it is possible that the kinetic parameters we document here for the low-affinity component represent CYP2B6-mediated nirvanol formation (Heyn et al., 1996), whereas the high-affinity component represents activity that has not been described before. To test the variability of the low- and high-affinity components of the reaction, we repeated the experiment using pooled HLMs from other liver preparations (HL10, HL16, and HL17). The data in all cases were best characterized by a two-site enzymatic activity. The initial kinetic parameter estimates derived from these data were 208.6 and 1060 μM for KM,1 andKM,2 and 46.9 and 471 pmol/min/mg protein for Vmax,1 andVmax,2, respectively. Because of the large (>20-fold) Vmax difference between the two activities and because the formation of nirvanol might be low in certain liver preparations, the high-affinity component would not be detected if experiments were conducted at high concentrations (in the millimolar range) and might be obscured by trace contamination of (S)-MP with nirvanol. This high-affinity component might be important in clinical settings, because of the relatively low in vivo plasma concentrations of (S)-MP (<15 μM) (Troupin et al., 1979) and because of the fact that nirvanol, not 4-OH-MP, is the major metabolite when MP is used chronically (Küpfer et al., 1984; Wedlund et al., 1984).
Nirvanol formation from (S)-MP.
Nirvanol-free (S)-MP was incubated for 60 min with pooled HLMs (HL2, HL8, and HL9). Data are presented as a Michaelis-Menten plot (A) and an Eadie-Hofstee plot (B). Dotted lines, high- and low-affinity components calculated by nonlinear regression.
Estimated kinetic parameters for the formation of nirvanol from nirvanol-free (S)-MP (0.5–200 μM), in the presence or absence of sulfaphenazole (25 μM), in pooled HLMs (HL2, HL8, and HL9)
To determine which P450 isoforms are involved in theN-demethylation of (S)-MP, we incubated (S)-MP (200 μM) with recombinant P450 isoforms (CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4) (table2). Of the isoforms tested, only CYP2B6 and CYP2C9 catalyzed the N-demethylation of (S)-MP to nirvanol. The role of CYP2C9 in the formation of nirvanol from (S)-MP was further evaluated by incubating sulfaphenazole (25 μM), a specific inhibitor of CYP2C9 (Baldwinet al., 1995), with (S)-MP across a wide range of concentrations (15–2000 μM) in HLMs. As demonstrated in fig.2A, sulfaphenazole inhibited the formation of nirvanol. Analysis of the Eadie-Hofstee plot (fig.2B) strongly suggested that sulfaphenazole preferentially removed the contribution of the high-affinity component, whereas the remaining low-affinity component was characterized by a linear relationship. Because of the convexity in the Eadie-Hofstee plot (fig.2B), which suggests positive cooperativity and the possibility of an activation of the enzyme, we were not able to precisely estimate the Vmax for nirvanol formation after sulfaphenazole treatment (table 1). To examine the possibility of mutual competitive substrate inhibition, we tested whether tolbutamide, a substrate probe for CYP2C9 (Relling et al., 1990), inhibits (S)-MP N-demethylation and whether (S)-MP itself inhibits CYP2C9-mediated tolbutamide hydroxylation. Indeed, tolbutamide competitively inhibited nirvanol formation from (S)-MP when lower substrate concentrations were used (<50 μM; 60–70% inhibition,Ki ∼ 31 μM) but had little or no effect at relatively higher substrate concentrations. (S)-MP also inhibited tolbutamide hydroxylation, although the inhibitory potency was low (calculated Ki values of 1.2 mM for recombinant CYP2C9 and >3 mM for HLMs).
Rate of nirvanol formation from (S)-MP (200 μM) by pooled HLMs and recombinant P450 isoforms
Effect of sulfaphenazole on nirvanol formation from (S)-MP.
Pooled HLMs from three livers (HL2, HL8, and HL9) were incubated, with or without sulfaphenazole (25 μM), at (S)-MP concentrations of 0.5–2000 μM. Data are presented as a Michaelis-Menten plot (A) and an Eadie-Hofstee plot (B).
In contrast to the data presented by Heyn et al.(1996), who observed no activity with any isoform other than CYP2B6, our results provide strong evidence that CYP2C9 is involved in the formation of nirvanol. This difference may be the result of the low activity of recombinant CYP2C9 in N-demethylation of (S)-MP. We found that the reaction catalyzed by recombinant CYP2C9 showed a low Vmax (17.4 ± 1.8 pmol/mg protein/min), whereas the KM value (150 ± 42 μM) was close to the averageKM,1 value (174.1 μM) obtained with mixed HLMs (table 1). Our data suggest that CYP2C9 may be an important catalyst of (S)-MP N-demethylation to nirvanol at clinical plasma concentrations resulting from the single 100-mg dose of racemic MP [containing 50 mg of (S)-MP] routinely used to determine the metabolic phenotype of CYP2C19. At these concentrations (1–25 μM), the predicted contribution of the high-affinity component would be 30% (calculated using the Michaelis-Menten equation for a two-site model). It is possible that the relevant in vivoconcentrations at the metabolic site in the liver are higher and that the contribution of CYP2C9 is lower than this estimate. Because we do not have reliable means of estimating the substrate concentrations at the active site in the liver, this question might be addressed by clinical studies that document the effects of a CYP2C9 inhibitor, such as fluconazole (Black et al., 1996; Mitra et al., 1996), on the partial metabolic clearance of (S)-MP to (S)-nirvanol.
The high-affinity/low-capacity component of (S)-MPN-demethylation (CYP2C9) appears to contribute significantly at low concentrations that may be present after a single dose used for metabolic phenotyping or during chronic therapy. Given the wide interindividual variability in the activities of CYP2C9 and CYP2B6, it is likely that the relative contributions of these enzymes to nirvanol formation would vary with the relative expression of each isoform in different livers. Under our experimental conditions with HLMs, approximately 90% of the rate of nirvanol formation can be explained by the low-affinity/high-capacity component. Although we recognize the need for highly specific substrate probes for CYP2B6 activity, we agree with Heyn et al. (1996) that (S)-MP at high concentrations (>1 mM) is useful as an in vitro probe for this isoform. However, because the low-affinityKM value (1911 μM) we found was 11 times greater than the KM value for the higher affinity component (174.1 μM), N-demethylation of (S)-MP is not likely to be a useful phenotyping tool for CYP2B6 in vivo, where concentrations of >1 mM are rarely encountered.
Footnotes
-
Send reprint requests to: David A. Flockhart, M.D., Ph.D., Division of Clinical Pharmacology, Georgetown University Medical Center, 3900 Reservoir Road, NW, Washington DC, 20007.
-
This work was supported in part by United States Public Health Service Grant T32-GM08386 from the National Institute of General Medical Sciences and by a fellowship award to J.-W.K. from the World Health Organization (WPRO 0630/95).
- Abbreviations used are::
- MP
- mephenytoin
- CYP or P450
- cytochrome P450
- HL
- human liver
- HLMs
- human liver microsomes
- Received August 13, 1997.
- Accepted April 17, 1998.
- The American Society for Pharmacology and Experimental Therapeutics