Abstract
Human colon carcinoma Caco-2 cells were used to study the induction of UDP glucuronosyltransferase (UGT) isoforms UGT1A6, UGT1A9, and UGT2B7 by aryl hydrocarbon receptor agonists and by antioxidant-type inducers with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) andt-butylhydroquinone (TBHQ), respectively. Early- (PF11) and late-passage clones (TC7) of Caco-2 cells, which show low and high constitutive UGT1A6 expression, respectively, were selected. The following results were obtained: 1) In Caco-2 cells UGT activity (4-methylumbelliferone as substrate) was significantly enhanced by 10 nM TCDD or 40 to 80 μM TBHQ and 2) duplex reverse-transcription-polymerase chain reaction analysis showed for the first time that the expression of human UGT1A6, UGT1A9, and UGT2B7 was enhanced by 40 to 80 μM TBHQ; both UGT1A6 and UGT1A9 were induced by 10 nM TCDD, whereas UGT2B7 was not induced by TCDD. The results suggest that at least two human UGTs (UGT1A6 and UGT1A9) are inducible by aryl hydrocarbon receptor agonists and even more isoforms (UGT1A6, UGT1A9, and UGT2B7) are inducible by antioxidant-type inducers in Caco-2 cells.
Studies in rodents demonstrated that the UDP glucuronosyltransferase (UGT)1 isoform UGT1A6 is regulated by aryl hydrocarbon receptor (AhR) agonists (Iyanagi et al., 1986) and by phenolic antioxidants/electrophiles = antioxidant-type inducers (Buetler et al., 1995). Some AhR agonists (for example, certain polycyclic aromatic hydrocarbons, β-naphthoflavone, etc.) are rapidly metabolized to reactive electrophiles and may therefore have mixed-type inducing properties. Hence, in the present investigation the dioxin TCDD, which does not generate electrophilic metabolites, andt-butylhydroquinone (TBHQ) were used as selective AhR- and antioxidant-type inducers, respectively. These two types of inducers appear to activate different pleiotropic gene transcription programs. AhR agonists induce both certain phase I (CYP1A1, CYP1A2, etc.) and phase II enzymes, whereas antioxidant-type inducers enhance phase II enzymes NAD(P)H quinone oxidoreductase-1 (NQO1), glutathioneS-transferase Ya, and UGTs. TCDD and TBHQ have therefore been termed bifunctional or monofunctional inducers, respectively (Prochaska and Talalay, 1988). TCDD and related compounds are ligands of the AhR, which has been characterized as an enhancer of gene transcription (Whitlock, 1993; Hankinson, 1995). Antioxidant-type inducers are believed to interact with redox-sensitive transcription factors that bind to antioxidant/electrophile response elements (ARE/EpRE; Nguyen et al., 1994; Jaiswal, 1994; Wasserman and Fahl, 1997).
Recently, evidence for marked tissue-specific differences of human UGT expression has been obtained (Strassburg et al., 1998). For example, in the human gastrointestinal tract, UGT1A7 was found to be expressed only in the gastric mucosa. (The updated nomenclature of UGT isoforms was used; Mackenzie et al., 1997). However, information on the regulation of human UGTs by AhR- and antioxidant-type inducers in different tissues is scarce. Interestingly, constitutive expression of UGT1A6 is low in rat liver with high inducibility by AhR agonists, whereas in testes there is high constitutive expression with no further inducibility (Münzel et al., 1994). In human liver and in intestinal biopsies UGT1A6 is constitutively expressed with high interindividual variability (Münzel et al., 1996; S. Schmohl, P.A. Münzel, K. Kälberer, K.E. Grund and K.W. Bock, unpublished results). However, TCDD inducibility has been observed in primary hepatocyte cultures and in colon carcinoma Caco-2 cells (Münzel et al., 1998). From the original Caco-2 cell line several clones with different states of differentiation have been isolated (Caro et al., 1995). For example, basal UGT1A6 expression is low in the early passage clone PF11, but it can be induced by TCDD. Therefore, this clone has been used previously for studies of the TCDD induction mechanism (Münzel et al., 1998). In the well differentiated, late passage clone TC7, constitutive UGT1A6 expression is relatively high with lower inducibility. Hence, there appears to be an inverse relationship between constitutive and inducible UGT expression. To our knowledge nothing is known about the inducibility of human UGTs by antioxidant-type inducers.
UGT1A6, conjugating planar phenols, and UGT1A9, conjugating bulky phenols (Ebner and Burchell, 1993), have been investigated previously because they appear to be functionally related in conjugating benzo(a)pyrene (BaP) diphenols to mono- and diglucuronides (Gschaidmeier et al., 1995). In addition to these family 1 members, UGT2B7 was studied as a major family 2 isoform that also conjugates BaP phenols (Jin et al., 1993). Moreover, this family 2 member catalyzes glucuronidation of a variety of drugs such as morphine (Coffman et al., 1998). The latter glucuronidation reaction received much interest because morphine-6-glucuronide has been shown to be more potent as an analgesic than the parent compound (Rossi et al., 1996).
The present study shows for the first time that at least three UGT isoforms (UGT1A6, UGT1A9, and UGT2B7) are induced by antioxidant-type inducers; at least two isoforms (UGT1A6 and UGT1A9) are induced by AhR agonists, whereas UGT2B7 is not induced by TCDD.
Materials and Methods
Chemicals and Reagents.
TBHQ was purchased from Fluka (Buchs, Switzerland) and dexamethasone from Sigma (St. Louis, MO). TCDD was obtained from Ökometric (Bayreuth, Germany). Avian myeloblastosis virus reverse transcriptase (Superscript RT) and [α-32P]dCTP (3000Ci/mmol) were provided by Amersham Pharmacia Biotech, Europe (Freiburg, Germany). TaqDNA polymerase was provided by Perkin-Elmer (Ueberlingen, Germany). Oligonucleotide primers were custom synthesized by Appligene (Illkirch, France). Deoxynucleotide triphosphates and oligo(dT15) were purchased from Boehringer Mannheim GmBH (Mannheim, Germany).
Caco-2 Cell Culture and Treatment.
Clones TC7 and PF11 derived from the colon carcinoma cell line Caco-2 (Caro et al., 1995) were obtained from Dr. Alain Zweibaum (Institut National de la Sante et de la Recherche Medicale, Villejuif, France) and grown on 100- × 20-mm Falcon tissue culture dishes (Becton-Dickinson, Heidelberg, Germany) in Dulbecco’s modified Eagle’s medium supplemented with 20% fetal calf serum (heat-inactivated at 56°C for 30 min), 25 mM glucose, and 1% nonessential amino acids (Life Technologies, Eggenstein, Germany). The medium was changed daily. Cells were treated with 10 nM TCDD or with 40 and 80 μM TBHQ (as indicated) when they reached preconfluence and were harvested after 72 h. Solvent controls contained 0.1% dimethyl sulfoxide (DMSO). Before harvest, cells were washed with 0.9% NaCl and stored at −80°C.
Duplex and Triplex Reverse Transcription-Polymerase Chain Reaction (RT-PCR) of Human UGT1A6, UGT1A9, and UGT2B7.
Total RNA of cell and tissue samples was extracted according toChomczynski and Sacchi (1987). Total RNA (0.1 μg/5 μl H2O) was heated at 70°C for 15 min and cooled on ice. RNA-derived cDNA synthesis of UGT1A6 (Münzel et al., 1996), UGT1A9, and UGT2B7 was carried out in a final volume of 1 μl containing 20 U/μl RNasin (Promega, Madison, WI), first strand buffer 1X, 10 mM DTT, 1 mM each deoxynucleotide triphosphate, 5 μM oligo(dT)15, and 10 U/μl avian myeloblastosis virus reverse transcriptase (Superscript RT Kit, Gibco BRL Life Technologies, Eggenstein, Germany). The samples were incubated at 42°C for 90 min and RT was inactivated by heating at 70°C for 15 min.
For PCR amplification of cDNA, 0.25 μl of the RT reaction mixture were added to a PCR master mix to bring the final volume to 12.5 μl. The final concentration in the PCR reaction mixture was 20 mM Tris-HCl (pH 8.4 at 25°C), 50 mM KCl, 2.5 mM MgCl2 (for UGT1A6 and UGT1A9) or 1.5 mM MgCl2 (for UGT2B7), 1 μM forward or reverse primers of the UGT1A6 and UGT1A9, 0.4 μM for UGT2B7, 400 μM deoxynucleotide triphosphates (dATP, dCTP, dGTP, and dTTP), 1 μCi [α-32P]dCTP and 0.625 U/12.6 μl Taq DNA polymerase. The primers used for amplification were the following: UGT1A6 (EMBL J04093; 17), forward primer, (1)-5′-ATGGCCTGCCTCCTTCGCTCATT-3′-(23), reverse primer (906)-5′-CCATTGATCCCAAAGAGAAAACC-3′-(928). For UGT1A9 (EMBL S55985), forward primer, (643)-5′-GAGGAACATTTATTATGCCACCG-3′-(665). The same reverse primer as for UGT1A6 was used. For UGT2B7 (EMBL J05428) the forward primer was (774)-5′-GACGTATGGCTTATTCGAAACTCCTGGAATTTTCAG-3′-(809) and the reverse primer was (1190)-5′-GCAATGTTATCAGGTTGATCGGCAAACAATGGAATC-3′ (1225). Human β-actin and glyceraldehyde phosphate dehydrogenase (GAPDH) were used as a basis for quantification of UGT expression. The forward primer for human β-actin (EMBL X00351) was (940)-5′-CTGGCGGCACCACCATGTACCCT-3′-(962) and the reverse primer was (1223)-5′-GGAGGGGCCGACTCGTCATACT-3′-(1145). For GAPDH (EMBL M33197) the forward primer was (430)-5′-CCCTCTGCTGATGCCCCCATGTTC-3′-(453) and the reverse primer (695)-5′-TTGCCCACAGCCTTGGCAGCGC-3′-(716).
PCR reactions were carried out in a Perkin-Elmer 2400 thermal cycler (PCR comprised 32 cycles for UGT1A6, 27 cycles for UGT1A9, 36 cycles for UGT2B7 including 24 cycles for β-actin and GAPDH) using the following conditions.
UGT1A6
The “hot start” modification was used (Münzel et al., 1996; to avoid nonspecific priming) by heating the master mix containing the cDNA samples to 94°C for 1 min; Taq DNA polymerase was heated to 90°C separately and added to the samples. Annealing was performed at 65°C for 40 s and extension at 72°C for 2 min. Thereafter a 10-min elongation step at 72°C was included.
UGT1A9
The PCR protocol for UGT1A9 was 94°C for 30 s, 61°C for 30 s, and 72°C for 1 min. The UGT1A9 protocol was preceded by a 3-min incubation of the reaction mixture at 94°C and followed by a 5-min elongation step at 72°C. Amplification was first carried out with UGT1A9 primers for 16 cycles. After addition of β-actin primers (0.4 μM), cycling was continued for another 20 cycles.
UGT2B7
The protocol was preceded by a 5-min incubation of the reaction mixture at 94°C, carried out for 12 cycles using the following cycling protocol: 94°C (30 s), 69°C (30 s), 72°C (1 min) and followed by a 3-min elongation step. After addition of β-actin primers (0.4 μM) and GAPDH primers (1.6 μM), the reaction mixture was incubated for 5 min. Thereafter, cycling was continued for another 24 cycles followed by a 7-min elongation step at 72°C.
Amplified cDNA products were separated by polyacrylamide gel electrophoresis using 6% native gels. They were sequenced to verify whether the correct UGT isoform was amplified. Radioactivity of the gels was evaluated by direct phosphor image analysis using a Fuji Bio-Image BAS2000 analyzer (Tokyo, Japan).
Nuclear Run-On Assay.
Caco-2 cells were grown on 145 × 20 mm collagen-coated culture dishes to 70% confluence. Cells were treated with 0.1% DMSO or 10 nM TCDD for 24 h; 20 culture dishes were harvested and nuclei were isolated as described by Phillips et al. (1988). The transcription reaction mixture was exactly as described by Sutter et al. (1991). Endonuclease-digested DNA samples (5 μg) of human UGT1A6 (BamHI/PvuII-fragment 700 bp, corresponding to exon I, Münzel et al., 1996), rat CYP1A1 (BamHI-fragment, 2.2 kb; EMBL X00469), human GAPDH (EcoRI/BamHI-fragment, 310 bp; EMBL J04038), and mouse β-actin (EcoRI/HindIII-fragment, 500 bp; EMBL X03672) were denatured by boiling in 0.3 M NaOH in a final volume of 50 μl for 10 min and chilled on ice; then 50 μl of cold 2 M ammonium acetate was added. The membrane (Hybond, Amersham Buchler Braunschweig, Germany) was soaked in sterile water for 20 min. Probes were mixed with 100 μl 10 mM Tris HCl (pH 8.0) and 1 mM EDTA to get a final volume of 200 μl, applied to the membrane using a slot-blot apparatus (Schleicher & Schuell, Dassel, Germany), and the wells were washed 2 to 3 times with 1 M ammonium acetate. After air drying of the membrane for at least 30 min, DNA was linked to the membrane using the stratalinker (Stratagene, La Jolla, CA; 1200 J for each side of the membrane). Prehybridization was performed for 2 h at 42°C in 0.2% SDS, 10 mM HEPES (pH 7.5), 0.3 M NaCl, 10 mM EDTA, 2X Denhardt’s solution, 50% formamide and herring sperm DNA (1 μg/ml). Hybridization with the labeled RNA transcripts (5–15 × 106 cpm) was carried out for 74 h at 42°C in the above buffer and washing was performed as described by Sutter et al. (1991). Thereafter, radioactivity of the gels was determined as described for duplex RT-PCR.
Enzyme Assays.
UGT activity was measured with 0.5 mM 4-methylumbelliferone (4-MUF) as substrate (Lilienblum et al., 1982). [UGT assays were also carried out with 0.5 mM 1-naphthol as substrate with similar results, except that UGT activity (4-MUF as substrate) was about 1.5-fold higher than UGT activity (1-naphthol as substrate)]. Caco-2 cells (7–10 mg protein) were homogenized with 500 μl, 0.25 M sucrose containing 10 mM Tris-HCl (pH 7.4) using a Dounce homogenizer and homogenates were stored at −80°C. No detergent activation of UGT activity was detectable in Caco-2 cell homogenates, suggesting that the enzyme was already activated by lysophosphatidylcholines generated from microsomal phospholipids by phospholipases. Therefore, addition of Brij 58 was omitted. The assays were performed at 37°C in the presence of 0.1 M Tris-HCl (pH 7.4) and 5 mM MgCl2 and 0.2 mg homogenate protein in a total assay volume of 0.5 ml. The reaction was started by addition of UDP-glucuronic acid (3 mM). 4-MUF glucuronide (and 1-naphthol glucuronide) was determined fluorometrically. The reaction was linear up to 6 min.
CYP1 ethoxyresorufin O-deethylase (EROD) activity was measured as described (Pohl and Fouts, 1980). Protein was determined according to Lowry et al. (1951).
Statistical analysis was performed using Student’s t test.
Results
The Caco-2 cell clone TC7 showed much higher basal expression of UGT activity (4-MUF as substrate) than clone PF11 (Table1). However, TCDD induction was higher in PF11 cells. Induction of CYP1 (EROD) activity by TCDD was high in both clones. As expected, there was only moderate induction of CYP1 activity by TBHQ; a small increase in EROD activity may be due to antioxidant-type induction of CYP1A2 (Eaton et al., 1995). Studies with Caco-2/TC7 cells showed significant induction of UGT activity by TBHQ and by TCDD (Fig. 1). After treatment with both TBHQ and TCDD, no additive effects were observed.
Induction of UGT (4-MUF as substrate) and EROD activities by treatment of Caco-2 cell clones TC7 and PF11 with TBHQ or TCDD
Induction of UGT activity (4-MUF as substrate) in Caco-2/TC7 cells by TBHQ and TCDD.
Preconfluent Caco-2/TC7 cells were grown with solvent alone (1), treated with 20, 40 and 80 μM TBHQ (2, 3, 4, respectively), with 10 nM TCDD (5), or with both, 40 μM TBHQ and 10 nM TCDD (6). Cells were harvested after 3 days. Means ± S.D. of five independent experiments are listed. *p < .05
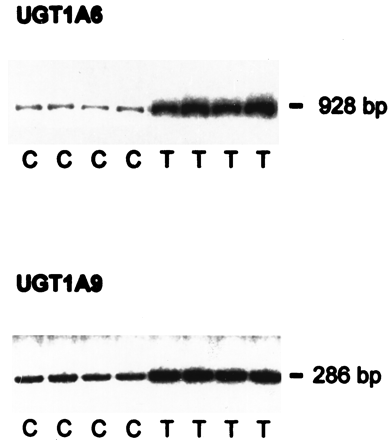
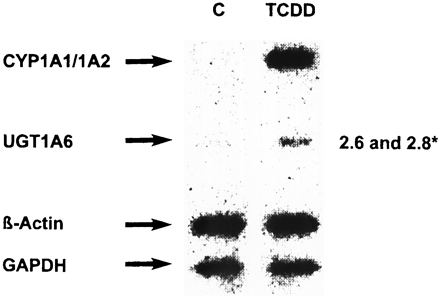
Analysis of the expression of individual UGTs with Caco-2/PF11 cells showed that at least two UGTs, UGT1A6 and UGT1A9, are inducible by TCDD (Fig. 2). Quantification of RT-PCR products showed ca. ∼4-fold induction of UGT1A6 and ca. ∼2.9-fold induction of UGT1A9 expression. To verify whether increased UGT mRNA was due to transcriptional activation, nuclear run-on analysis was performed (Fig. 3). As expected, CYP1A1 expression was markedly elevated by TCDD treatment for 24 h. Because CYP1A1 transcripts were undetectable in untreated nuclei, induction factors could not be determined. UGT1A6 was also transcriptionally activated but to a smaller extent. After normalization to β-actin or to GAPDH, induction factors of 2.6 or 2.8, respectively, were found in four independent experiments. These findings suggest that the increase of UGT1A6 mRNA is due to transcriptional activation.
Induction of human UGT1A6 and UGT1A9 expression by TCDD in Caco-2/PF11 cells.
Preconfluent Caco-2/PF11 cells were grown for 4 days as untreated solvent controls containing 0.1% DMSO or treated with 10 nM TCDD. RT-PCR analysis was carried out as described in Materials and Methods.
Transcriptional regulation of UGT1A6 expression in Caco-2/PF11 cells.
Cells were treated with 10 nM TCDD for 24 h. Nuclei were isolated and nuclear run-on analysis was carried out. *Numbers represent means of the ratio of TCDD versus C of four independent experiments, determined by densitometry, and normalized to β-actin and GAPDH, respectively.
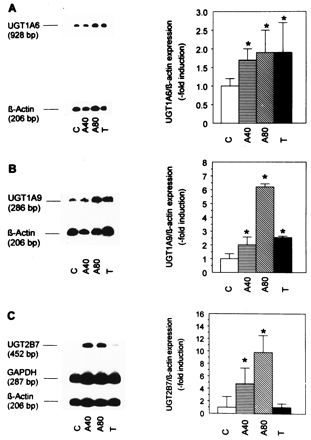
Because TC7 cells showed appreciable basal UGT activity, this clone was chosen for the induction studies of UGT isoforms by TBHQ. As shown in Fig. 4, the three isoforms studied were clearly inducible by 40 to 80 μM TBHQ. Both UGT1A6 and UGT1A9 were induced by 10 nM TCDD, whereas UGT2B7 expression was not induced by TCDD.
Influence of TBHQ and TCDD on the expression of UGT isoforms UGT1A6 (A), UGT1A9 (B), and UGT2B7 (C).
Preconfluent Caco-2/TC7 cells were grown as untreated solvent controls (C), treated with the antioxidant (A) 40 and 80 μM TBHQ (A40, A80), or with TCDD (T), as indicated. They were harvested after 3 days. Representative duplex or triplex RT-PCR blots are shown on the left. They were quantitated by PhosphorImaging and means of the results of several experiments are shown on the right. Data represent means ± S.D. of four experiments with UGT1A6 and of three experiments with UGT1A9 and UGT2B7. *p < .05
Discussion
Induction studies in Caco-2 cells utilizing selective duplex RT-PCR methods demonstrated for the first time that multiple human UGTs are induced by AhR-and antioxidant-type inducers. It could be shown that both UGT1A6 and UGT1A9 appear to be induced by TCDD, in particular in the PF11 clone isolated from early passage Caco-2 cells. Induction was less pronounced in the TC7 clone, probably due to higher constitutive UGT expression. It is intriguing that basal UGT expression has been found to be inversely related to inducibility (Münzel et al., 1994). In addition, basal expression of UGTs appears to be dependent upon the differentiation state of enterocytes, in line with previous immunohistochemical findings in duodenal biopsies using polyclonal anti-UGT antibodies. They showed that staining of UGT protein was high at the villous tip of duodenal biopsies but was low in crypt cells (J. Buchthal, K.-E. Grund and K.W. Bock, unpublished results). Induction of UGT1A6 and UGT1A9 by AhR agonists may explain enhanced paracetamol (Bock et al., 1993) and propranolol glucuronidation in smokers (Walle et al., 1987), respectively.
At least three UGT isoforms (UGT1A6, UGT1A9, and UGT2B7) were demonstrated to be inducible by TBHQ in Caco-2/TC7 cells, but UGT2B7 expression was not induced by TCDD treatment. The antioxidant-type induction mechanism may be interesting with regard to current chemoprevention programs, for example, against colon carcinoma (Boone et al., 1990; Reddy et al., 1993). Chemopreventive induction mechanisms are known to be triggered by a large variety of constituents of our plant diet, including polyphenolic flavonoids in onions such as quercetin, organosulfur compounds in garlic, and isothiocyanates in broccoli (Wattenberg, 1983; Prestera and Talalay, 1995). These adaptive mechanisms probably evolved as a consequence of feeding on a plant diet (Nebert, 1991). Hence, electrophilic metabolites derived from dietary plant constituents appear to trigger an adaptive response that enhances the antioxidant defense, the latter including ancillary enzymes such as UGTs (Sies, 1993). Induction of UGTs by flavonoids has been shown to determine the level of hormones in cells such as testosterone in prostate cancer cells (Sun et al., 1998) and is known to decrease the bioavailability of many dietary constituents and drugs such as morphine. UGTs have also been shown to be responsible in part for resistance to chemotherapeutic drugs such as daunorubicin (Gessner et al., 1990) and mycophenolic acid (Franklin et al., 1996). Glucuronidation may also facilitate detoxification of dietary contaminants such as the carcinogen BaP (Bock, 1991). Apart from the proximate carcinogenic BaP-7,8-dihydrodiol, multiple phenols and quinones represent the majority of BaP metabolites. Quinones such as BaP-3,6-quinone are known to undergo quinone/quinol redox cycles (Lilienblum et al., 1985). On the other hand, they are also reduced to diphenols by NQO1, subsequently conjugated to mono- and diglucuronides by human UGT1A6 and UGT1A9 (Gschaidmeier et al., 1995), and eliminated via the bile. Hence, the enzymes induced by antioxidant-type inducers (UGTs, NQO1, and GSTs) may be key players in the detoxification of BaP quinones.
AhR- and antioxidant-type inducers trigger distinct but different adaptive responses (Jaiswal, 1994). TCDD-type inducers are ligands of the AhR, which is known to induce both phase I and phase II enzymes of drug metabolism. The mechanism responsible for antioxidant-type induction (which selectively enhances phase II enzymes) is not well characterized. In studies of rat glutathione S-transferase Ya (Nguyen et al., 1994) and of rat and human NQO1, a DNA-binding motif responsible for antioxidant-type induction has been identified and termed antioxidant-response element (ARE; Nguyen et al., 1994; Jaiswal, 1994). The consensus sequence TGACnnnGC resembles the AP1 binding motif TGACTCA. In the case of the human NQO1 it has also been shown that the leucine zipper proteins Nrf-1 and Nrf-2 positively regulate and c-Fos and Fra-1 negatively regulate gene expression (Venugopal and Jaiswal, 1996). DNA domains responsible for antioxidant-type induction of UGTs have not yet been identified. No consensus ARE sequence is found in the regulatory region of human UGT1A6; however, ARE-like sequences appear to be present in this region (Münzel et al., 1998). Transfection experiments are currently under way (similar to those carried out to characterize AhR-mediated induction) to identify DNA sequences responsible for antioxidant-type induction of UGT1A6.
In conclusion, the results show for the first time that at least three human UGTs (UGT1A6, UGT1A9, and UGT2B7) are inducible by antioxidant-type inducers and at least two UGTs (UGT1A6 and UGT1A9) are inducible by AhR agonists. The Caco-2 cell model appears, therefore, to be useful to identify the DNA domain(s) responsible for antioxidant-type induction of UGTs.
Acknowledgments
We thank Dr. Alain Zweibaum (Institut National de la Santé et de la Recherche Medicale U-178, Villejuif, France) for providing Caco-2 cell clones, and S. Beck-Gschaidmaier and B. Gregg for expert technical assistance.
Footnotes
-
Send reprint requests to: Prof. Dr. Karl Walter Bock, Institute of Toxicology, University of Tübingen, Wilhelmstrasse 56, D-72074 Tübingen, Germany
-
This work was supported by the Deutsche Forschungsgemeinschaft (DFG).
- Abbreviations used are::
- AhR
- aryl hydrocarbon receptor
- BaP
- benzo(a)pyrene
- EROD
- ethoxyresorufinO-deethylase
- GAPDH
- glyceraldehyde phosphate dehydrogenase
- 4-MUF
- 4-methylumbelliferone
- NQO1
- NAD(P)H quinone oxidoreductase
- TBHQ
- t-butylhydroquinone
- TCDD
- 2,3,7,8-tetrachlorodibenzo-p-dioxin
- UGT
- UDP glucuronosyltransferase
- DMSO
- dimethyl sulfoxide
- RT-PCR
- reverse transcription-polymerase chain reaction, RT-PCR
- Received July 8, 1998.
- Accepted February 3, 1999.
- The American Society for Pharmacology and Experimental Therapeutics