Abstract
ABT-378 is a potent in vitro inhibitor of the HIV protease and is currently being developed for coadministration with another HIV protease inhibitor, ritonavir, as an oral therapeutic treatment for HIV infection. In the present study, the effect of ritonavir, a potent inhibitor of cytochrome P-450 (CYP) 3A, on the in vitro metabolism of ABT-378 was examined. Furthermore, the effect of ABT-378-ritonavir combinations on several CYP-dependent monooxygenase activities in human liver microsomes was also examined. ABT-378 was found to undergo NADPH- and CYP3A4/5-dependent metabolism to three major metabolites, M-1 (4-oxo) and M-3/M-4 (4-hydroxy epimers), as well as several minor oxidative metabolites in human liver microsomes. The mean apparent Km andVmax values for the metabolism of ABT-378 by human liver microsomes were 6.8 ± 3.6 μM and 9.4 ± 5.5 nmol of ABT-378 metabolized/mg protein/min, respectively. Ritonavir inhibited human liver microsomal metabolism of ABT-378 potently (Ki = 0.013 μM). The combination of ABT-378 and ritonavir was much weaker in inhibiting CYP-mediated biotransformations than ritonavir alone, and the inhibitory effect appears to be primarily due to the ritonavir component of the combination. The ABT-378-ritonavir combinations (at 3:1 and 29:1 ratios) inhibited CYP3A (IC50 = 1.1 and 4.6 μM), albeit less potently than ritonavir (IC50 = 0.14 μM). Metabolic reactions mediated by CYP1A2, CYP2A6, and CYP2E1 were not affected by the ABT-378-ritonavir combinations. The inhibitory effects of ABT-378-ritonavir combinations on CYP2B6 (IC50 = >30 μM), CYP2C9 (IC50 = 13.7 and 23.0 μM), CYP2C19 (IC50 = 28.7 and 38.0 μM), and CYP2D6 (IC50 = 13.5 and 29.0 μM) were marginal and are not likely to produce clinically significant drug-drug interactions.
Discovery and development of HIV protease inhibitors is a major advance in the treatment of HIV-1 infection and AIDS. Several HIV protease inhibitors have been developed recently for the treatment of HIV infection (Roberts et al., 1990; Vacca et al., 1994; Kempf et al., 1995; Patick et al., 1996;Vacca and Condra, 1997). Therapy with ritonavir, a potent inhibitor of HIV-1 protease, produced a rapid and sustained decline of plasma viral RNA and a concomitant elevation of CD4 cells in HIV-infected individuals (Danner et al., 1995). Due to the high replication and mutation rates of HIV, sustained high plasma concentrations of protease inhibitors have been shown to be necessary to prevent the emergence of resistant virus (Molla et al., 1996). Thus, it is crucial to maintain high plasma levels of the protease inhibitors to delay the development of drug resistance. Due to limited oral bioavailability and poor pharmacokinetic characteristics of many of the currently used protease inhibitors, new efforts are being made to design more potent protease inhibitors with improved pharmacokinetic properties.
In vitro metabolism studies have demonstrated that the metabolism of ritonavir is mediated predominantly by cytochrome P-450 (CYP)2 3A group of enzymes with a minor contribution from CYP2D6 (Kumar et al., 1996). Furthermore, ritonavir is a potent inhibitor of CYP3A-mediated biotransformations (nifedipine oxidation, terfenadine hydroxylation, 17α-ethynylestradiol hydroxylation; Kumar et al., 1996). HIV protease inhibitors saquinavir and indinavir are metabolized primarily by CYP3A (Chiba et al., 1997;Fitzsimmons and Collins, 1997) and have poor pharmacokinetic profiles due to low oral bioavailability, which is presumed to be the result of high metabolic clearance. In vitro ritonavir potently inhibited the human liver microsomal metabolism of saquinavir (IC50 = 0.25 μM) and indinavir (IC50 = 2.2 μM; Kempf et al., 1997). In humans, a significant increase in plasma saquinavir levels were observed when coadministered with ritonavir, leading to a 50-fold increase in area under the curve (AUC) and a 22-fold increase in the maximal plasma concentration (Cmax; Hsu et al., 1998a). A substantial increase in plasma drug levels was also observed with indinavir on coadministration with ritonavir (Hsu et al., 1998b). The improved pharmacokinetics of other protease inhibitors on coadministration with ritonavir may have implications for the therapy of HIV infection with combinations of ritonavir and other protease inhibitors. This is especially important due to orthogonal resistance patterns and synergistic action obtained with some of the combinations of protease inhibitors (Molla et al., 1996).
ABT-378 (Fig. 1), an analog of ritonavir, was designed to minimize interaction of the inhibitor with valine-82 of the HIV protease, the predominant site of mutation in ritonavir-treated patients (Molla et al., 1996; Sham et al., 1998). ABT-378 is a potent inhibitor of wild-type and mutant HIV protease (Ki = 1.3–28 pM) and is also active against mutant HIV selected by ritonavir in vivo (EC50 ≤ 0.06 μM). The oral bioavailability of ABT-378 in animal models was low and ABT-378 was rapidly cleared from plasma (Marsh et al., 1997), probably due to oxidative metabolism. On coadministration, small doses of ritonavir were found to produce significant increases in AUC and Cmax of ABT-378 in both rat and dog (Marsh et al., 1997). In early clinical trials, similar results have been observed in humans (Lal et al., 1997,1998). The present study investigates the metabolic basis of the inhibitory effect of ritonavir on the in vitro metabolism of ABT-378 in human liver microsomes. Because the therapy of HIV infection involves administration of multiple drugs, some of which may be CYP substrates, the effect of ABT-378-ritonavir combinations on several CYP-dependent monooxygenase activities in human liver microsomes was also examined in this study.
Structures of ABT-378 and its major human liver microsomal metabolites.
Materials and Methods
Chemicals.
ABT-378 was uniformly labeled with carbon-14 in the carbonyl carbon beta to the 2,6-dimethylphenoxy group of the molecule (53.8 mCi/mmol; Fig. 1) and was found to be >97% radiochemically pure. [14C]Ritonavir, [14C]phenacetin, [14C]dextromethorphan, and [3H]terfenadine were also synthesized at Abbott Laboratories (Abbott Park, IL). [14C]Tolbutamide and [14C]chlorzoxazone were obtained from Amersham International (Arlington Heights, IL). [14C]S-Mephenytoin was obtained from ChemSyn Laboratories (Lenexa, KS). Coumarin, troleandomycin, terfenadine, phenacetin, tranylcypromine, tolbutamide, quinidine, 4-methylpyrazole, glucose 6-phosphate, glucose 6-phosphate dehydrogenase, and β-NADP were obtained from Sigma Chemical Co. (St. Louis, MO). Ketoconazole, furafylline, sulfaphenazole, and chlorzoxazone were obtained from Research Biochemicals International (Natick, MA). Human B-lymphoblastoid-derived CYP microsomes andS-mephenytoin were obtained from Gentest Corporation (Woburn, MA). Anti-CYP3A4 antibodies were obtained from Dr. Jerome Lasker (Mt. Sinai Medical Center, New York, NY). Transplant-quality human liver tissue was obtained from the International Institute for the Advancement of Medicine (IIAM; Exton, PA). Liver microsomes were prepared by ultracentrifugation.
Incubations.
[14C]ABT-378 was combined with unlabeled ABT-378 in methanol to prepare appropriate stock solutions. Individual incubations consisted of 0.1 mg/ml microsomal protein, [14C]ABT-378 (final methanol concentration < 0.7%, v/v) in 100 mM phosphate buffer (pH 7.4) with final concentrations of 5 mM magnesium chloride, 5 mM glucose 6-phosphate, 1 mM β-NADP, and 1 U/ml glucose 6-phosphate dehydrogenase. The drug, buffer, and microsomes were mixed and kept at 37°C for 5 min, and the reaction was started by adding the NADPH-generating system. The reaction was stopped by adding an equal amount of acetonitrile and vortexing. The incubations with lymphoblastoid microsomes were conducted essentially as described above with 20 μM [14C]ABT-378 and 3.3 mg/ml microsomal protein, with an incubation time of 30 min.
HPLC.
Separations were achieved at ambient temperature with a Beckman Ultrasphere 5-μm, 4.6 × 250 mm C-18 column connected to an Alltech Ultrasphere 5-μm C-18 guard column. A linear gradient of 25 to 55% acetonitrile in buffer (25 mM ammonium acetate, pH adjusted to 4.8 with formic acid) over 57 min was used as column eluent at a flow rate of 1 ml/min. Radioactivity in the column effluent was monitored with a Flo-One/Beta model A-500 radioactivity flow detector (Packard Instruments, Meriden, CT) equipped with a 0.25-ml flow cell. A ratio of column effluent to liquid scintillator (Ultima Flo M, Packard Instruments) of 1:3 was used.
Kinetics.
A concentration range of 0.3 to 50 μM with an incubation period of 10 min was used. Due to lack of solubility, concentrations >50 μM could not be used. The kinetic parameters were calculated by a weighted Lineweaver-Burk method by using EnzymeKinetics v1.3 software (Trinity Software, Campton, NH). The kinetic parameters were determined with liver microsomes from four subjects: IEN016, IE4420, IEG710, and IAY148, as well as pooled microsomes, which were prepared by mixing equal mg amounts of the aforementioned four liver microsomes. The kinetics of ABT-378 metabolism by CYP3A4 lymphoblastoid microsomes were determined with 0.1 to 50 μM ABT-378 with an incubation period of 10 min. The protein concentration was 0.15 mg/ml, which was equivalent to 7.2 pmol of CYP3A4/ml incubation.
Metabolic Correlation.
Correlation of the rate of ABT-378 metabolism with CYP isoform-specific activities in the microsomes prepared from a panel of human livers (n = 10) was studied at a concentration of 25 μM ABT-378 (0.1 μCi/ml) and 0.1 mg of microsomal protein/ml. Statistical analysis was performed with Instat 2.01 software (GraphPad, San Diego, CA).
Chemical Inhibition.
Methanolic stock solutions of inhibitors were added just before the addition of ABT-378 (7 μM, approximately equal to itsKm). An equivalent quantity of methanol was added for control incubations. In the case of the mechanism-based inhibitors, troleandomycin and furafylline, the mixture of microsomes, inhibitor, and the NADPH-generating system was preincubated for 10 min at 37°C before the addition of ABT-378. TheKi values were determined by coincubating various concentrations of the inhibitor (0–50 nM ritonavir or 0–100 nM ketoconazole) with various concentrations of ABT-378 (2.5–10 μM) in the presence of pooled liver microsomes and an NADPH-generating system. The Ki value for the inhibition of the metabolism of [14C]ritonavir by ABT-378 was determined by coincubating various concentrations of [14C]ritonavir (0.5–2 μM) with various concentrations of ABT-378 (0–60 μM) in the presence of pooled liver microsomes (0.25 mg of protein/ml) and an NADPH-generating system. The sample work-up and analyses were as described previously (Kumar et al., 1996).
Immunoinhibition.
Microsomes (0.02 mg/ml), antibodies, and phosphate buffer were combined and kept for 30 min at ambient temperature. ABT-378 (7 μM) was then added and the mixture was kept at 37°C for another 5 min. The reaction was started by adding the NADPH-generating system, and the incubation was conducted at 37°C for 20 min. The ratio of preimmune IgG and anti-CYP3A4 IgG was varied to achieve a range of anti-CYP IgG/CYP ratios (range: 0–20) while keeping the total amount of IgG added constant.
Effect on CYP Activities.
The effect of the ABT-378-ritonavir combination on various CYP isoform-dependent monooxygenase activities was determined as follows. Individual incubations consisted of human liver microsomal protein in 100 mM phosphate buffer (pH 7.4) with either an NADPH-generating system or NADPH. In all assays, the substrate concentrations were approximately equal to their respective Kmvalues. The assay conditions are summarized in Table1. Phenacetin O-deethylation, coumarin 7-hydroxylation, tolbutamide methylhydroxylation,S-mephenytoin 4′-hydroxylation, dextromethorphanO-demethylation, chlorzoxazone 6-hydroxylation, and terfenadine oxidation (t-butyl hydroxylation plus carboxylation) were used as isoform-specific marker activities for CYP1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4/5, respectively. The effect on CYP2B6-dependent S-mephenytoin N-demethylation was examined by using CYP2B6 B-lymphoblastoid microsomes.
Isoform-specific CYP assays: Assay conditions and analytical methods
Results
As observed previously (Kumar et al., 1999), ABT-378 was metabolized by human liver microsomes to three major (M-1, 4-oxo-ABT-378, M-3/M-4, epimers of 4-hydroxy-ABT-378) and several minor metabolites via NADPH-dependent pathways (Fig. 1). The rate of metabolism of ABT-378 was linear for up to 10 min of incubation at a protein concentration of 0.1 mg of microsomal protein/ml. Hence, 0.05 or 0.1 mg/ml microsomal protein and a 10-min incubation period were used for all experiments except where specifically mentioned otherwise. Kinetic parameters could not be calculated for metabolitesM-3/M-4 because metabolite M-1 (a subsequent oxidation product of metabolites M-3/M-4), as well as two dihydroxylated metabolites were formed during the course of incubation from metabolites M-3/M-4. Hence, all the kinetic parameters were calculated based on the rate of disappearance of ABT-378. In a panel of human liver microsomes (n = 10), the rate of metabolism of ABT-378 was 5.42 ± 4.80 nmol/mg of protein/min. Metabolite M-4 was the major metabolite formed by all 10 liver microsomes examined and accounted for 40 to 50% of the total metabolites. The mean apparent rates of formation ofM-1, M-3, and M-4 were 0.65 ± 0.64, 1.02 ± 0.74, and 2.28 ± 1.74 nmol/mg/min, respectively.
Microsomes derived from B-lymphoblastoid cells transfected with CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9-arg, CYP2C9-cys, CYP2C19, CYP2D6, and CYP2E1 cDNA did not metabolize ABT-378. CYP3A4 and CYP3A5 microsomes metabolized ABT-378 and formed all of the three major metabolites as well as all the minor metabolites. These data suggest the primary role of CYP3A4 and CYP3A5 in the metabolism of ABT-378 toM-1 and M-3/M-4.
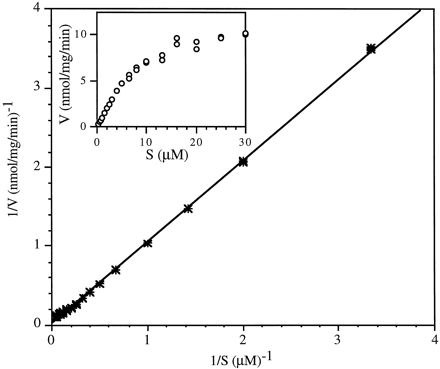
The metabolism of ABT-378 followed monophasic Michaelis-Menten kinetics (Fig. 2). In the concentration range tested, the mean apparent Km andVmax values for the metabolism of ABT-378 by human liver microsomes were 6.8 ± 3.6 μM (range 1.6–10.1 μM) and 9.4 ± 5.5 nmol of ABT-378 metabolized/mg protein/min (range 2.2–13.9 nmol metabolized/mg protein/min), respectively (Table2). The mean intrinsic clearance was 1.37 ± 0.31 ml/mg of protein/min (range 1.01–1.76 ml/mg of protein/min). The kinetics were also determined with pooled microsomes, and the kinetic parameters were in the same range as those determined with individual microsomes. Expressed CYP3A4 microsomes exhibited aKm of 1.6 μM and aVmax of 29.3 nmol/nmol of CYP/min.
Kinetics of human liver microsomal metabolism of ABT-378 (Subject ID: IE4420).
Kinetics of the metabolism of ABT-378 by human liver microsomes and CYP3A4 lymphoblastoid microsomes
The rate of metabolism of ABT-378 by a panel of 10 human liver microsomes was studied to correlate the data with previously determined isoform-specific CYP activities. The correlation parameter used was the linear regression coefficient (r). A two-tailed Student’st test for paired data was performed to calculateP values. The metabolism of ABT-378 correlated with erythromycin N-demethylation (CYP3A, r = 0.980, P < .001), coumarin 7-hydroxylation (CYP2A6,r = 0.674, P < .05), andS-mephenytoin 4′-hydroxylation (CYP2C19, r = 0.817 P < .01; Table 3). However, there were coincidental correlations between several isoforms in this liver bank. For example, there was a correlation between CYP3A and CYP2A6 as well as CYP2C19 (Table 3). Hence the role of each CYP isoform in the metabolism of ABT-378 was further established by chemical and immunoinhibition experiments as described below.
Correlation of the rate metabolism of ABT-378 with isoform-specific CYP activities in a panel of human liver microsomes (n = 10)
The effect of isoform-selective CYP inhibitors/substrates was examined at 7 μM ABT-378 (Halpert et al., 1994; Newton et al., 1995; Bourrie et al., 1996; Wienkers et al., 1996). Furafylline (CYP1A2, 5 μM), coumarin (CYP2A6, 100 μM), sulfaphenazole (CYP2C9/10, 1 μM), tranylcypromine (CYP2C19, 50 μM), quinidine (CYP2D6, 2 μM), and 4-methylpyrazole (CYP2E1, 20 μM) did not affect the metabolism of ABT-378 (Table 4). Ketoconazole (CYP3A, 1 μM, 80.4% inhibition) and troleandomycin (CYP3A, 200 μM, 84.3% inhibition) were very effective inhibitors of the metabolism of ABT-378 as well as the formation of the three major metabolites. Ritonavir (0.1 μM) was also a very effective inhibitor of the metabolism of ABT-378. The potent inhibition of the metabolism of ABT-378 by CYP3A inhibitors further confirms the major role of CYP3A. The lack of inhibition by isoform-selective inhibitors and the inability of specific CYP-containing microsomes to metabolize ABT-378 indicates that the correlation obtained between ABT-378 metabolism and CYP2A6/CYP2C19 activities is probably coincidental.
Effect of isoform-selective CYP inhibitors on the metabolism of ABT-378 by human liver microsomes
The effect of antihuman-CYP3A4 Ig on the metabolism of ABT-378 by pooled microsomes was studied at varying ratios of antibodies to microsomal protein, while keeping the total amount of antibody added at a constant level. The anti-CYP3A4 antibodies used in this study have been shown to produce greater than 80% inhibition of nifedipine oxidation, a CYP3A4 marker activity, at an IgG/CYP ratio of 2.5 (J. Lasker, personal communication). At an anti-IgG/CYP ratio of 3, anti-CYP3A4 antibodies inhibited 92.6% of the metabolism of ABT-378, and at higher ratios completely abolished the metabolism. These results further confirmed that the members of the CYP3A subfamily are probably the exclusive contributors to the metabolism of ABT-378 in human liver microsomes.
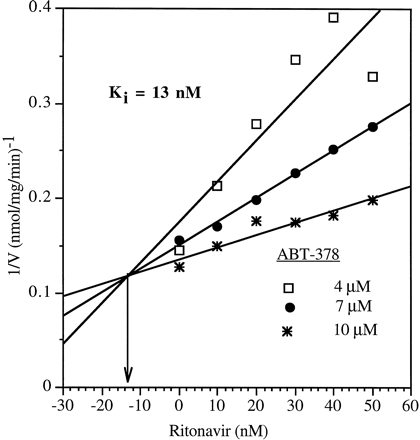
The metabolism of ABT-378 was inhibited by both ritonavir and ketoconazole in a concentration-dependent manner. TheKi values for the inhibition were determined by Dixon plots. Ritonavir inhibited the metabolism of ABT-378 with a Ki value of 0.013 μM. The nature of this inhibition was competitive as indicated by the intersecting lines in the Dixon plot (Fig.3). Even though a low protein concentration (0.05 mg/ml) was used in this study, it is possible that at low concentrations of ritonavir, the assumption of [inhibitor]≫[enzyme] is violated. Ketoconazole inhibited the metabolism of ABT-378 with a Ki value of 0.20 μM. ABT-378 inhibited the human liver microsomal metabolism of ritonavir very weakly, with an estimatedKi of 130 μM, indicating that ABT-378 may not have a significant inhibitory effect on ritonavir in vivo on coadministration of both compounds.
Effect of ritonavir on the human liver microsomal metabolism of ABT-378: Dixon plot.
The effect of the ABT-378-ritonavir combination on isoform-specific CYP-dependent monooxygenase activities in human liver microsomes was examined (Table 5 and Fig.4). ABT-378/ritonavir concentration ratios of 3:1 and 29:1 were chosen to encompass the wide spectrum of concentrations that are likely to occur in humans on coadministration of these two drugs. Previous studies have shown that ritonavir is a potent inhibitor of CYP3A and also inhibits CYP2D6, CYP2C9, and CYP2C19 (Kumar et al., 1996). In the current study, ABT-378 was found to be a much weaker inhibitor of CYP3A, CYP2D6, CYP2C9, and CYP2C19 than ritonavir (Fig. 4). At 30 μM, the inhibition produced by ABT-378 was 7.3% of CYP1A2, 14.7% of CYP2A6, 28.8% of CYP2C9, 29.7% of CYP2C19, 41.7% of CYP2D6, 6.7% of CYP2E1, and 73.9% of CYP3A activities, and there was no apparent inhibitory effect on CYP2B6 activity. Consequently, the combination of ABT-378 and ritonavir was a less potent inhibitor of CYP3A, CYP2D6, CYP2C9, and CYP2C19 activities than ritonavir alone. CYP1A2, CYP2A6, and CYP2E1 isoforms were not inhibited by ABT-378-ritonavir combination, with only a marginal effect on CYP2B6 activity. The 3:1 and 29:1 ratios of ABT-378 and ritonavir inhibited CYP2C9-dependent tolbutamide hydroxylation with an IC50 of 13.7 and 23.0 μM, respectively, compared with 8.0 μM ritonavir. The 3:1 and 29:1 ratios of ABT-378 and ritonavir inhibited CYP2C19-dependent S-mephenytoin 4′-hydroxylation with an IC50 of 28.7 and 38.0 μM, respectively, compared with 13.0 μM ritonavir. The 3:1 and 29:1 ratios of ABT-378 and ritonavir inhibited CYP2D6-dependent dextromethorphan O-demethylation with an IC50 of 13.5 and 29.0 μM, respectively, compared with 2.5 μM ritonavir. The 3:1 and 29:1 ratios of ABT-378 and ritonavir inhibited CYP3A-dependent terfenadine hydroxylation with an IC50 of 1.1 and 4.6 μM, respectively, compared with 0.14 μM ritonavir.
Effect of ABT-378-ritonavir combination on isoform-specific CYP-dependent monooxygenase activities
Effect of ABT-378, ritonavir, and their combination on the isoform-specific CYP-dependent monooxygenase activities in human liver microsomes.
Discussion
The results obtained in the current study demonstrate that ABT-378 undergoes CYP-dependent biotransformation to three major metabolites (M-1, M-3, and M-4) as well as several minor metabolites in human liver microsomes, consistent with a previous human liver microsomal metabolism study (Kumar et al., 1999). Members of the CYP3A subfamily (CYP3A4 and CYP3A5) were found to be the enzymes responsible for the formation of all the metabolites of ABT-378. Ritonavir was found to be a very potent inhibitor of CYP3A-mediated biotransformation of ABT-378 with a very lowKi value (0.013 μM).
The high metabolic lability precludes the use of ABT-378 alone as an effective antiviral agent for the treatment of HIV infection. The potent in vitro inhibition of the metabolism of ABT-378 by ritonavir indicates that on coadministration of ritonavir with ABT-378, the former would potentially inhibit the metabolism of the latter, thereby reducing the clearance of ABT-378. This has been demonstrated to be true in a rat model, where the AUC and Cmaxwere severalfold higher on coadministration of [14C]ABT-378 and ritonavir compared with when [14C]ABT-378 was administered alone (Marsh et al., 1997). This potent pharmacokinetic enhancement of ABT-378 by ritonavir is the rationale for coadministration of ritonavir with ABT-378 as a therapeutic treatment for AIDS and HIV infection. Because the Ki value for the inhibition of ABT-378 metabolism is very low (0.013 μM), smaller doses of ritonavir than used therapeutically (600 mg b.i.d., resulting in a plasma concentration range of 9–36 μM; Hsu et al., 1997) may be sufficient to produce the desired pharmacokinetic enhancement of ABT-378. In early human studies, a substantial increase in exposure to ABT-378 was observed when 50 to 200 mg of ritonavir was coadministered with ABT-378 (Lal et al., 1997, 1998). Based on these early human studies, it is evident that at equitherapeutic doses, the plasma levels of ritonavir after the dosing of the combination would be far lower than those observed during ritonavir-alone therapy.
The effect of the ABT-378-ritonavir combinations on isoform-specific CYP-dependent monooxygenase activities indicated that ABT-378 is a much weaker inhibitor of CYP3A, CYP2D6, CYP2C9, and CYP2C19 than ritonavir (Fig. 4). The results obtained also indicate that the combination is less potent in inhibiting CYP3A, CYP2D6, CYP2C9, and CYP2C19 activities than ritonavir alone and that the inhibitory activity of the combination is primarily due to the effect exerted by the ritonavir component. As with ritonavir, the ABT-378-ritonavir combination did not have an inhibitory effect on CYP1A2-, CYP2A6-, and CYP2E1-mediated biotransformations, indicating that this combination is not likely to inhibit the in vivo metabolic reactions mediated by these enzymes.
The inhibitory potency of the combination for CYP2C9- and CYP2C19-mediated reactions was lower than that produced by ritonavir. Previous studies have shown that ritonavir increases the AUC of CYP2D6 substrate desipramine by 2.4-fold (Hsu et al., 1998c). Because, in vitro, an ABT-378-ritonavir combination is severalfold less potent than ritonavir in inhibiting CYP2D6, this combination may not produce clinically significant drug-drug interactions in vivo when coadministered with CYP2D6 substrates.
The in vitro IC50 values for the inhibition of CYP3A-mediated terfenadine hydroxylation were 1.1 and 4.6 μM for 3:1 and 29:1 ratios of the ABT-378-ritonavir combination, respectively. These values are severalfold higher than those obtained with ritonavir (IC50 = 0.14 μM,Ki = 0.017 μM; Kumar et al., 1996). As predicted from in vitro inhibition results, ritonavir, when coadministered with the protease inhibitor saquinavir, a high-clearance CYP3A substrate, increased the AUC of saquinavir by >50-fold (Merry et al., 1997; Hsu et al., 1998a). An even greater increase in the AUC values was observed when ABT-378 was coadministered with ritonavir (Lal et al., 1997, 1998). This dramatic increase in AUC by ritonavir is probably due to its potent inhibition of CYP3A-mediated biotransformations of these compounds. However, with CYP3A substrates, such as clarithromycin, for which a significant alternate route of clearance exists (urinary excretion of the unchanged drug), the magnitude of interaction (77% increase in AUC) produced by ritonavir was lower (Ouellett et al., 1998).
From the current data, it appears that the CYP3A-inhibitory effect of the ABT-378-ritonavir combination is largely due to the effect of the ritonavir component. In this regard, it should be noted that ritonavir had widely variable effects on CYP3A that are not uniformly predictable from in vitro data. First, ritonavir appears to induce CYP3A, inasmuch as its clearance increases approximately 2-fold with multiple dosing (Hsu et al., 1997). This is observed with ritonavir alone and when administered with ABT-378 or saquinavir. Indeed, the multiple-dosing enhancement of the saquinavir AUC is approximately 20-fold, as compared with the >50-fold effect observed with single dosing (Hsu et al., 1998a). Beyond the complication of enzyme induction, which offsets inhibition, are the differences in substrate sensitivities to ritonavir inhibition. ABT-378 is exquisitely sensitive to ritonavir inhibition, whereas 17α-ethinylestradiol is quite resistant (IC50 = 2 μM; Kumar et al., 1996). The reasons for the in vitro substrate differences are not known, although a pattern emerged from the in vivo studies. Clinically, the largest interactions have been observed for agents that have the highest intrinsic clearances and large first-pass extraction ratios (ABT-378 and saquinavir), moderate effects are observed for drugs with medium intrinsic clearances (rifabutin, 3.5-fold increase in AUC, Cato et al., 1998; indinavir, 3-fold increase in AUC, Hsu et al., 1998b), and little or no effects are generally observed for agents with low intrinsic clearances (alprazolam, Hsu et al., 1998c). For example, alprazolam (CLint ∼3 liters/h), which is thought to be metabolized only by CYP3A (Wright et al., 1997), was not affected when coadministered with a 500-mg twice daily regimen of ritonavir (Hsu et al., 1998c). This is paradoxical because the in vitro IC50 in human liver microsomes for the inhibition of alprazolam metabolism by ritonavir was 0.2 μM (data not shown). Similarly, another low-clearance (∼7 liters/h) CYP3A substrate, methadone (in vitro IC50 = 0.05 μM ritonavir,Iribarne et al., 1998), was unaffected by coadministered ritonavir in vivo (Hsu et al., 1998c). From these observations for ritonavir, at equitherapeutic doses, it is expected that this combination will produce smaller inhibitory effects on high-clearance drugs than will ritonavir alone, and that little to no effects will be observed with low-intrinsic-clearance, low-first-pass CYP3A substrates.
In summary, at therapeutic doses, the combination of ABT-378-ritonavir is much weaker in inhibiting CYP-mediated biotransformations as compared with ritonavir alone. At therapeutic doses, this combination is likely to inhibit CYP3A-mediated biotransformations, albeit much less potently than ritonavir alone. Metabolic reactions mediated by CYP1A2, CYP2A6, and CYP2E1 will not be affected by the ABT-378-ritonavir combination. The effect of the ABT-378-ritonavir combination on CYP2B6, CYP2C9, CYP2C19, and CYP2D6 will be marginal and may be of clinical significance only for drugs with a narrow therapeutic window. Because ABT-378 is intended to be given together with ritonavir, other coadministered CYP3A inhibitors such as ketoconazole may not produce clinically significant effects. However, there is potential for alteration of ABT-378 disposition by coadministered drugs that are CYP3A inducers (e.g., rifampicin and phenobarbital; Okey, 1990; Hsu et al., 1998c).
Acknowledgments
We thank Dr. A. D. Rodrigues for the characterization of the human liver bank and Drs. A. Hsu, R. Bertz, R. Lal, and E. Sun for helpful discussions. We thank Drs. S. Roberts and K. Marsh for reviewing the manuscript.
Footnotes
-
Send reprint requests to: Dr. G. Richard Granneman, Clinical Pharmacokinetics and Toxicokinetics, AP-13A, Abbott Laboratories, Abbott Park, IL 60064. E-mail:rick.g.granneman{at}abbott.com
-
↵1 J.D. was a recipient of an Abbott Summer (1996) Research Internship.
- Abbreviations used are::
- CYP
- cytochrome P-450
- AUC
- area under the curve
- Cmax
- maximal plasma concentration
- Received January 29, 1999.
- Accepted May 6, 1999.
- The American Society for Pharmacology and Experimental Therapeutics