Abstract
Acetonitrile is an organic solvent commonly used to increase the solubility of lipophilic substrates for in vitro studies. In this study, we examined its effect on four reactions (diclofenac hydroxylation, tolbutamide methyl hydroxylation, phenytoin hydroxylation, and celecoxib methyl hydroxylation) catalyzed by human liver microsomes and by the recombinant CYP2C9. In both cases, the effect of acetonitrile on activity was found to be substrate-dependent. Namely, it increased diclofenac 4′-hydroxylase and tolbutamide methyl hydroxylase activities, but decreased celecoxib methyl hydroxylase activity in a concentration-dependent manner. By comparison, hydroxylation of phenytoin was resistant to its effect. The presence of acetonitrile (3%, v/v) gave rise to a lowerKm and a higherVmax for diclofenac hydroxylase in both liver microsomes and recombinant CYP2C9 preparations (87 and 52% increase inVmax/Km ratio, respectively). On the other hand, the inhibitory effect of the solvent (1%, v/v) toward celecoxib hydroxylase was characterized by a decrease in Vmax (human liver microsomes) or a change in both Km andVmax (rCYP2C9), leading to 25 and 46% decrease inVmax/Km for both systems. The results of this study underscore the need for careful evaluation of solvent effects before initiation of inhibition or cytochrome P450 reaction phenotyping studies.
In recent years, in vitro drug metabolism studies have played an increasingly important role in the evaluation of new drug entities. Typically, various in vitro systems are used to screen for metabolic stability, identification of metabolites, and determination of kinetic parameters (Km and Vmax), and inhibition constants (Ki). The data are used for the purposes of lead candidate selection, based on characterization of metabolic pathways, identification of enzymes responsible for metabolism, estimation of in vivo intrinsic clearance, and prediction of drug-drug interactions (Lin and Lu, 1997).
Because in vitro studies are carried out in aqueous physiological buffers, potential problems with solubility may be encountered when the compounds of interest are highly lipophilic. The poor solubility may limit the concentration range to be studied and, thus, make it difficult to evaluate reactions characterized by high apparentKm. Therefore, it is common practice to dissolve compounds in solvents. However, solvents have the potential of altering enzyme-substrate interactions. It is known that some organic solvents can affect the activity of enzymes involved in the metabolism of exogenous compounds, due to solvation properties or competition for the enzyme in question (Gerhards and Gibian, 1967; Teschke et al., 1975). More recently, increasing attention has been drawn to the effect of organic solvents on the activity of cytochrome P450 (CYP)1 enzymes because of the importance of those enzymes in drug metabolism (Cotreau-Bibbo et al., 1996; Draper et al., 1997; Chauret et al., 1998; Hickman et al., 1998; Busby et al., 1999; Coller et al., 1999). Methanol, dimethyl sulfoxide, and acetonitrile, when exceeding 1% (v/v), have been found to inhibit or stimulate the CYP-mediated metabolism of several substrates in human liver microsomes (Chauret et al., 1998). The change in enzyme activity due to the presence of an organic solvent may compromise the reliability and interpretation of in vitro data. For instance, polyethylene glycol and acetone have been shown to affect the metabolism of tamoxifen and alprazolam, leading to variability in the kinetic parameter estimates (Cotreau-Bibbo et al., 1996). In addition, the inhibitory effect of dimethylformamide on CYP2C19 activity led to flunitrazepam be erroneously reaction phenotyped (Coller et al., 1999).
Acetonitrile is a commonly used organic solvent for in vitro studies. Interestingly, unlike other solvents that are inhibitory toward CYP2C9 activity, acetonitrile stimulates tolbutamide hydroxylation in human liver microsomes, a reaction mediated by CYP2C9 (Chauret et al., 1998;Hickman et al., 1998; Busby et al., 1999; Palamanda et al., 2000). CYP2C9 is abundant in human liver microsomes and has been shown to catalyze the oxidation of many drugs such as tolbutamide, warfarin, flurbiprofen, phenytoin, hexobarbital, and diclofenac (Goldstein and de Morais, 1994; Tracy et al., 1996; Inoue et al., 1997; Miners and Birkett, 1998; Yamazaki et al., 1998). Therefore, we examined the effect of acetonitrile on the metabolism of four structurally diverse CYP2C9 substrates (tolbutamide, diclofenac, phenytoin, and celecoxib), determined the resultant change in kinetic parameters, and estimated its influence on the CYP reaction phenotype of celecoxib.
Materials and Methods
Chemicals and Enzyme Sources.
Diclofenac, tolbutamide, and 3-hydroxy tolbutamide were obtained from Research Biochemicals International (Natick, MA). 1′-Hydroxy diclofenac was obtained from Gentest Corporation (Woburn, MA). 5,5-Diphenylhydantoin (phenytoin, sodium salt), 5-(4′-hydroxyphenyl)-5-phenylhydantoin (pHPPH), and 5-(4′-methylpheny)-5-phenylhydantoin (MPHT) were purchased from Aldrich Chemical Co. (Gillingham, Dorset, UK). Flurbiprofen, chlorpropamide, β-nicotinamide adenine dinucleotide phosphate (β-NADP+), β-nicotinamide adenine dinucleotide phosphate reduced form (NADPH), EDTA, glucose 6-phosphate, and glucose 6-phosphate dehydrogenase were obtained from Sigma Chemical Co. (St. Louis, MO). Acetonitrile was provided by Fisher Scientific (Pittsburgh, PA). All other reagents were of analytical or HPLC grade.
Pooled human liver microsomes (from 10 subjects) and a microsomal preparation of an individual organ donor were obtained from The International Institute for the Advancement of Medicine (IIAM, Exton, PA) and Gentest Corporation (Woburn, MA), respectively. A bank of fully characterized human liver microsomes (n = 16 different organ donors) was purchased from Xenotech LLC (Kansas City, KS). The recombinant CYP2C9, CYP3A4, and mouse ascites containing monoclonal antibodies (MAbs) raised against CYP2C9 MAb2C9a and CYP3A4 MAb3A4a were prepared in-house.
Microsomal Incubations.
The incubation for the metabolism of diclofenac, tolbutamide, and celecoxib was carried out in the same way. The incubation mixtures (final volume of 0.5 ml) consisted of the following: 0.1 M potassium phosphate buffer (pH 7.4), 10 mM MgCl2, 1 mM EDTA, 1.0 mM NADP+, 10 mM d-glucose 6-phosphate, d-glucose 6-phosphate dehydrogenase (Sigma Type VII, from baker's yeast, 2.0 U/ml), microsomal protein (0.10–0.50 mg/ml for human liver microsomes and 5–25 pmol CYP/ml for recombinant enzymes), substrate (concentration varied with compound), and acetonitrile (0–5%, v/v). The reaction was started by the addition of the NADPH-generating system, after a 3-min preincubation, and terminated with 2 ml of acetonitrile. The respective internal standards (flurbiprofen for diclofenac, chlorpropamide for tolbutamide, and diclofenac for celecoxib, 50 μl of 100 mM stock) were added to the sample before centrifugation. The supernatant was transferred to a clean tube and evaporated to dryness in an Automation Environmental SpeedVac system (Savant Instruments, Inc., Holbrook, NY). The residue was reconstituted in 150 μl of aqueous solution of acetonitrile (30%) for HPLC analysis.
For the metabolism of phenytoin, the incubation mixture (final volume of 0.5 ml) contained 10 mM MgCl2, 1 mM EDTA, 1 mM NADPH, and 150 mM Tris-HCl buffer (pH 7.4). The reaction was initiated by the addition of NADPH (final concentration of 1 mM) and terminated by the addition of 0.5 ml of acetonitrile followed by 2 ml of ethyl acetate. The internal standard, MPHT (50 μl of 5 μM), was added to the samples before extraction. The organic layer was transferred and evaporated to dryness in Speedvac (Savant Instruments Inc., Holbrood, NY). The residues were reconstituted in 100 μl of 30% acetonitrile aqueous solution for liquid chromatography-mass spectrometry (LC-MS) analysis. The recovery of all analytes was greater than 95%. Phenytoin hydroxylase activity was measured using Tris-HCl as the assay buffer because of low turnover in the presence of phosphate buffer (data not shown).
All substrates in stock solutions (100 times more concentrated in 50% acetonitrile) were first added to the test tubes and the solvent was allowed to evaporate at room temperature before the addition of the remaining assay components.
Immunoinhibition Studies.
The influence of acetonitrile on the P450 reaction phenotyping via immunoinhibition of celecoxib hydroxylation in human liver microsomes was also evaluated. Mouse ascites, fluid containing antibodies against CYP2C9 or CYP3A4, were diluted with potassium phosphate buffer (1:1, 1:2, 1:4, 1:8, 1:16, 1:32, and 1:64 dilution). An aliquot (20 μl) of each diluted ascites solution was incubated with 50 μg of human liver microsomal protein (50 μl) at room temperature for 20 min before the addition of MgCl2, EDTA, celecoxib, and phosphate buffer in the presence and absence of acetonitrile (final concentration of 3%, v/v). The inhibitory potency of each antibody preparation was confirmed after coincubation with the appropriate recombinant CYP (rCYP; Mei et al., 1999).
Concentration of Substrates in Reaction Mixtures in the Presence and Absence of Acetonitrile.
Five-microliter aliquots of 100-time-concentrated stock solution of substrates in 50% acetonitrile (2, 10, 100, and 1.5 mM for diclofenac, phenytoin, tolbutamide, and celecoxib, respectively) were placed in test tubes and the solvent was allowed to evaporate to dryness. Various amounts of human liver microsomes (50 μl) were added directly to the dry residue and vortexed briefly. Four hundred microliters of the respective buffers were added to each tube, followed by the addition of 50 μl of the same buffer containing 0, 10, 30, and 50% acetonitrile to yield final solvent concentrations of 0, 1, 3, and 5% in a 500-μl total volume. The mixture was vortexed and subjected to centrifugation (3000g for 20 min) to remove any particles in the mixture. Aliquots (100-μl) of supernatant were taken and added to two volumes of acetonitrile to precipitate microsomal proteins. The concentration of each substrate in the supernatant was determined by the HPLC or LC-MS method described below. The same amount of each substrate dissolved in 30% acetonitrile aqueous solution was used as the reference for quantitation.
Kinetic Analysis.
Estimates of apparent Km andVmax values were obtained by fitting the untransformed data to a one- or two-enzyme model (Grafit 3,Leatherbarrow, 1992). After initial kinetic parameter estimates were obtained, the data were also analyzed by linear transformation (Eadie-Hofstee plot) to confirm a single Kmmodel.
Sample Analysis.
Diclofenac, celecoxib, and their respective metabolites were separated on a reversed phase C18 column (Betasil, 4.6 × 150 mm, 5 μm) using a Shimadzu LC-10AS HPLC system. The mobile phase consisted of 0.05% aqueous phosphoric acid (solvent A) and acetonitrile/H2O (90:10) in 0.05% phosphoric acid (solvent B) and was delivered at a constant flow rate of 1.0 ml/min. The initial mobile phase consisted of 30% solvent B, which increased linearly to 80% over 13 min. The elution of the analytes was monitored by UV detection (274 nm for diclofenac and its metabolite, 254 nm for celecoxib and its metabolite). The same mobile phase was applied to the analysis of tolbutamide and its metabolite, but the elution was carried out on a MetaChem (Torrance, CA) ODS-3 column (4.6 × 150 mm, 5 μm). The initial mobile phase consisted of 30% of solvent B, which increased linearly to 70% over 10 min, and the elution was monitored at 230 nm.
Phenytoin and its metabolite were analyzed by LC-MS, which was performed on a Sciex (Concord, Ontario, Canada) Model API 150 single quadruple mass spectrometer interfaced via a Sciex heated nebulizer probe to a liquid chromatograph consisting of a Perkin-Elmer Cetus Instruments (Norwalk, CT) 200 series quaternary pump and an autoinjector. The separation of phenytoin and pHPPH was accomplished on a MetaChem (Torrance, CA) ODS-3 column (2.1 × 50 mm, 5 μm). The mobile phase, consisting of water (solvent A), acetonitrile (solvent B), and 50 mM NH4Ac, pH 4.5 (solvent C), was delivered at a flow rate of 0.5 ml/min with a linear increase of solvent B from 25 to 65% over 2 min. The composition of solvent C (10%) was kept constant during the gradient elution. The column eluant was directed to a heated nebulizer probe operating at 450°C. The nebulizing gas pressure, auxiliary gas flow, and curtain gas flow were set at 80 psi, 1.2 liter/min and 1.0 liter/min, respectively. Single ion monitoring (SIM) experiments in negative mode were performed using dwell time of 150 ms/transition to detect ions atm/z 267 (pHPPH), 265 (MPHT), and 251 (phenytoin).
Statistical Analysis of Data.
The statistical significance in enzyme activities between the various acetonitrile concentrations was determined using a single factor ANOVA with a P < .05 indicating a significant difference. The enzyme kinetic parameters (Km,Vmax, andVmax/Km) determined in the presence and absence of the solvent were evaluated for statistical significance by means of a Student's ttest, and the statistical difference between the various groups of data is denoted by *P < .05.
Results
Effect on CYP2C9 Activity.
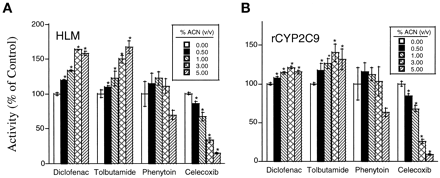
Acetonitrile showed different effects on the metabolism of four CYP2C9 substrates (Fig. 1). For instance, diclofenac 4′-hydroxylase and tolbutamide methyl hydroxylase activity were increased, whereas celecoxib methyl hydroxylase decreased with increasing acetonitrile concentration. However, phenytoin hydroxylase was resistant to the effect of the solvent. This effect was observed with both native human liver microsomes and insect cell microsomes containing recombinant CYP2C9, although activation was more pronounced with human liver microsomes than with the recombinant enzyme.
Effect of acetonitrile on product formation for four substrates after incubation with native human liver microsomal preparations (A) and recombinant CYP2C9 (B).
Diclofenac (20 μM), tolbutamide (1000 μM), phenytoin (100 μM), and celecoxib (15 μM) were incubated with human liver microsomes (0.1, 0.25, 0.25, and 0.1 mg protein/ml, respectively) or recombinant CYP2C9 (5, 25, 25, and 5 pmol rCYP2C9/ml, respectively) for 10, 30, 30, and 10 min, respectively. The respective control rates (acetonitrile = 0%) are 1540 ± 30, 243 ± 13, 9.13 ± 0.01, and 249 ± 4.1 pmol/min/mg protein after incubation with human liver microsomes, and 24.6 ± 0.33, 4.21 ± 0.04, 0.10 ± 0.01, and 12.6 ± 0.4 pmol/min/pmol rCYP after incubation with recombinant CYP2C9. Values represent a mean ± S.D. (triplicate measurements). *Significant difference from control (P < .05). ACN, acetonitrile.
The concentration of diclofenac and tolbutamide in the reaction mixture did not vary with the amount of acetonitrile (Table1), indicating that the stimulation of the hydroxylase activity for these two substrates is not attributable to increased dissolution of the substrates. However, the solvent appeared to facilitate the dissolution of phenytoin and celecoxib because lower substrate concentrations were measured in reaction mixtures without acetonitrile (Table 1). Obviously, the decreased activity of celecoxib hydroxylase is not associated with the change in substrate concentration due to the presence of the solvent.
Concentration of diclofenac, tolbutamide, phenytoin, and celecoxib in the reaction mixture in the presence of 0, 1, 3, and 5% acetonitrile
Effect on Enzyme Kinetics.
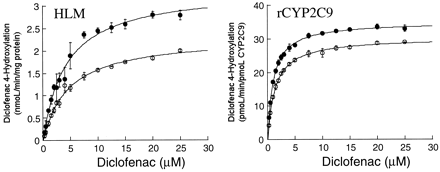
The effect of acetonitrile on CYP2C9-mediated catalysis was investigated further using the hydroxylation of diclofenac and methyl hydroxylation of celecoxib. As for the activation effect, the presence of acetonitrile (3%, v/v) gave rise to a lowerKm value and a higherVmax value for diclofenac hydroxylase in liver microsomes, resulting in a substantial increase (87%) in intrinsic clearance. The effect was less significant (52% increase inVmax/Km ratio) with recombinant CYP2C9, which also reflected a significant change in both Km andVmax values (Fig.2 and Table2). By comparison, the inhibitory effect of acetonitrile (1%, v/v) on celecoxib hydroxylase activity in the recombinant CYP2C9 was characterized by a change in bothKm and Vmaxvalues, suggesting mixed-type inhibition (Fig.3 and Table 2). However, the presence of 1% acetonitrile only decreased the Vmaxvalue of the reaction in human liver microsomes, which was consistent with noncompetitive inhibition (Fig. 3 and Table 2).
Rate of formation of 4′-hydroxy diclofenac by a pool of human liver microsomes (HLM) and rCYP2C9 in the presence (●) and absence (○) of acetonitrile (3%, v/v).
The data represent the mean ± S.D. from three independent determinations.
Kinetic parameters for the hydroxylation of diclofenac and celecoxib in human liver microsomes and recombinant CYP2C9 determined in the presence and absence of acetonitrile
Rate of formation of methyl hydroxy celecoxib by a pooled preparation of human liver microsomes (HLM) and rCYP2C9 in the presence (●) and absence (○) of acetonitrile (1%, v/v).
The data represent the mean ± S.D. from three independent determinations.
Effect on CYP Reaction Phenotyping of Celecoxib Hydroxylation.
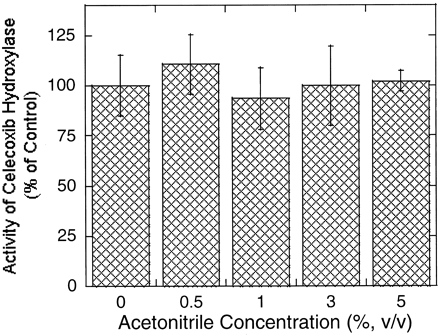
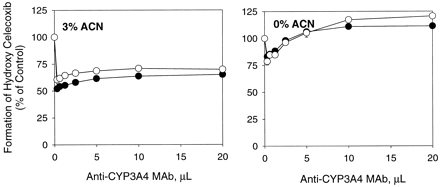
In addition to CYP2C9, CYP3A4 also plays a role in the hydroxylation of celecoxib in human liver microsomes (Tang et al., 2000). Unlike CYP2C9, CYP3A4 was resistant to the effect of acetonitrile as shown in Fig.4. No significant change was observed in the formation of hydroxy celecoxib over the acetonitrile concentration range examined (0.5–5%, v/v). The different susceptibility of these two isoforms to the effect of the solvent led to the overestimation of the contribution of CYP3A4 to the reaction (Fig.5). Namely, mouse ascites containing anti-CYP3A4 monoclonal antibody suppressed the rate of hydroxy celecoxib formation by approximately 40 to 50% and 15 to 25% in the presence and absence of acetonitrile (3%, v/v), whereas anti-CYP2C9 monoclonal antibody inhibited the reaction by 67 and 85% under the same conditions (Figs. 5 and 6). Interestingly, anti-CYP3A4 antibody showed maximum inhibitory potency against celecoxib hydroxylation at low concentrations and the inhibition became less significant with increasing antibody concentration, especially when acetonitrile was absent (Fig. 5B).
Effect of acetonitrile on the rate of methyl hydroxy celecoxib formation catalyzed by recombinant CYP3A4.
The incubation was carried out for 20 min in the presence of celecoxib (20 μM) and rCYP3A4 (20 pmol/ml). The results are expressed as mean ± S.D. from triplicate determinations. The control rate (acetonitrile = 0%) of hydroxy celecoxib formation is 0.65 ± 0.09 pmol/min/pmol rCYP.
Concentration-dependent inhibition of methyl hydroxy celecoxib formation in human liver microsomes by anti-CYP3A4 MAb in the presence and absence of acetonitrile (3%, v/v).
An individual human liver microsomal sample HG6 (●) and a pooled human liver microsomal sample HHM-0259 (○) were used. Each data point represents the mean ± S.D. of triplicate determinations (the S.D. bars for most data are less than the size of the symbol). ACN, acetonitrile.
Concentration-dependent inhibition of methyl hydroxy celecoxib formation in human liver microsomes by anti-CYP2C9 MAb in the presence and absence of acetonitrile (3%, v/v).
An individual human liver microsomal sample HG6 (●) and a pooled human liver microsomal sample HHM-0259 (○) were used. Each data point represents the mean ± S.D. of triplicate determinations (the S.D. bars for most data are less than the size of the symbol). ACN, acetonitrile.
Discussion
It is now known that different CYP isoforms are differentially affected by organic solvents. For instance, phenacetinO-deethylase (CYP1A2) and coumarin 7-hydroxylase (CYP2A6) are quite resistant to dimethyl sulfoxide, but chlorzoxazone 6-hydroxylase (CYP2E1) is very susceptible to the solvent (Chauret et al., 1998). For a given substrate, it has also been found that an individual CYP can have different susceptibility to different solvents (Draper et al., 1997; Chauret et al., 1998; Hickman et al., 1998). However, it is not known whether the effect of an organic solvent on a particular CYP is substrate-dependent. Diclofenac, tolbutamide, phenytoin, and celecoxib are structurally diverse CYP2C9 substrates. The first three compounds have been well established as the probes for CYP2C9 activity in human liver (Leemann et al., 1993; Veronese et al., 1993; Bort et al., 1999). Celecoxib is principally oxidized by CYP2C9 in human liver microsomal preparations, although CYP3A4 plays a minor role (Tang et al., 2000). This study showed that acetonitrile, a commonly used organic solvent for in vitro studies, exerted different effects on the metabolism of these four compounds. Namely, it activated the hydroxylation of diclofenac and tolbutamide, but inhibited the reaction of celecoxib, while eliciting a negligible effect on the metabolism of phenytoin. The same effect of acetonitrile on tolbutamide hydroxylase (human liver microsomes) and diclofenac hydroxylase (rCYP2C9) activity has been reported by Chauret et al. (1998), Hickman et al. (1998), Busby et al. (1999), and Palamanda et al. (2000).
The effect of acetonitrile can lead to an erroneous estimation of kinetic parameters. A lower Km value and a higher Vmax value were obtained for diclofenac 4′-hydroxylation in native liver microsomes, whereas the opposite was observed with celecoxib methyl hydroxylation (Table 2). This is an important observation, becauseKm and Vmaxvalues are the cornerstones of in vitro and in vivo correlations (Houston, 1994; Carlile et al., 1999). For a new chemical entity that behaves like diclofenac, the presence of acetonitrile would give rise to an overestimation of intrinsic clearance. Conversely, underestimation would occur if a compound were to behave like celecoxib. Cotreau-Bibbo et al. (1996) have also observed a solvent effect with acetone. Namely, acetone stimulated the production of α-hydroxyalprazolam in human liver microsomes, resulting in a significant increase in Vmax. Clearly, improper use of an organic solvent can significantly compromise the in vitro determination of intrinsic clearance.
Involvement of multiple CYP isoforms in the metabolism of a compound has been well documented (Nielsen et al., 1996; Yumibe et al., 1996;Crewe et al., 1997; Rochat et al., 1997). For instance, CYP2D6, CYP2C9, and CYP3A4 contribute to 4-hydroxylation of tamoxifen in human liver microsomes (Crewe et al., 1997). Formation of descarboethoxyloratadine from loratadine was mediated by both CYP3A4 and CYP2D6 (Yumibe et al., 1996). On the basis of the nominal specific content of individual CYP proteins in native human liver microsomes (Shimada et al., 1994; Imaoka et al., 1996), the relative contribution of an individual CYP to a given reaction can be quantitatively estimated using heterologously expressed CYP proteins (Rodrigues, 1999). This estimation can also be achieved by means of immunoinhibition (Gelboin et al., 1988; Mei et al., 1999). Evidently, the variable susceptibility of different CYPs to the effect of an organic solvent present in the incubation system could discount the accuracy of reaction phenotyping, as illustrated by the effect of acetonitrile on the metabolism of celecoxib in this study. We have shown that, in addition to CYP2C9, CYP3A4 also plays a role in celecoxib hydroxylation in human liver microsomes. Its contribution varies with individual liver microsomal preparations (Tang et al., 2000). By comparison to CYP2C9, the CYP3A4-catalyzed metabolism of celecoxib was refractory to the effect of acetonitrile. As a result, the presence of acetonitrile brought about an overestimation of the contribution of CYP3A4 in the reaction phenotyping of celecoxib. For a pooled microsomal preparation, the contribution of CYP3A4 accounted for ∼40% in the presence of 3% (v/v) acetonitrile versus less than 20% in the absence of the solvent. The presence of acetonitrile appeared to affect the interaction of anti-CYP3A4 monoclonal antibody with the enzyme. Interestingly, without acetonitrile, the monoclonal antibodies against human CYP3A4 suppressed hydroxy celecoxib formation by ∼20% at low concentrations, and its inhibitory effect diminished as the concentration of ascites increased. When the volume of ascites reached >5 μl, a small but significant increase in the rate of hydroxy celecoxib formation was observed. However, this stimulation was reduced in the presence of acetonitrile. It should be noted that anti-CYP3A4 ascites did not stimulate celecoxib hydroxylase activity catalyzed by rCYP2C9 (data not shown). Thus, it is unlikely that the antibody stimulated CYP2C9 in human liver microsomes. Therefore, the effect of anti-CYP3A4 ascites on human liver microsomal celecoxib hydroxylase activity cannot be explained at this time.
The substrate-dependent effect of acetonitrile observed in this study may be associated with different binding sites on the enzyme. Six substrate recognition sites (SRSs) have been identified for CYP2C9 and other CYP2C gene subfamily proteins (Gotoh, 1992). These SRSs span residues 96–117, 198–205, 233–240, 286–304, 359–369 and 470–477. A few critical residues, or even a single amino acid, within an SRS may define the ability of CYP2C9 to metabolize a particular substrate or group of related compounds (Miners and Birkett, 1998). Different CYP2C9 substrates may bind to different SRSs on the enzyme and show different responses to site-directed mutation at different SRS. For example, the Ile359Leu substitution in SRS5 of CYP2C9 markedly decreases the hydroxylation of tolbutamide and phenytoin (Veronese et al., 1993) but not that of diclofenac (Wong et al., 1996). Similarly, the Phe114Leu substitution (in SRS1) decreases CYP2C9-like warfarin hydroxylation and inhibition by sulfaphenazole without affecting diclofenac hydroxylation (Haining et al., 1996). Like any protein molecule, the native (aqueous-based) conformation of these SRSs is maintained by the interaction of several noncovalent forces, including hydrogen bonding, ionic, hydrophobic, and van der Waals interactions. Disruption of such forces, as a result of addition of organic solvents to an aqueous enzyme solution, may lead to conformational transition of these SRSs. This change may alter the binding affinity of substrates to their respective SRSs, affecting catalytic turnover, or switching substrate specificity. If the SRSs on CYP2C9 respond to the effect of acetonitrile (or other organic solvents) differently, it would not be surprising to expect substrate-dependent solvent effects.
To the best of our knowledge, this is the first report of a substrate-dependent solvent effect on CYP activity. The results of this study highlight the importance of careful selection of the type and amount of solvent to be used in an in vitro study. Moreover, evaluation of a particular CYP/solvent pair should be performed with more than one substrate.
Footnotes
-
Send reprint requests to: Cuyue Tang, Ph.D., Dept. of Drug Metabolism, Merck Research Laboratories, Sumneytown Pike, P.O. Box 4, WP75A-203, West Point, PA 19486-0004. E-mail:cuyue_tang{at}merck.com
- Abbreviations used are::
- CYP
- cytochrome P450
- rCYP
- recombinant cytochrome P450
- MAb
- monoclonal antibody
- SRSs
- substrate recognition sites
- pHPPH
- 5-(4′-hydroxyphenyl)-5-phenylhydantoin
- MPHT
- 5-(4′-methylpheny)-5-phenylhydantoin
- LC-MS
- liquid chromatography-mass spectrometry
- Received December 3, 1999.
- Accepted February 15, 2000.
- The American Society for Pharmacology and Experimental Therapeutics