Abstract
Canines are used extensively in the pharmaceutical industry for the preclinical screening of novel therapeutics, yet comparatively little is known about the phase 2 metabolism in this species. In humans, morphine is known to undergo extensive metabolism by glucuronidation, and the UDP-glucuronosyltransferase isoform, which catalyzes the formation of morphine-3-O-glucuronide and morphine-6-O-glucuronide is UGT2B7. This study was designed to investigate the glucuronidation of morphine using dog liver microsomes. Liver microsomes from beagle dogs catalyzed the glucuronidation of morphine-3(and 6)-O-glucuronide at rates 4 to 10 times that of rhesus monkey and human liver microsomes. The Km of morphine using beagle dog liver microsomes was ∼270 μM, which is similar to that found for expressed human UGT2B7. The Vmax for morphine, using dog liver microsomes, was 27 nmol/min/mg of protein. Flunitrazepam inhibited the glucuronidation of morphine in dog liver microsomes, and the Ki was 40 μM, which is similar to human UGT2B7 for other substrates. The effects of detergents were also investigated with dog liver microsomes, and Brij 35 and Brij 58 were found to be the best detergents to use for maximal activation of the dog liver morphine UGT. These studies suggest that dog has a UGT2B isoform similar to human UGT2B7.
The detoxication of xenobiotics and endobiotics by glucuronidation generally leads to more water soluble compounds. Conjugation with glucuronic acid is facilitated by a family of enzymes, the UDP-glucuronosyltransferases (UGTs).1 To date, over 35 different UGT isoforms have been cloned and expressed from rats, rabbits, monkeys, and humans (Mackenzie et al., 1997). UGTs have been divided into two distinct subfamilies, UGT1A and UGT2 (Mackenzie et al., 1997). The UGT1A subfamily in rats and humans is encoded from a gene where the unique first exons are spliced alternatively to common exons 2 through 5 (Ritter et al., 1992; Emi et al., 1995). This gives rise to many novel UGT isoforms that catalyze the glucuronidation of opioids, bilirubin, estrogens, and amines. The UGT2 family is encoded by individual genes, and this family catalyzes the glucuronidation of steroids, opioids, and carboxylic acids (Hague et al., 1991).
Dogs are used extensively in the pharmaceutical industry, but very little is known about the UGTs in this species. Sommerer et al. (1988)demonstrated that dog liver microsomes catalyzed a bilirubin monoglucuronide/monodiester at rates higher than the diglucuronide and monoglucuronide combined. Schmoldt et al. (1987) investigated the glucuronidation of digitoxin in dog liver microsomes and found that several compounds, including testosterone and pregnanediol, were noncompetitive inhibitors. Oguri et al. (1996) purified a phenobarbital-inducible UGT isoform from dog liver that catalyzed the glucuronidation of testosterone and morphine, but the 50-kDa protein only catalyzed the formation of morphine-3-O-glucuronide.
We have demonstrated for the first time, to our knowledge, that beagle male liver microsomes catalyzed the glucuronidation of morphine at both the 3-hydroxy and the 6-hydroxy positions, and that the enzyme was inhibited by flunitrazepam (FNZ).
Materials and Methods
Dog Liver and Microsomes.
Liver microsomal preparations were prepared by standard differential centrifugation methods from female monkey and male dog liver (beagles). All microsomal samples were stored at −80°C until use. Female human liver was purchased from the International Institute for the Advancement of Medicine (Exton, PA).
Chemicals.
UDP-[U-14C]glucuronic acid (255 mCi/mmol) was purchased from ICN Pharmaceuticals (Irvine, CA). Morphine sulfate, M3G, M6G, FNZ, and UDPGA were purchased from Sigma Chemical Co. (St. Louis, MO). Protein assay reagents were obtained from Bio-Rad (Hercules, CA). The detergents NMG, OTG, OBG, CHAPS, CHAPSO, Brij 35 and Brij 58, and Triton X-114 were purchased from Pierce (Rockford, IL). All other reagents were of analytical grade.
Microsomal Assays.
The glucuronidation of morphine was determined using the method described by Puig and Tephly (1986). Briefly, the enzyme assay mixture contained 20 μg of hepatic microsomal protein with 0.5 mg of CHAPS/mg of protein, 50 mM Tris-HCl (pH 8.4), 10 mM MgCl2, 2 mM UDPGA (0.25 μCi/100 μl), and 5 mM morphine in a total volume of 100 μl. Samples were incubated at 37°C for either 10 min for kinetics and to obtain the rate of formation of glucuronides or for 2 h to obtain, isolate, and identify M6G. Reactions were terminated with 5 ml of ice-cold 1 M ammonium acetate, pH 9.2.
The kinetic analysis of morphine was determined using optimal assay conditions for pH, protein concentration, and reaction times yielding linear product formation. Kinetic parameters were calculated using the program entitled Enzyme Kinetics (ChemSW, Fairfield, CA).
HPLC Analysis.
The HPLC system consisted of a Shimadzu SIL-10AD equipped with dual LC-10AD pumps and a Shimadzu diode array SPD-M10A detector. The morphine glucuronides were separated on a Zorbax RX-C8 column (4.6 mm × 25 cm; Hewlett-Packard) and detected at 220 nm. The mobile phase, a modification of that reported by Stone et al. (1998), consisted of acetonitrile/20 mM phosphate buffer, pH 2.1 (7:93 v/v) with a flow rate of 0.5 ml/min. Separations were obtained under isocratic conditions at ambient temperature. Retention times for M3G and M6G were 7.7 and 9.9 min, respectively, and were verified with those of the authentic standards. The individual peaks were collected and counted for radioactive content using a Beckman scintillation counter with scintillation mixture (Budget Solve).
Results and Discussion
Dog liver microsomes catalyzed the glucuronidation of morphine at very high rates (Table 1), in accordance with other studies that show that dog liver microsomes have extremely high rates of glucuronidation (Schmoldt et al., 1987; Sommerer et al., 1988; Oguri et al., 1996). In this study, the glucuronidation rates varied in the liver microsomes obtained from three male beagle dogs (designated 99 M, 116 M, and 123 M). However, these rates exceeded those from monkey or human liver microsomes by 4- to 10-fold (Table 1). These data suggest that the amount of constitutive UGT proteins was greater in the dog or that the efficiency of the UGT enzymes responsible for the glucuronidation of morphine was extremely high.
Glucuronidation of morphine by dog, monkey, and human liver microsomes
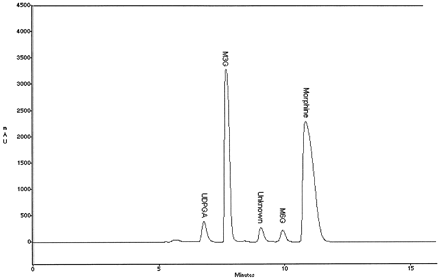
HPLC analysis was used to investigate whether dog liver microsomes catalyzed both M3G and M6G formation (Fig.1). It is known that the ratio of M3G to M6G in human liver microsomes is 8:1 and that the responsible isoform is UGT2B7 (Coffman et al., 1997). In this study, liver microsomes from male beagles catalyzed the formation of M3G as well as M6G, and the ratio was approximately 50:1. A kinetic analysis of morphine glucuronidation was performed, and the Kmwas 267 μM for total morphine glucuronides (i.e., M3G and M6G) and the Vmax was 27 nmol/min/mg (dog 116 M). The Km of morphine using recombinant human UGT2B7 and recombinant cynomolgus monkey UGT2B18 has been reported to be 450 and 330 μM, respectively (Green et al., 1997; Coffman et al., 1998).
HPLC chromatogram of morphine glucuronides formed by dog liver microsomes.
Assay and HPLC procedures are described in Materials and Methods. The retention times of M3G and M6G were 7.7 and 9.9 min, respectively. Because [14C]UDPGA was used, M3G and M6G peaks were radioactive, whereas the peaks labeled “morphine” and “unknown” had no radioactivity associated with them.
FNZ is a known in vitro inhibitor of morphine glucuronidation in rats (Thomassin and Tephly, 1990). In this study, morphine glucuronidation was inhibited in dog liver microsomes by approximately 40% using 50 μM FNZ. In human liver microsomes, the glucuronidation of morphine was inhibited by approximately 30% (Table2). The Ki of FNZ in dog liver microsomes was 40 μM, which is similar to that reported by Cheng et al. (1998) for UGT2B7 with several estrogen compounds.
Inhibition of morphine glucuronidation by FNZ in dog and human liver microsomes
UGTs are located in the endoplasmic reticulum of cells and are subject to latency (Clarke and Burchell, 1994). Detergents are commonly used to activate (0.1 mg of detergent/mg of protein) or solubilize (0.5 mg of detergent/mg of protein) UGT proteins, and Green and Tephly (1996) have demonstrated that detergents have an inhibitory effect on stably expressed UG1A4. Several detergents were investigated on the rate of morphine glucuronide formation using dog liver microsomes (Fig.2). Brij 35 and Brij 58 (used at 0.1 and 0.5 mg of detergent/mg of protein) were the best detergents for the maximal activation of morphine glucuronidation in dog liver microsomes (115 nmol/min/mg of protein) compared with native microsomes (12 nmol/min/mg of protein). Triton X-114 and Luberol were poorer activators of the morphine UGT.
Effects of several detergents on the glucuronidation of morphine using dog liver microsomes.
Assays are described in Materials and Methods. Native microsomes catalyzed morphine glucuronidation at a rate of 12 nmol/min/mg of protein. Values are averages from duplicate incubations. ▪, 0.5 mg of detergent/mg of protein; ■, 0.1 mg of detergent/mg of protein.
In summary, dog liver microsomes catalyzed the glucuronidation of morphine at extremely high rates and catalyzed the formation of both M3G and M6G, with a ratio of approximately 50:1, respectively. This is the first time, to our knowledge, that male beagle liver microsomes have been shown to catalyze the formation of M6G, which is 50 times more potent as an analgesic than morphine itself. FNZ inhibited morphine glucuronidation in dog liver microsomes, similar to its effect on UGT2B7. UGT2B7 is one of the most important UGT enzymes, because it metabolizes many different classes of compounds, including opioids, carboxylic acids, estrogens, and androgens (Coffman et al., 1997; Cheng et al., 1998). These data suggest that a UGT isoenzyme is present in the male dog liver, and its expression is similar in catalytic properties to monkey UGT2B18 and human UGT2B7.
Footnotes
-
Send reprint requests to: Christopher King, Ph.D., Merck & Co., P.O. Box 2000, RY80-A9 Rahway, NJ 07065. E-mail:christopher_king{at}merck.com
- Abbreviations used are::
- UGT
- UDP-glucuronosyltransferase
- NMG
- nonanoyl-N-methylglucamide
- OTG
- octyl symbol 98 β-thioglucopyranoside
- OBG
- octyl symbol 98 β-glucoside
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- CHAPSO
- 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonate
- UDPGA
- UDP-glucuronic acid
- M3G
- morphine-3-O-glucuronide
- M6G
- morphine-6-O-glucuronide
- FNZ
- flunitrazepam
- Received December 20, 1999.
- Accepted February 28, 2000.
- The American Society for Pharmacology and Experimental Therapeutics