Abstract
The objective of this study was to evaluate the utility of brain tissue slices to determine the effect of plasma and brain tissue nonspecific binding on the brain-to-plasma ratio (Kp). Mouse or rat brain slices (400 μm) were prepared using a McIlwain tissue chopper (Surrey, UK) and incubated with 1 μg/ml of compound at 37°C either in a physiological buffer to determine the buffer-to-slice concentration ratio, i.e., unbound fraction in brain tissue (fu,slice), or in plasma to determine the slice-to-plasma concentration ratio (Cslice/Cplasma). The unbound fraction in plasma, fu,plasma, was determined using equilibrium dialysis. In vitro-in vivo correlation of the brain-to-plasma ratio was examined for 13 and eight model compounds in mice and rats, respectively. Cslice/Cplasma and fu,plasma/fu,slice predicted the Kp in rats, and Cslice/Cplasma predicted the Kp in FVB mice for non-P-glycoprotein substrates within 3-fold but overpredicted Kp for P-glycoprotein substrates by more than 3-fold. However, Cslice/Cplasma predicted the Kp in mdr1a/1b knockout mice for both non-P-glycoprotein and P-glycoprotein substrates. Our present study demonstrates that a brain slice method can be used to differentiate whether a compound having a low Kp is due to the effect of low nonspecific binding to brain tissue relative to plasma proteins or because of efflux transport at the blood-brain barrier.
Brain is separated from the systemic circulation by two barriers: a blood-brain barrier (BBB) and a blood-cerebrospinal-fluid barrier (BCSFB). The BBB and BCSFB represent physical and enzymatic barriers that restrict and regulate the penetration of compounds into and out of the brain and maintain the homeostasis of the brain microenvironment (Davson and Segal, 1995). Brain penetration is essential for compounds where the site of action is within the central nervous system (CNS). For targets outside the CNS, BBB penetration may need to be minimized to reduce CNS-related side effects (Chen et al., 2003).
The brain-to-plasma concentration ratio (Kp) is the most commonly used parameter for measuring brain penetration in a drug discovery setting. A large Kp is considered a favorable property of a good CNS compound. This approach implies that a compound having a high Kp penetrates into brain tissue better than one having a low Kp. However, according to the definition of Kp (Kp = Cbrain/Cplasma) and unbound fraction in plasma (fu,plasma = Cu,plasma/Cplasma) and brain (fu,brain = Cu,brain/Cbrain), the following equation can be obtained: 
Kp,in, defined as the ratio of fu,plasma over fu,brain, can be considered the “intrinsic” partition coefficient between brain and plasma. It is determined by nonspecific binding in brain and plasma and is not related to BBB properties. Kp,free, defined as the ratio of the free brain concentration (Cu,brain) over the free plasma concentration (Cu,plasma), delineates BBB properties and governs the relationship between free brain and plasma concentrations. Kp,free represents a better parameter than Kp to assess brain penetration or brain bioavailability for CNS compounds (Liu and Chen, 2005). However, it is difficult to determine Kp,free experimentally. As a surrogate approach in drug discovery, Kp is often used to select compounds with good brain penetration. However, using Kp introduces the caveat that a high Kp can be due to a high Kp,in or Kp,free and a low Kp can be due to a low Kp,in or Kp,free. Therefore, when Kp is used to select CNS compounds, it is critical to ensure that a low Kp is due to a low Kp,free but not because of a low Kp,in. To overcome the limitation of Kp, we propose determining Kp,in and then indirectly evaluating Kp,free using eq. 1.
Kp,in may be calculated from fu,plasma and fu,brain, which can be determined using in vitro approaches such as equilibrium dialysis, ultrafiltration, and ultracentrifugation from plasma and brain tissue homogenate (Fichtl et al., 1991; Kalvass and Maurer, 2002; Maurer et al., 2005). Recently, Maurer et al. (2005) demonstrated that nonspecific binding in brain homogenate and plasma can be used to predict in vivo Kp for 23 of 33 tested compounds within a 3-fold range. The concern of estimating fu,brain from brain homogenate is that brain homogenization may change brain binding properties by unmasking binding sites that are not normally accessible to a drug in vivo. In addition, the unbound fraction in brain tissue may not be directly extrapolated from the unbound fraction determined from diluted brain tissue homogenate.
Brain slices have been used to study neural physiology for almost half a century (Collingridge, 1994). Cellular structure is maintained in slices, but the blood-brain barrier is not functional because a compound can directly penetrate into the brain slices from the incubation medium (Newman et al., 1988). The use of brain slices to estimate the fu,brain has been reported in the literature (Van Peer et al., 1981). In that study, 1000-μm brain slices were incubated for 1 h, and the buffer-to-slice ratio was assumed to be equal to the in vivo fu,brain. Brain slices have also been used to study the partition coefficient between slice and incubation buffer (Ooie et al., 1997; Gredell et al., 2004). Based on the same concept, Kakee et al. (1996) used brain slices to estimate the apparent distribution volume in brain. Our main concern was that diffusion into brain tissue may be time-dependent and whether 1 h was sufficient time for the 1000-μm brain slices to reach equilibrium. No extensive validation study was reported for the brain slice method. In the present study, the experimental conditions for a brain slice uptake study and the utility of brain slices to determine Kp,in are evaluated.
Materials and Methods
Chemicals. Supplies of midazolam, 9-hydroxyrisperidone, and metoclopramide were obtained from Pfizer Global Material Management (Groton, CT). Caffeine, fluoxetine, propranolol, theobromine, sulpiride, thiopental, quinidine, zolpidem, and theophylline were obtained from Sigma-Aldrich (St. Louis, MO). Propoxyphene was obtained from US Pharmacopeia (Rockville, MD). N[3-(4′-Fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS) and methoxy-3-[(2-phenyl-piperadinyl-3-amino)-methyl]-phenyl-N-methylmethane-sulfonamide (CP-141938) were synthesized at Pfizer Global Research and Development Laboratories with a purity greater than 98%. All other chemicals used in the experiments were of the highest available grade.
Animal Experiments. Male FVB (wild type) and mdr1a/1b (–/–,–/–) mice (20–30 g) were obtained from Taconic (Germantown, NY). All animals were housed in a controlled temperature and humidity environment with an alternating 12-h light and dark cycle with free access to food and water.
Mice received a 10-mg/kg subcutaneous dose of NFPS, propranolol, theobromine, or theophylline. The doses were prepared in 0.9% saline and delivered in a volume of 2 ml/kg. Animals were sacrificed in a CO2 chamber. Blood samples were collected in heparin-treated tubes at designated times between 10 min and 24 h via cardiac puncture. After centrifugation (3000 rpm, 10 min) of the blood, plasma was isolated. Brain tissue was harvested and rinsed with saline immediately after collection. The plasma and brain samples were stored at –20°C before analysis.
Protein Binding. The unbound fraction in plasma and brain homogenate was determined using a 96-well equilibrium dialysis method reported previously (Kalvass and Maurer, 2002). Briefly, Sprague-Dawley rat plasma and brain tissues were obtained on the day of the study. Brain tissue was homogenized in 2 volumes (w/v) of 100 mM sodium phosphate buffer. Plasma, brain homogenate, and phosphate buffer (for equilibrium controls) were adjusted to pH 7.4 and then spiked with compound (500 ng/ml), and 150 μl of the spiked matrix was added to individual wells of the dialysis apparatus. The receiver side contained 150 μl of phosphate buffer. The 96-well equilibrium dialysis apparatus was maintained on a rotator in a Thermo Forma Stericult incubator (Marietta, OH) at 37°C for 5 h. Ten microliters of either plasma or brain homogenate and 50 μl of buffer were taken from the apparatus and added to silanized glass vials in a 96-well block containing 100 μl of acetonitrile fortified with internal standard. The samples were then vortexed, centrifuged, and stored at –20°C before analysis. The unbound fractions determined from diluted brain tissue homogenates were corrected to yield an estimate of unbound fraction in the intact brain tissue using a previously published method (Kalvass and Maurer, 2002).
Brain Slices. After rats and mice were sacrificed in a CO2 chamber, whole brain was obtained and immediately stored in ice-cold physiological buffer (122 mM NaCl, 25 mM NaHCO3, 10 mM glucose, 3 mM KCl, 1.4 mM CaCl2, and 1.2 mM MgSO4, pH 7.4; Ooie et al., 1997). Cerebral cortex was dissected from whole brain, and the hemispheres were separated. Coronal brain slices of the cortex (400 μm) were prepared using a McIlwain Tissue Chopper (Surrey, UK). The slices were immediately transferred to ice-cold buffer using a paintbrush to prevent injury to the slices. One rat brain slice or two mouse brain slices were added to a silanized 25-ml scintillation vial containing either buffer or plasma spiked with 1 μg/ml of a test compound. The incubation was conducted on a rotator inside a Stericult incubator with 95%/5% O2/CO2 and relative humidity of 75% at 37°C. At designated time points, 100 μl of incubation buffer or plasma was transferred to an Eppendorf tube. The buffer Eppendorf contained 100 μl of acetonitrile to wash the pipette tip for nonspecifically bound drug. The brain slices were removed and rinsed in a silanized vial with the buffer. Rat or mouse slices were then transferred to a test tube containing 975 or 488 μl of phosphate buffer. Samples were stored at –20°C until analysis.
Sample Analyses. Whole brain tissues were homogenized in 4 volumes (w/v) of water. Twenty microliters of plasma or brain homogenate, 20 μl of dimethyl sulfoxide, and 200 μl of acetonitrile containing an internal standard were mixed in silanized 96-well glass tubes. The samples were then centrifuged at 3000 rpm for 10 min, and the supernatant was used for HPLC/MS/MS analysis.
For the protein binding samples, a mixed matrix method was used. Plasma and brain donor samples (containing 10 μl of plasma or brain homogenate and 100 μl of acetonitrile in silanized 96-well glass vials) were mixed with 50 μl of buffer and 50 μl of 50% methanol/water and vortexed. Likewise, 150 μl of receiver sample (containing 50 μl of receiver buffer and 100 μl of acetonitrile in silanized 96-well glass) were mixed with 10 μl of plasma or brain homogenate and 50 μl of 50% methanol/water and vortexed. Standards were made in a like manner. One-hundred and sixty microliters of mixed matrix (100 μl of acetonitrile, 50 μl of buffer, and 10 μl of plasma or brain homogenate) and 50 μl of standard stock solutions were mixed and vortexed. Twenty microliters of internal standard in acetonitrile was then added to standards and samples and vortexed. The 96-well plate was then centrifuged at 3000 rpm for 10 min and the supernatant was analyzed by HPLC/MS/MS.
Brain slices were homogenized in phosphate buffer at an approximately 40-fold dilution where the estimated brain slice weights were 25 and 12.5 mg for rat and mouse, respectively. All incubation buffer, plasma, and brain homogenate samples were diluted to the linear range of the standard curves and then precipitated with acetonitrile containing an internal standard, followed by centrifugation at 3000 rpm for 10 min. The supernatant was then analyzed by HPLC/MS/MS for all except caffeine, theobromine, and theophylline. The supernatant of these three compounds was dried down with N2 at room temperature. The residual was reconstituted with 30 to 50 μl of acetonitrile, vortexed, and underwent HPLC/MS/MS analysis.
The HPLC/MS/MS system consisted of either a Shimadzu ternary pump (Shimadzu LC-10A; Kyoto, Japan) or an Agilent quaternary pump HPLC system (Hewlett Packard, Palo Alto, CA), an autosampler, and a PE Sciex API 3000 or 4000 (Perkin-Elmer Sciex Instruments, Foster City, CA) mass spectrometer with a turbo ion spray interface (PE-Sciex, Thornhill, ON, Canada). Sample injection volume was 10 μl. HPLC/MS/MS conditions for the 13 compounds can be found in Table 1. For all of the assays, the concentration of samples was within the linear range of quantitation. The relative accuracy was between 80 and 120%.
HPLC/MS/MS conditions for all 13 compounds
Protein normalization was performed to correct for dilution of brain homogenate. The Pierce BCA protein assay (Rockford, IL) was conducted on all of the brain homogenate samples. The weights of brain slices were calculated by comparing the protein concentration of the brain homogenate to a control brain homogenate containing a known weight of brain (Newman et al., 1988).
Results
Optimization of Brain Slice Uptake Conditions. The effect of brain slice thickness on Cslice/Cplasma was evaluated in the present study. Using fluoxetine as a model substrate, the Cslice/Cplasma for slices with a thickness of 200, 300, 400, and 500 μm was similar after incubation for 6 h. Because some of the 200-μm and 300-μm slices appeared slightly damaged at the end of 6-h incubation, 400-μm slices were used in the present study.
The time to reach equilibrium between the slices and incubation buffer and the slices and plasma was determined for eight model compounds: caffeine, CP-141938, fluoxetine, NFPS, propranolol, quinidine, theobromine, and theophylline. fu,slice decreased over time before reaching equilibrium between 1 and 6 h. As representative examples, Fig. 1, A and C, shows the time course of fu,slice for caffeine and propranolol, respectively. Likewise, Cslice/Cplasma increased over time and reached equilibrium between 1 and 6 h. Figure 1, B and D, shows the time course of Cslice/Cplasma for caffeine and propranolol, respectively. For those compounds not extensively binding to brain tissue, such as caffeine, the equilibrium of brain slices and incubation medium can be achieved at 1 h after incubation. However, for those compounds extensively binding to brain tissue, such as propranolol, 2 to 6 h were needed to achieve equilibrium.
The effect of fresh and previously frozen plasma on Cslice/Cplasma was examined using caffeine and fluoxetine as model compounds. The Cslice/Cplasma of caffeine was 0.364 ± 0.05 and 0.382 ± 0.03 in fresh plasma and previously frozen rat plasma, respectively. The Cslice/Cplasma of fluoxetine was 20.1 ± 2.9 and 20.6 ± 6.9 for fresh plasma and previously frozen plasma, respectively. No statistical difference was observed for the Cslice/Cplasma values of caffeine (p = 0.65) or fluoxetine (p = 0.91) between fresh and previously frozen plasma, indicating previously frozen plasma can be used for the brain slice uptake study.
Time course of unbound brain slice fraction (A) or brain slice-to-plasma ratio (B) for caffeine is shown. Time course of unbound brain slice fraction (C) or brain slice-to-plasma ratio (D) for propranolol.
Correlation of Predicted Kp,in from Brain Slices and in Vivo Observed Kp. Eight compounds, caffeine, CP-141938, fluoxetine, NFPS, propranolol, quinidine, theobromine, and theophylline were selected to evaluate the utilities of a brain slice method. The selection of these model compounds was based on their physicochemical properties, BBB permeability, in vivo Kp values, and P-gp transporter activity. All the rat-related and mouse-related parameters are presented in Tables 2 and 3, respectively. All in vivo rat Kp values calculated from the area under the curve ratio of brain concentration and plasma concentration were obtained from previous studies (Liu et al., 2005). Kp data for quinidine were from the literature (Kusuhara et al., 1997). In vivo mouse Kp values for NFPS, propranolol, theobromine, and theophylline were determined in the present study. All other mouse Kp values were obtained from the literature (Smith et al., 2001; Doran et al., 2005). All in vivo mouse Kp values were calculated from the area under the curve ratio of brain concentration and plasma concentration.
The parameters of eight model compounds in rats
The parameters of thirteen model compounds in mice
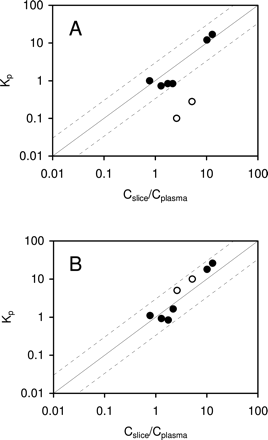
Direct and indirect brain slice methods were evaluated. The in vitro and in vivo values were considered to be consistent if the values were within 3-fold. This criterion was chosen to allow for differences as a result of experimental error and for actual differences that would be considered to be of little pharmacologic consequence (Maurer et al., 2005). The direct brain slice method uses Cslice/Cplasma to estimate Kp,in. The relationship between rat Kp and Cslice/Cplasma for the eight model compounds are exhibited in Fig. 2A. Six of the eight model compounds, caffeine, fluoxetine, NFPS, propranolol, theobromine, and theophylline, were non-P-gp substrates. The Cslice/Cplasma values for the six non-P-gp substrates were within 3-fold of the observed in vivo Kp. CP-141938 and quinidine were P-gp substrates. The Cslice/Cplasma values of CP-141938 and quinidine were 9- and 5-fold greater than their in vivo Kp, respectively. The relationship between Kp and Cslice/Cplasma in FVB mice and mdr1a/1b gene knockout mice for the eight model compounds are presented in Fig. 3, A and B, respectively. For the six non-P-gp substrates, the Cslice/Cplasma values were within 3-fold of the observed in vivo Kp in FVB and mdr1a/1b knockout mice. The Cslice/Cplasma for the two P-gp substrates, quinidine and CP-141938, were 17- and 26-fold greater than the observed Kp in FVB mice, respectively, but were within 3-fold of the observed Kp in mdr1a/1b knockout mice. These results demonstrate that in rats and wild-type mice, Cslice/Cplasma was consistent with the in vivo Kp for non-P-gp substrates but overpredicted the in vivo Kp for P-gp substrates. In P-gp knockout mice, however, Cslice/Cplasma was consistent with Kp for both non-P-gp and P-gp substrates.
Relationship between in vivo Kp and Cslice/Cplasma (A), fu,plasma/fu,slice ratio (B), and fu,plasma/fu,homogenate ratio (C) in rats. Solid and open symbols represent non-P-gp substrates (caffeine, fluoxetine, NFPS, propranolol, theobromine, and theophylline) and P-gp substrates (CP-141938 and quinidine), respectively. Solid and dashed lines represent unity and 3-fold boundaries, respectively.
The indirect slice method uses fu,plasma/fu,slice to predict Kp,in. The relationship between rat Kp and fu,plasma/fu,slice for the eight model compounds is shown in Fig. 2B. The fu,plasma/fu,slice values for five of the six non-P-gp substrates were within 3-fold of the observed Kp. Only NFPS showed 4-fold of difference between fu,plasma/fu,slice and Kp. The fu,plasma/fu,slice for the two P-gp substrates CP-141938 and quinidine were 16- and 12-fold, respectively, greater than the Kp. Therefore, both the direct and indirect brain slice methods were able to predict in vivo Kp for non-P-gp substrates but overpredicted P-gp substrates in P-gp-competent animals.
Comparison of Brain Slices Method and Brain Homogenate Method. To compare the brain slice and brain homogenate methods, we used the brain homogenate method to examine the eight model compounds in rats. The relationship between Kp and unbound fraction in plasma over the unbound fraction determined from brain homogenate ratio (fu,plasma/fu,homogenate) for the eight model compounds is shown in Fig. 2C. The fu,plasma/fu,homogenate for four of the six non-P-gp substrates (caffeine, fluoxetine, theobromine, and theophylline) were within 3-fold of in vivo Kp. However, the fu,plasma/fu,homogenate for two non-P-gp substrates, NFPS and propranolol, overpredicted 27-fold and underpredicted 4-fold the Kp, respectively. The fu,plasma/fu,homogenate for the two P-gp substrates CP-141938 and quinidine were 8- and 9-fold, respectively, greater than the Kp.
Relationship between in vivo Kp and brain slice-to-plasma ratio in FVB mice (A) and mdr1a/1b knockout mice (B), respectively. Solid and open symbols represent non-P-gp substrates (caffeine, fluoxetine, NFPS, propranolol, theobromine, and theophylline) and P-gp substrates (CP-141938 and quinidine), respectively. Solid and dashed lines represent unity and 3-fold boundaries, respectively.
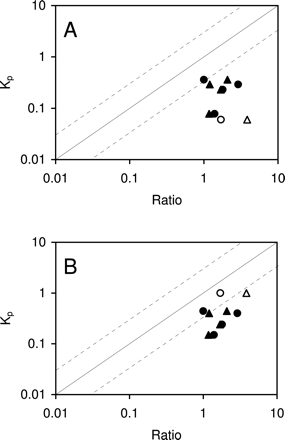
Furthermore, we selected additional five compounds, midazolam, 9-hydroxyrisperidone, sulpiride, thiopental, and zolpidem, for which the brain homogenate method seemed unable to predict Kp (Maurer et al., 2005), to examine whether the brain slice method was able to better predict Kp,in. The correlation between in vivo Kp and Cslice/Cplasma and the correlation between in vivo Kp and the fu,plasma/fu,homogenate in FVB and mdr1a/1b knockout mice is presented in Fig. 4, A and B, respectively. Midazolam, sulpiride, thiopental, and zolpidem were not P-gp substrates, whereas 9-hydroxyrisperidone was a P-gp substrate. For these five compounds, fu,plasma/fu,homogenate overpredicted the in vivo Kp in FVB and mdr1a/1b knockout mice greater than 3-fold. However, Cslice/Cplasma was able to predict within 3-fold of the observed in vivo Kp for thiopental in FVB and mdr1a/1b mice and for 9-hydroxyrisperidone in mdr1a/1b mice. For midazolam, zolpidem, and sulpiride, both methods were unable to predict in vivo Kp within 3-fold. These results indicate the brain slice method is comparable with or slightly better than the brain homogenate method in predicting Kp.
Discussion
The objective of the present study was to assess the utility of brain slices to determine Kp,in, a parameter dependent on nonspecific binding in brain tissue and plasma proteins. The main conclusions of this work are: 1) for 400-μm brain slices, the equilibrium can be achieved in 6 h; 2) Kp,in can be estimated from Cslice/Cplasma (direct brain slice method) or fu,plasma/fu,slice (indirect brain slice method) within 3-fold of error; and 3) similar accuracy was observed between brain slice and brain homogenate methods.
Relationship between Kp and Cslice/Cplasma ratio (circles) and between Kp and fu,plasma/fu,homogenate (triangles) for 9-hydroxyrisperidone, midazolam, sulpiride, thiopental, and zolpidem in FVB mice (A) and mdr1a/1b knockout mice (B), respectively, is shown. Solid and open symbols represent non-Pgp substrates and Pgp substrates, respectively. Solid and dashed lines represent unity and 3-fold boundaries, respectively.
Our study indicates that the optimal conditions for brain slice thickness and incubation time in the tissue binding study are 400 μm and 6 h, respectively. Brain slices have been used to assess brain tissue binding in the literature (Van Peer et al., 1981), where 1000-μm brain slices were incubated for 1 h. The rate of diffusion and time to achieve equilibrium was a concern for that study. To address these issues, the effect of brain slice thickness and the duration of incubation were assessed.
Some of the brain slices with thickness less than 400 μm appeared to be damaged during the incubation process; therefore, 400-μm brain slices were selected for all of the studies. For those compounds not extensively binding to brain tissue, such as caffeine, the equilibrium of brain slices and the incubation medium was achieved at 1 h after incubation. However, for those compounds extensively binding to brain tissue, such as propranolol, 2 to 6 h were needed to achieve equilibrium. These observations are consistent with the literature. In a study of brain penetration for quinolone antimicrobial agents, the time to reach equilibrium for 300-μm brain slices was less than 1 h (Ooie et al., 1997). Similar equilibrium time was observed for 2-deoxyglucose in 540-μm brain slices (Newman et al., 1988). In addition, for 300-μm brain slices, the equilibrium was achieved at 2 to 6 h after incubation for propofol (Gredell et al., 2004). To guarantee that equilibrium was achieved, all of our incubations were conducted for 6 h. Our study also demonstrated that similar fu,slice and Cslice/Cplasma are observed in fresh prepared plasma and previously frozen plasma. Therefore, previously frozen plasma was also used in this study.
The present study indicates that brain slices can be used to determine the intrinsic brain-plasma partition Kp,in. Direct and indirect brain slice methods have been examined to estimate Kp,in. The direct brain slice method measures Kp,in using the slice-plasma concentration ratio (Cslice/Cplasma), which was obtained by incubation of brain slices in plasma. To our knowledge, this method represents a novel in vitro approach to estimate Kp,in. This method was validated by the observation that in rats and wild-type mice Cslice/Cplasma was consistent with the in vivo Kp for non-P-gp substrates but overpredicted the in vivo Kp for P-gp substrates. In P-gp knockout mice, Cslice/Cplasma was consistent with both non-P-gp and P-gp substrates. These results are expected from eq. 1. In wild-type animals, Kp,free is equal to unity for non-P-gp substrates, and Kp,free is less than unity for P-gp substrates, resulting in a Kp,in equal to Kp for non-P-gp substrates and a Kp,in greater than Kp for P-gp substrates, assuming no other transporter is playing a significant role in brain penetration (Liu and Chen, 2005). In P-gp knockout animals, Kp,free is equal to unity for both non-P-gp and P-gp substrates, resulting in a Kp,in equal to Kp for both non-P-gp and P-gp substrates.
The indirect brain slice method calculates Kp,in from the ratio of fu,plasma/fu,slice, where fu,plasma and fu,slice are determined from equilibrium dialysis and the brain slice method, respectively. For non-P-gp substrates, fu,plasma/fu,slice was consistent with the in vivo observed Kp, but for P-gp substrates, fu,plasma/fu,slice was greater than the in vivo observed Kp in rats. The direct and indirect brain slice methods seem to do equally well in estimating Kp,in, but the direct method does not require the determination of fu,plasma.
Our study indicates there is an agreement between the brain slice and brain homogenate methods for Kp,in prediction. The brain homogenate method has been reported in the literature to predict in vivo Kp (Fichtl et al., 1991; Kalvass and Maurer, 2002; Maurer et al., 2005). The homogenization process is of concern because it may alter binding properties by unmasking binding sites that are not accessible to a drug in intact brain tissue. Therefore, the reliability of calculating the unbound fraction in the intact brain tissue from the unbound fraction determined from a diluted brain tissue homogenate remains uncertain.
In addition, many lipophilic compounds in current CNS drug discovery programs often have low plasma unbound fractions and brain unbound fractions. Nonspecific adsorption to the dialysis apparatus and poor recovery frequently hinders the accurate measurement of the unbound fraction (Ward and Azzarano, 2004). The indirect brain slice method offers an alternative and possibly more accurate approach to assess the unbound fraction in brain from intact brain tissue, as fu,slice. A brain slice maintains brain cellular structure but has no barrier between the incubation media and the tissues. Early studies demonstrated that BBB-nonpermeable compounds, such as inulin, can penetrate into interstitial space in brain slice (Newman et al., 1988). Consistent with this assertion, we have observed that there was no significant difference for brain uptake of P-gp substrates CP-141938 and quinidine in P-gp knockout and wild-type mice brain slices, although 50- and 36-fold differences were observed in their Kp values in P-gp knockout and wild-type mice. A brain slice incubation can be conducted in silanized glass vials, and no dialysis membrane is involved, which further reduces the nonspecific adsorption for lipophilic compounds. The direct brain slice method further reduces the interference of adsorption and improves accuracy of the prediction by omitting the need to determine fu,plasma.
In rats, there was a good correlation between the in vivo Kp and predicted Kp using brain slice and brain homogenate methods for all model compounds except NFPS, whose fu,plasma/fu,homogenate was 27-fold lower than the observed Kp, whereas the direct and indirect brain slice method were within 3- and 4-fold of error, respectively. This discrepancy was due to the brain slice unbound fraction being approximately 7-fold greater than the value measured using brain homogenate. Whether the lower brain unbound fraction measured using the brain homogenate method was caused by greater access to binding sites normally inaccessible in intact brain tissue or nonspecific binding to the dialysis apparatus remains to be determined.
In mice, the prediction using the direct brain slice method is consistent with that using the brain homogenate method. Both methods overpredicted the in vivo Kp for midazolam, sulpiride, and zolpidem, indicating that a mechanism other than P-gp-mediated efflux transport may cause the lower brain penetration for this group of compounds. For thiopental and 9-hydroxyrisperidone, the brain slice method seems to be in better agreement with Kp. These results indicate the brain slice method is comparable with or probably slightly better than the brain homogenate method to predict Kp. Although brain slices offer advantages in theory, more studies are needed to examine whether the brain slice method is superior to the brain homogenate method in a drug discovery setting.
There are at least two utilities of a brain slice technique to study brain penetration in CNS drug discovery setting. One is to determine whether a compound having a low Kp is due to low nonspecific binding in brain tissue relative to plasma proteins, i.e., low Kp,in, or because of an efflux transporter at the BBB, i.e., low Kp,free. If the estimated Kp,in of a compound is within 3-fold of the observed in vivo Kp, the low in vivo Kp is likely due to low binding in brain tissue relative to the plasma proteins, indicating that brain penetration is not impaired. If the estimated Kp,in of a compound is greater than 3-fold of the in vivo Kp, the low Kp is likely due to an efflux transporter at the BBB, and the compound should be considered to have impaired brain penetration. In contrast to using in vitro techniques or transgenic animal models to assess whether a specific transporter causes a low Kp, the brain slice approach is a mechanism-independent method. Therefore, it is useful in a CNS drug discovery program to screen out efflux transporter substrates. To elucidate the mechanism of impaired brain penetration, other in vitro and in vivo methods are needed to investigate the underlying mechanism of low BBB penetration.
The other utility is to use fu,slice and total in vivo brain concentration to estimate the brain interstitial fluid drug concentration or free brain concentration. This may be used as a surrogate approach in place of resource intensive brain microdialysis studies to estimate free brain concentration. The data from Ooie et al. (1997) support this approach. The slice-to-medium concentration ratios for norfloxacin, ofloxacin, fleroxacin, and pefloxacin were 1.9, 1.34, 1.3, and 1.3, respectively. The calculated fu,slice, the reciprocal of slice-to-medium ratios, are 0.5, 0.73, 0.8, and 0.75, respectively. They are within 3-fold of the observed in vivo fu,brain, 1.3, 0.57, 0.37, and 0.47, which were determined using brain microdialysis for each compound. Brain slices may represent a particularly useful approach in drug discovery to evaluate whether the lack of efficacy in an in vivo pharmacological model is due to insufficient free drug concentration in the brain. More studies are needed to assess the utilities of this application.
In conclusion, the present study demonstrates that the brain slice technique can be used to determine intrinsic brain-to-plasma partitioning determined solely by nonspecific binding to assess brain penetration issues in a drug discovery setting. A direct and an indirect brain slice method have been developed and validated. Brain slices represent a mechanism-independent approach to assess whether a low brain-to-plasma ratio is due to nonspecific binding in plasma and brain or because of efflux transport at the BBB. It may also be used to estimate brain free concentrations.
Acknowledgments
We thank Steven Li, Jeffery Van Deusen, Michelle Vanase-Frawley, and Donald Critchett for technical assistance and Jae Lee and Bill Smith for support of this project.
Footnotes
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.105.007914.
-
ABBREVIATIONS: BBB, blood-brain barrier; BCSFB, blood-cerebrospinal fluid barrier; CNS, central nervous system; NFPS, N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy) propyl]sarcosine; CP-141938, methoxy-3-[(2-phenyl-piperadinyl-3-amino)-methyl]-phenyl-N-methyl-methane-sulfonamide; HPLC/MS/MS, high-performance liquid chromatography/tandem mass spectrometry; P-gp, P-glycoprotein.
- Received October 18, 2005.
- Accepted February 15, 2006.
- The American Society for Pharmacology and Experimental Therapeutics