Abstract
The active forms of all marketed hydroxymethylglutaryl (HMG)-CoA reductase inhibitors share a common dihydroxy heptanoic or heptenoic acid side chain. In this study, we present evidence for the formation of acyl glucuronide conjugates of the hydroxy acid forms of simvastatin (SVA), atorvastatin (AVA), and cerivastatin (CVA) in rat, dog, and human liver preparations in vitro and for the excretion of the acyl glucuronide of SVA in dog bile and urine. Upon incubation of each statin (SVA, CVA or AVA) with liver microsomal preparations supplemented with UDP-glucuronic acid, two major products were detected. Based on analysis by high-pressure liquid chromatography, UV spectroscopy, and/or liquid chromatography (LC)-mass spectrometry analysis, these metabolites were identified as a glucuronide conjugate of the hydroxy acid form of the statin and the corresponding δ-lactone. By means of an LC-NMR technique, the glucuronide structure was established to be a 1-O-acyl-β-d-glucuronide conjugate of the statin acid. The formation of statin glucuronide and statin lactone in human liver microsomes exhibited modest intersubject variability (3- to 6-fold; n = 10). Studies with expressed UDP glucuronosyltransferases (UGTs) revealed that both UGT1A1 and UGT1A3 were capable of forming the glucuronide conjugates and the corresponding lactones for all three statins. Kinetic studies of statin glucuronidation and lactonization in liver microsomes revealed marked species differences in intrinsic clearance (CLint) values for SVA (but not for AVA or CVA), with the highest CLintobserved in dogs, followed by rats and humans. Of the statins studied, SVA underwent glucuronidation and lactonization in human liver microsomes, with the lowest CLint (0.4 μl/min/mg of protein for SVA versus ∼3 μl/min/mg of protein for AVA and CVA). Consistent with the present in vitro findings, substantial levels of the glucuronide conjugate (∼20% of dose) and the lactone form of SVA [simvastatin (SV); ∼10% of dose] were detected in bile following i.v. administration of [14C]SVA to dogs. The acyl glucuronide conjugate of SVA, upon isolation from an in vitro incubation, underwent spontaneous cyclization to SV. Since the rate of this lactonization was high under conditions of physiological pH, the present results suggest that the statin lactones detected previously in bile and/or plasma following administration of SVA to animals or of AVA or CVA to animals and humans, might originate, at least in part, from the corresponding acyl glucuronide conjugates. Thus, acyl glucuronide formation, which seems to be a common metabolic pathway for the hydroxy acid forms of statins, may play an important, albeit previously unrecognized, role in the conversion of active HMG-CoA reductase inhibitors to their latent δ-lactone forms.
HMG1-CoA reductase inhibitors, also called “statins”, which target the rate-limiting enzyme in cholesterol biosynthesis, are used widely for the treatment of hypercholesterolemia and hypertriglyceridemia (Mauro, 1993). Except for simvastatin (SV) and lovastatin (LV), all currently available statins are administered as the pharmacologically active hydroxy acid forms. SV and LV are inactive δ-lactones, which, upon conversion to their respective hydroxy acids (SVA and LVA), serve as potent competitive inhibitors of HMG-CoA reductase (Duggan and Vickers, 1990). All statins undergo varying degrees of metabolism in both animals and humans (Vickers et al., 1990b; Everett et al., 1991;Dain et al., 1993; Halpin et al., 1993; Cheng et al., 1994; Le Couteur et al., 1996; Boberg et al., 1998; Black et al., 1999), catalyzed primarily by the cytochrome P450 system (Wang et al., 1991;Boberg et al., 1997; Prueksaritanont et al., 1997, 1999). Other reported biotransformation pathways include lactonization of statin hydroxy acids and β-oxidation at the common dihydroxy heptanoic or heptenoic acid side chain (Vickers et al., 1990b; Halpin et al., 1993;Boberg et al., 1997; Black et al., 1999; Prueksaritanont et al., 2001); in recent studies, it has been shown that the CoA thioester conjugate of the hydroxy acid side chain probably serves as an intermediate in these processes (Prueksaritanont et al., 2001).
In a recent study, glucuronide conjugates of CVA have been reported in animals (Boberg et al., 1998), indicating that the heptenoic acid side chain also may be subject to glucuronidation. To date, however, no other marketed statins have been reported to form glucuronides in animals or humans. In preliminary studies on the metabolism of SVA in dogs, we found high levels of SV and modest concentrations of SVA glucuronide in specimens of bile. Moreover, the glucuronide conjugate of SVA seemed to be unstable upon standing. Based on these observations, we speculated that acyl glucuronidation might be a common metabolic pathway for statins and that spontaneous cyclization of the resulting conjugate may contribute to the lactonization observed for all statins.
Based upon these considerations, we set out to characterize the glucuronidation of statins using SVA, AVA, and CVA as model compounds in liver microsomal preparations from both animals and humans. These three statins were selected for study since they have been reported to undergo significant lactonization in vivo in animals and/or humans (Vickers et al., 1990a,b; Boberg et al., 1998; Kantola et al., 1998). The identity of the UGTs, which catalyze the glucuronidation of these agents, was investigated with the aid of expressed enzymes, and the stability of the acyl glucuronide in vitro was studied in the case of the SVA conjugate. Finally, the significance of the glucuronidation pathway in the disposition of SVA in vivo was examined using the dog as an animal model.
Experimental Procedures
Materials.
SV, SVA, and [14C]SVA, with a specific activity of 50 μCi/μmol (Fig. 1), were synthesized at Merck Research Laboratories (Rahway, NJ). AVA and CVA were extracted from commercial sources, and their identity and purity were confirmed by infrared and NMR spectroscopy. Brij 58 and UDPGA were obtained from Sigma Chemical Co. (St. Louis, MO). CD3CN (99.8 atom % D) and D2O (99.8 atom % D) were purchased from Isotec, Inc. (Miamisburg, OH), and CF3COOD (99.5 atom % D) was obtained from Aldrich Chemical Co. (Milwaukee, WI). All other reagents were of analytical or HPLC grade. Human recombinant UGTs were obtained from GENTEST (Woburn, MA) and Panvera (Madison, WI). Human liver microsomes were purchased from XenoTech, LLC (Kansas City, KS) and GENTEST, whereas those from male Sprague-Dawley rats (∼260–320 g) and beagle dogs (9–11 kg) were prepared in-house, as described previously (Prueksaritanont et al., 1997), and were pooled from four to six animals before use.
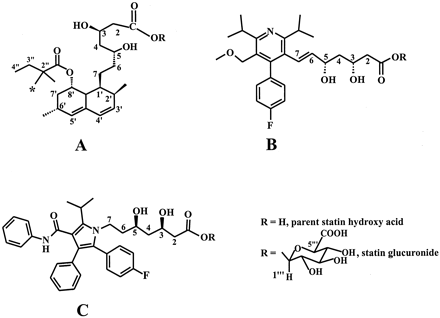
Structures of statin hydroxy acids and their acyl glucuronide conjugates for SVA (A), CVA (B), and AVA (C).
✻, indicates the position of 14C label.
In Vitro Metabolism of Statins.
A typical incubation mixture, in a final volume of 0.3 ml, contained 0.3 mg (dog) or 0.45 mg (human or rat) of liver microsomal protein, preincubated for 15 min with 0.045 mg of Brij 58, 20 mM MgCl, 5 mM UDPGA, and 0.05 M Tris buffer, pH 7.0. The preincubation step with Brij 58 was found to be optimal for high enzyme activity. Unless otherwise specified, the reaction was started by the addition of SVA, AVA, or CVA following a 3-min preincubation at 37°C and was conducted for up to 60 min. Control experiments were performed by excluding either the microsomes or UDPGA from the incubation mixtures. The reaction was terminated at appropriate time intervals by the addition of 0.8 ml of acetonitrile (ACN). The ACN extracts were evaporated to dryness and reconstituted, just before analysis, in the mobile phase (20% ACN in 25 mM ammonium acetate buffer, pH 4.5) for analysis by the HPLC method described below. Kinetic studies were conducted using 0.2 to 200 μM statins in liver microsomal preparations from humans, rats, and dogs. The incubation mixtures were incubated for 45 min at 37°C.
Incubations with human recombinant UGTs were performed using the same conditions as described above for human liver microsomes, except that the mixture contained 0.3 mg of UGTs and was incubated for up to 60 min. Control incubations using microsomes isolated from the same cell line containing the vector, but without a cDNA insert, also were included.
For the purpose of isolation and purification of the statin glucuronides, large-scale incubations of the statins (100 μM; 20 × 0.5-ml incubation) were carried out with dog liver microsomes (2 mg/ml) and UDPGA (5 mM) for 60 min. The ACN extracts were evaporated to dryness and reconstituted for analysis by LC-MS and LC-NMR spectroscopy.
Stability of SVA Acyl Glucuronide.
The acyl glucuronide of SVA was isolated by HPLC (see Analytical Procedures for conditions) from an in vitro incubation with dog liver microsomes, SVA (100 μM), and UDPGA (5 mM). Duplicate 0.5-min fractions (∼0.5 ml) containing the SVA glucuronide were collected by a fraction collector (Foxy 200; ISCO, Inc., Lincoln, NE) into tubes containing 0.3 ml of buffer, with a specific pH value between 4 to 8. The resulting mixtures then were injected immediately onto an HPLC column (with an autosampler set at 5°C) at about 2.5-h intervals over an 8-h period. For each pH mixture, the time for the first injection was considered as the starting time (time = 0).
In Vivo Metabolism and Excretion of SVA.
All studies were reviewed and approved by the Merck Research Laboratories Institutional Animal Care and Use Committee. Beagle dogs (n = 3 each; 9–14 kg) were surgically prepared with common bile-duct cannulae and were housed individually in metabolism cages with an extracorporeal reservoir on their back for bile collection. [14C]SVA was administrated intravenously at 1.2 mg/kg, and bile was collected in a bag containing 0.5 M ammonium acetate buffer, pH 4.5 (∼10% of total bile volume), continuously every hour over a period of 10 h and during the next 10 to 24 h. Urine samples also were collected during 0 to 8, 8 to 24, and 24 to 48 h postdose. The bile and urine samples were frozen immediately on dry ice and kept at −20°C for later analysis.
Analytical Procedures for Statins and Metabolites.
SVA, CVA, AVA, and their metabolites were analyzed using published HPLC methods (Prueksaritanont et al., 1999) with minor modifications. In brief, samples held in an autosampler set at 5°C were chromatographed on a C18 Zorbax column (150 × 4.6 mm, 5 μm; Waters, Inc., Milford, MA) preceded by a C18 guard column, with a linear gradient of ACN and 25 mM ammonium acetate, pH 4.5. The eluate was monitored by UV absorption at 240 nm (SVA and AVA) or 280 nm (CVA) and by an on-line β-RAM radioactivity detector (IN/US Systems, Tampa, FL). Due to the unavailability of authentic standards for glucuronide conjugates of statins, quantitation of these metabolites in the in vitro incubation mixtures was accomplished using standard curves for their respective parent statins, assuming identical extraction recoveries and extinction coefficients between the parent drug and its corresponding glucuronide conjugate. For the three statins, standard curves showed satisfactory linearity and precision (<15% coefficient of variation). The limits of assay detection were 5 pmol (on column) for all three statins.
Levels of total radioactivity in bile and urine samples were determined by direct measurement of samples using a scintillation counter (Packard Instrument Company, Inc., Downers Grove, IL). Concentrations of SV, SVA glucuronide, and SVA in bile samples were estimated based on total radioactivity and metabolite profiling studies (using HPLC with an on-line IN/US β-RAM radioactivity detector).
Identification of the statin metabolites was accomplished by using LC-MS techniques (HP-1050 gradient system; Hewlett Packard, San Fernando, CA; Finnigan MAT LCQ ion trap mass spectrometer; Thermo Finnigan MAT, San Jose, CA). Separation of the metabolites was carried out on a Betasil C18 column (2 × 150 mm, 5 μm), with a linear gradient of ACN and 0.1% formic acid (30% ACN to 80% ACN in 20 min) delivered at a constant flow rate of 0.2 ml/min. Mass spectral analyses were performed using electrospray ionization in the negative ion mode (for SVA and AVA glucuronide conjugates) or positive ion mode (for statin lactones and CVA glucuronide). The electrospray ionization voltage was set at 4 kV, with the heated capillary temperature held at 230°C.
For NMR studies, the dried extract from in vitro incubates containing the metabolite were reconstituted in approximately 250 μl of 30% ACN/70% water in 1 mM ammonium acetate, pH 4.5 (SVA), or 10% ACN/90% water/0.1% CF3COOD (AVA and CVA) before injection. All NMR spectra were acquired under stopped-flow conditions. Once the apex of the metabolite peak was detected, the HPLC pump was stopped after a precalibrated delay time, at the end of which the metabolite was located in the NMR flow cell. The HPLC conditions were optimized such that the LC peak volume matched that of the NMR flow cell volume (60 μl) to maximize the signal-to-noise ratio of the NMR spectrum. Deuterated mobile phase was used for all LC-NMR runs, and no solvent suppression techniques were applied. The following HPLC conditions were used for LC-NMR studies: Symmetry C18, 5-μm, 3.9 × 150-mm column (SVA) or Phenomenex phenylhexyl, 5-μm, 2 × 150-mm column (AVA and CVA); flow rate, 1.0 ml/min (SVA) or 0.3 ml/min (CVA and AVA); and UV detection at 239 nm (SVA), 244 nm (AVA), or 283 nm (CVA). Separation of glucuronide metabolites from parent statins was achieved using the following gradient conditions: SVA, 0 to 1 min at 30% A, 1 to 18 min 30 to 81% A, 18 to 22 min at 81% A, and a 22.1-min return to 30% A; AVA/CVA, 0 to 3 min at 10% A, 3 to 30 min 10 to 64% A, 30.1 to 35 min at 90% A, and a 35.1-min return to 10% A, where A is 90% CD3CN + 10% D2O + 0.1% CF3COOD and B is 90% D2O + 10% CD3CN + 0.1% CF3COOD. The parent LC-NMR spectra were obtained by injecting ∼25 μg of the corresponding statin under the same LC conditions as used for the metabolite. 1H chemical shifts (in parts per million) are referenced relative to residual CD2HCN at 1.99 ppm. NMR spectra were obtained using an Inova (11.7 T/500 MHz) 51-mm, narrow-bore spectrometer (Varian, Inc., Palo Alto, CA) equipped with a 60-μl flow probe (Varian, Inc.).
Data Analysis.
Apparent Km andVmax values were estimated using a nonlinear regression program (Enfit; Biosoft, Ferguson, MO). The CLint values were estimated by dividingVmax byKm.
Results
Glucuronidation of Statins in Dog Liver Microsomes.
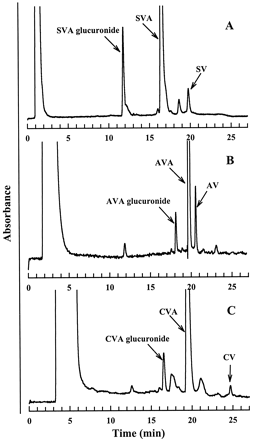
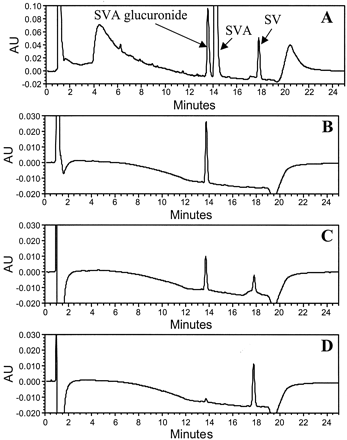
Figure 2A illustrates a typical HPLC chromatogram derived from incubates of SVA with dog liver microsomes in the presence of UDPGA. Two major products with an UV spectrum similar to that of SVA were observed. The nonpolar product, which eluted after SVA, was identified as the lactone SV based on the identical HPLC retention time and UV spectrum compared with the authentic standard. The more polar metabolite, upon LC-MS analysis, afforded an [M − H]− ion at m/z 611, 176 mass units higher than the [M − H]− ion of SVA, suggesting that it was a glucuronide conjugate of SVA. Except for a small amount of SV (<0.6% of initial concentration), the two metabolites were not detectable in control incubations that lacked liver protein or UDPGA. The results suggested that the glucuronide conjugate of SVA was formed enzymatically, whereas SV was formed both by enzymatic (major) and chemical (minor) processes under the conditions used. The enzymatic reaction for both products was mediated by UDPGA-dependent enzyme(s).
Representative HPLC-UV profiles of metabolites of SVA (A), AVA (B), and CVA (C) in dog liver microsomal incubates.
Incubations were carried out at 37°C for 45 min using dog liver microsomes (1 mg/ml) and statins (100 μM) with UDPGA (5 mM).
As was the case with SVA, two metabolites of AVA or CVA (one more polar and the other less polar than the corresponding parent) were detected when AVA or CVA was incubated with human liver microsomes supplemented with UDPGA (Fig. 2, B and C). Both metabolites afforded an UV spectrum identical to that of the corresponding parent and were not detected in the absence of microsomes or UDPGA. LC-MS analysis indicated that the more polar metabolites of AVA and CVA gave an [M − H]− ion at m/z 733 and an [M + H]+ species at m/z636, respectively, which corresponded to an addition of 176 mass units to their respective parent statin. In each case, collisional activation of these ions led a loss of 176 Da (data not shown), suggesting that the metabolites in question were the statin glucuronides. The nonpolar metabolites of AVA and CVA afforded [M + H]+ions at m/z 541 and 442, which were 18 mass units less than the corresponding ions from AVA and CVA, respectively. Based on the HPLC retention time and UV and MS spectra, the nonpolar metabolites were assigned as AV and CV, the lactone derivatives of AVA and CVA, respectively.
For all three statins, formation of the glucuronide conjugate and lactone was linear with time up to 1 h. The formation of each glucuronide was highest at an incubation pH of 7.0 (data not shown). At incubation buffer pH values higher than 7, formation of the lactones became more prevalent compared with that of the glucuronide conjugates. Lactone formation also seemed to increase when the incubation mixture was left overnight at room temperature. For these reasons, all incubations in subsequent experiments were performed at pH 7.0, and samples were analyzed immediately (with an autosampler set at 5°C during analysis) after incubation.
NMR Identification of Statin Glucuronides.
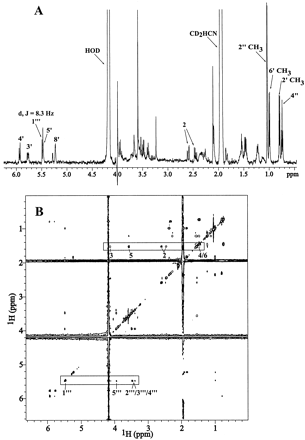
LC-NMR experiments were performed to identify the site of conjugation of the statin glucuronides. Under the HPLC conditions used for LC-NMR in this study, the full-width-at-half-height LC peak volumes for the glucuronides of SVA, CVA, and AVA were 156, 75, and 69 μl, respectively, which contained ∼10 to 15 μg of the conjugates in the active volume of the flow cell. The results described below support the conclusion that the glucuronide conjugates of these statins formed upon incubation with dog liver microsomes are β-1-O-acyl glucuronides (Fig. 1). The anomeric proton (labeled 1‴) of SVA glucuronide exhibits a 1H chemical shift of 5.48 ppm (Figs. 3, A and B; Table1). Through-bond proton connectivities of the heptanoic acid side chain and the glucuronide moiety in SVA glucuronide are shown in Fig. 3B and were identified by total correlation NMR spectroscopy (Summers et al., 1986). Similar to the SVA glucuronide, the anomeric protons of the glucuronide conjugates of CVA and AVA have a 1H chemical shift of 5.51 and 5.53 ppm, respectively. For all three statins, the 1‴ proton was a doublet, with a scalar coupling constant of 8.1 or 8.3 Hz indicative of a β-anomer. The 1‴ chemical shifts are consistent with an acyl glucuronide rather than an ether glucuronide at the 3 or 5 positions since the anomeric proton in an alkyl ether glucuronide would have a δ < 4.5 ppm (Boberg et al., 1998). Relative to the parent statin, protons at position 2 (α to the carboxyl group) also underwent a concomitant downfield shift in the glucuronide conjugate (Table 1).
One-dimensional 1H (A) and total correlation NMR spectroscopy (B) spectra of SVA glucuronide.
Only key protons are identified in both spectra.
1H Chemical shifts of key protons in the heptanoic/heptenoic acid side chain of all three statins and their corresponding glucuronides
NMR also indicated that all three statin glucuronides were in the open acid form. Protons 3 and 5 have distinct chemical shifts characteristic of the lactone or the hydroxy acid form. For example, protons 3 and 5 appeared in SV (the lactone form of SVA) at 4.25 and 4.64 ppm, respectively, and in SVA at 4.19 and 3.58 ppm, respectively. The chemical shifts of methine protons 3 and 5 (Table 1) are indicative of the open acid forms in all three statin glucuronides. The chemical shifts of protons in the remainder of the statin molecules were practically unchanged.
Metabolism of Statins by Human Liver Microsomes.
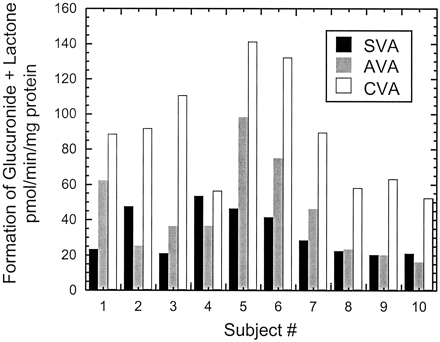
As was the case with dog liver microsomes, formation of the statin acyl glucuronide and the corresponding lactone in human liver microsomes was dependent on the presence of both microsomal protein and UDPGA. Due to the apparent instability of the acyl glucuronides of the three statins studied, the sum of the acyl glucuronide and the corresponding lactone was used to calculate rates of total glucuronidation for each statin. For all three statins, formation rates of the acyl glucuronide conjugates + the lactones in 10 human livers exhibited 3- to 6-fold variation and were higher with CVA than with SVA or AVA in most livers (Fig. 4). On average, the summed rates of glucuronidation + lactonization were 32 ± 13, 44 ± 27, and 88 ± 32 pmol/min/mg of protein for SVA, AVA, and CVA, respectively. The formation rates of glucuronide + lactone of AVA seemed to correlate with that of CVA (r2, 0.7). However, no correlation was observed for the rates of glucuronidation + lactonization between SVA and CVA or between SVA and AVA (r2, <0.2).
Formation of acyl glucuronide conjugates and lactones of SVA, AVA, and CVA in human liver microsomes obtained from 10 different individuals.
Incubations were carried out in duplicate at 37°C for 40 min using liver microsomes (1.5 mg/ml) and statins (200 μM) with UDPGA (5 mM).
Metabolism of Statins by Recombinant UGT Isoforms.
Studies on the metabolism of statins by commercially available human recombinant UGT isoforms indicated that both UGT1A1 and UGT1A3 catalyzed the formation of acyl glucuronide conjugates of SVA, AVA, and CVA. As was the case in the liver microsomal preparations, formation of the corresponding lactones also was observed with UGT1A1 and UGT1A3 in the presence of UDPGA. Per milligram of protein, the rate of glucuronidation and lactonization of SVA, AVA, or CVA was approximately the same for UGT1A1 (5–7 pmol/min/mg) and 1A3 (2 pmol/min/mg) under the experimental conditions used. All other UGTs tested (UGT1A4, UGT1A6, UGT2B7, and UGT2B15), as well as the control microsomes, failed to produce either the glucuronide or the lactone of any statin to appreciable extent (≤0.5 pmol/min/mg).
Kinetic Studies.
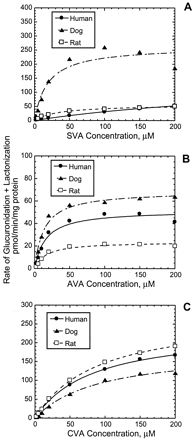
The rates of formation of the acyl glucuronide conjugates and the lactones of the three statins in human, dog, and rat liver microsomes were best described by single-enzyme Michaelis-Menten kinetics over the substrate concentration range studied (Figs.5, A–C). In the case of SVA, species differences were observed in the kinetics of UDPGA-dependent metabolism. In dogs, this reaction was mediated by an enzyme(s) of higher affinity and capacity than that in humans but of comparable affinity and higher capacity than that in rats (Table2). As a result, the CLint of SVA glucuronidation + lactonization was ∼4- to 35-fold higher in dogs than in rats and humans, respectively. In contrast, species differences were not as marked for the UDPGA-dependent metabolism of AVA and CVA; the CLint ranged from 2 to 6 μl/min/mg in all three species (Table 2). The glucuronidation of AVA was mediated by UGTs with high affinity (Km, ∼10–15 μM) in all species examined. In human liver microsomes, the rate of glucuronide + lactone formation was lower for SVA (0.4 μl/min/mg) than for either AVA or CVA (2.9–3.3 μl/min/mg).
Formation of acyl glucuronide conjugates and lactones of SVA (A), AVA (B), and CVA (C) in dog, rat, and human liver microsomes as a function of the substrate concentration.
Incubations were carried out in duplicate at 37°C for 40 min using liver microsomes (1.5–2 mg/ml) and various concentrations of statins with UDPGA (5 mM).
Kinetic parameters for UDPGA-dependent metabolism of statins in liver microsomal preparations
Stability of SVA Acyl Glucuronide.
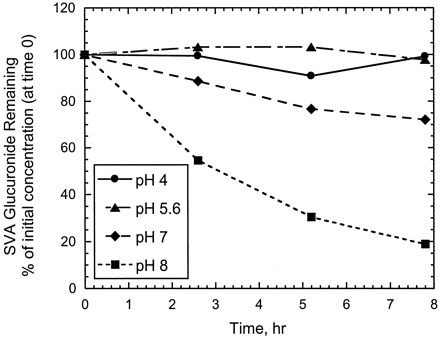
The SVA acyl glucuronide isolated from in vitro incubations (Fig.6A) was found to be relatively stable at 5°C in buffers over pH 4 to 5.6 for at least 8 h after isolation (Figs. 6B and 7). At pH values of 7 or higher, the isolated acyl glucuronide converted rapidly to SV (Figs. 6, C and D, and 7). In fact, formation of SV was observed in the first injection done immediately after the isolated glucuronide fraction was added to pH 8 buffer. Under the conditions (5°C) and in all buffers tested, there was no evidence for the formation of SVA or acyl migration products of the glucuronide conjugate of SVA (Fig. 6, B–D). At higher temperatures (37°C), however, the glucuronide levels decreased more rapidly, and at pH values >8, both SV and SVA were formed (data not shown).
Representative HPLC-UV profiles of a dog liver microsomal incubate with SVA (200 μM) and UDPGA (5 mM) (A), an isolated fraction of SVA glucuronide collected in pH 4 buffer (B), an isolated fraction of SVA glucuronide collected in pH 7 buffer (C), and an isolated fraction of SVA glucuronide collected in pH 8 buffer (D).
AU, absorbance unit..
Stability of SVA glucuronide conjugate as a function of time in different pH buffers at 5°C.
In Vivo Formation of SVA Acyl Glucuronide in Dogs.
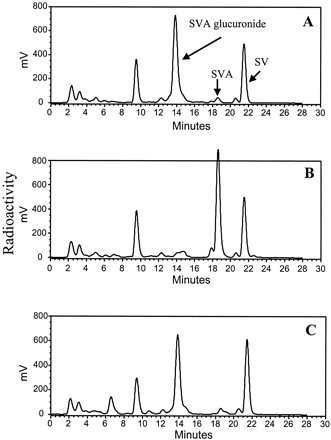
In dogs, within 24 h following i.v. administration of [14C]SVA, about 65 and 5% of total radioactivity was found in bile and urine, respectively. As was reported previously (Vickers et al., 1990a,b), metabolite profiling studies of bile and urine samples indicated that unchanged SVA accounted for less than 5% of the total radioactivity recovered, suggesting that SVA underwent extensive metabolism in dogs. The studies also indicated the presence of the acyl glucuronide conjugate of SVA and its lactone SV in bile (Fig. 8A). The identity of SVA glucuronide in bile was based on the HPLC retention time, the UV and LC-MS spectra, and the formation of free SVA upon treatment with β-glucuronidase. Unlike the chemical degradation process, which led to SV, the enzymatic hydrolysis of SVA glucuronide resulted in unchanged parent SVA (Fig. 8B). Using the present bile collection scheme, the acyl glucuronide (∼20% of dose) and SV (∼14% of dose) were among major metabolites in bile, accounting for about ∼30 and ∼20%, respectively, of the total radioactivity recovered (Table 3). As was the case in vitro, levels of the acyl glucuronide conjugate in bile decreased, and concentrations of SV increased when samples of bile were allowed to stand or when an aliquot of bile (which was initially buffered to pH 4.5 to prevent degradation) was treated with sodium hydroxide to pH 7 (Fig. 8C).
Representative HPLC-radioactivity profiles of metabolites of SVA in bile samples collected during the first 2 h postdose, buffered immediately to pH 4.5 without (A) and with (B) treatment with β-glucuronidase, and in bile samples adjusted to pH 7 (C).
Recovery of SVA metabolites and total radioactivity in bile collected during 0 to 10 h after administration of [14C]SVA (1.2 mg/kg i.v.) to dogs
Discussion
This article provides the first experimental evidence for the glucuronidation of SVA, AVA, and CVA in liver microsomal preparations from animals and humans and of glucuronidation of SVA in vivo in dogs. To date, only glucuronide conjugates of CVA, but not AVA and SVA, have been documented in dog bile (Boberg et al., 1998), whereas significant lactone formation was reported to occur in vivo following administration of SVA or CVA to dogs (Vickers et al., 1990a; Boberg et al., 1998) and of CVA and AVA to humans (Kantola et al., 1998, 1999). Thus far, the lactonization of statins generally has been attributed to cyclization of acyl-CoA thioester intermediates (Halpin et al., 1993;Boberg et al., 1998) and to chemical equilibration processes (Kantola et al., 1999), and the former pathway has been confirmed recently in vitro (Prueksaritanont et al., 2001). Based on the present in vitro and in vivo results, we hypothesize that the lack of reports on glucuronidation of SVA and AVA (and possibly other statin acids) most likely is a consequence of the relative ease of spontaneous cyclization of the statin acyl glucuronides and that the glucuronidation pathway contributes, at least in part, to the formation of statin lactones.
In the current study, LC-NMR techniques were used to characterize the acyl glucuronide conjugates of three statins. LC-NMR is well suited to such applications since it has been demonstrated to be a powerful nondestructive tool for the structural characterization of unstable metabolites (Lindon et al., 2000). In the in vitro experiments, it was shown that the formation of each statin lactone was dependent on the presence of both microsomal protein and UDPGA, suggesting that lactonization of the statin hydroxy acid was mediated by glucuronidation followed by elimination of the glucuronic acid moiety. This glucuronidation-dependent lactonization was later confirmed when it was found that SVA glucuronide converted rapidly to SV upon standing. Since the glucuronide-to-lactone conversion occurred readily in the physiological pH range (pH 7–8), this mechanism has in vivo relevance and probably contributes to the formation of SV from SVA in vivo. Lactonization by the glucuronidation pathway also is expected to occur for AVA and CVA since both compounds undergo significant glucuronidation and lactonization under the same in vitro incubation conditions. In addition, the acyl glucuronide conjugates of these statins also were unstable and underwent conversion primarily to the corresponding lactone forms upon standing. In the case of CVA, the glucuronide conjugate was found to undergo hydrolysis to regenerate the parent statin at pH 7.4 or above (data not shown). The data suggest that, in vivo, CVA glucuronide would undergo conversion to CVA in addition to lactonization to CV. It is noteworthy that the acyl glucuronide conjugates of these statins, at physiological pH, preferentially undergo lactonization compared with acyl migration, a process commonly observed for acyl glucuronides of numerous carboxylic acid-containing compounds (Spahn-Langguth, 1992). It was also found that all statin glucuronides were converted to their respective parent acids when treated with β-glucuronidase.
Although two isomeric ether-linked glucuronides of CVA, conjugated by the two hydroxyl groups of the heptenoic side chain, were identified following the administration of CVA to dogs (Boberg et al., 1998), these conjugates were not detected at significant levels under the present liver microsomal incubation conditions to allow definite characterization. Similarly, glucuronide conjugates other than the acyl glucuronide were not formed to any appreciable extent when SVA or AVA was incubated with liver microsomal preparations or when SVA was administered to dogs. In recombinant UGT systems, the acyl glucuronide also was a major metabolite for each statin. Considering that the total glucuronidation (glucuronide + lactone formation) of SVA did not correlate with that of AVA or CVA in the 10 human livers examined in this study, UGTs other than UGT1A1 and 1A3 might also be involved in the glucuronidation of these statins in human liver microsomes. Based on the kinetic studies with human liver microsomes, which showed apparently monophasic characteristics, these responsible UGTs may posses Km values in a comparable range to those observed in human liver microsomes.
The relevance of the in vitro findings in dog liver microsomes to the in vivo situation was addressed in studies on the glucuronidation of SVA in the dog. Consistent with the in vitro results, the biliary recovery of SVA glucuronide following administration of [14C]SVA to dogs was substantial, accounting for about 20% of an i.v. dose. Indeed, this figure of 20% may underestimate the fraction of the dose converted to the conjugate if hydrolysis or lactonization occurred to regenerate SVA or to form SV, respectively. The present in vitro finding of more efficient glucuronidation in liver microsomes obtained from dogs than rats also is in agreement with our preliminary in vivo studies, which showed higher biliary levels of SVA glucuronide + SV in dogs than in rats following i.v. administration of [14C]SVA (data not shown). Thus, it may be anticipated that the formation of SVA glucuronide following SVA administration probably would be lower in humans than in dogs. In contrast, based on the present in vitro results, it would not be anticipated that marked species differences in the rates of glucuronidation of AVA and CVA would occur in vivo. In this regard, it is important to point out that in dogs, the combined biliary recoveries of CVA glucuronide (∼5%) and the corresponding lactone CV (18%) accounted for >20% of the intraduodenal dose (Boberg et al., 1998) and that relatively high levels of CV and lactone metabolites thereof were observed following administration of CVA to humans (Jemal et al., 1999; Kantola et al., 1999). In the case of AVA, the areas under the plasma concentration versus time curves of the lactone (AV) also were high and similar to those of AVA following AVA administration to healthy subjects (Kantola et al., 1998). Unfortunately, published in vivo data are not available to permit a comparison between the rates of AVA glucuronidation and lactonization in animals or between the amounts of CVA and AVA glucuronides and their lactones formed in humans.
The present study suggests that, in addition to the well known P450-mediated oxidation (Wang et al., 1991; Boberg et al., 1997;Prueksaritanont et al., 1997) and β-oxidation processes (Vickers et al., 1990b; Halpin et al., 1993; Boberg et al., 1997; Black et al., 1999; Prueksaritanont et al., 2001), glucuronidation constitutes a common metabolic pathway for statins. Quantitatively, there seem to be differences in the relative contributions of these pathways to the metabolism of different statins in different species. For most statins, β-oxidation has been shown to be a major pathway in rodents (Arai et al., 1988; Vickers et al., 1990a; Halpin et al., 1993; Black et al., 1998; Boberg et al., 1998; Black et al., 1999), whereas in humans, P450-mediated oxidative metabolism has been regarded as a major pathway of biotransformation (Igel et al., 2001). However, based on the findings of the present study, this assumption may not be valid for all statins and is particularly unlikely to hold true in the case of CVA, which is a low-clearance compound in humans (Mück, 2000). In support of the latter point, our preliminary study using human liver microsomes fortified with NADPH (Prueksaritanont et al., 1999) showed that the CLint of CVA oxidative metabolism (∼ 10 μl/min/mg of protein) was in a comparable range to the CLint of glucuronidation, whereas that of AVA oxidation (∼50 μl/min/mg of protein) (data not shown) was much higher than the value for UDPGA-dependent metabolism (Table 2). In the case of AVA, the value for the CLint under oxidative conditions (in the presence of NADPH) in our study was comparable to that reported in the literature (∼80 μl/min/mg;Jacobson et al., 2000).
The present results also suggest that the metabolism of statins is complex, involving acid/lactone interconversion by various pathways, as proposed in Fig. 9. The statin lactones are hydrolyzed to their open acids chemically or enzymatically by esterases or the newly identified paraoxonases (Vickers et al., 1990a; Billecke et al., 2000; Draganov et al., 2000). The statin acids are converted to the corresponding lactones by the acyl glucuronide intermediate, as demonstrated in this study, and by the CoASH-dependent pathway (Prueksaritanont et al., 2001). Both acyl glucuronide and acyl CoA derivatives may revert to the statin acids by hydrolysis. Similar considerations apply to oxidative metabolites of the statins. Indeed, the lactone forms of oxidative metabolites of CVA and glucuronide conjugates of the oxidative metabolites of CVA and AVA have been identified in animals and/or humans following administration of CVA and AVA, respectively (Le Couteur et al., 1996; Boberg et al., 1998; Black et al., 1999). Jacobson et al. (2000) recently hypothesized that most of the hydroxy acid metabolites present in human plasma following AVA administration are converted from metabolites of AV (following lactonization of AVA), which are formed by CYP3A. In the latter study, the mechanism responsible for the lactonization of AVA was thought to be of a chemical nature, and no data on in vivo formation of AV metabolites were provided.
Proposed metabolic pathways of statins.
Overall, the present study demonstrates that, in addition to the well known P450-mediated oxidation and β-oxidation processes, glucuronidation also is a common metabolic pathway for the three statins examined. Additionally, this study provides evidence that glucuronidation may play an important role in mediating the lactonization of statins in vivo, and highlights the complexities of statin metabolism associated with hydroxy acid/lactone interconversion. This interconversion process clearly will need to be taken into account in assessing mechanistic aspects of drug-drug interactions involving statins.
Acknowledgments
We thank Drs. A. Jones and C. Raab and Greg Gatto and Nathan Yu for the synthesis and purification of [14C]SVA and also J. Brunner and K. Michel for animal experiments.
Footnotes
- Abbreviations used are::
- HMG
- hydroxymethylglutaryl
- SV
- simvastatin
- LV
- lovastatin
- SVA
- hydroxy acid form of simvastatin
- LVA
- hydroxy acid form of lovastatin
- CVA
- cerivastatin
- UGT
- UDP glucuronosyltransferase
- AVA
- atorvastatin
- UDPGA
- UDP-glucuronic acid
- HPLC
- high-pressure liquid chromatography
- ACN
- acetonitrile
- LC-MS
- liquid chromatography-mass spectrometry
- CLint
- intrinsic clearance
- AV
- the lactone form of atorvastatin
- CV
- the lactone form of cerivastatin
- P450
- cytochrome P450
- Received October 25, 2001.
- Accepted January 18, 2002.
- The American Society for Pharmacology and Experimental Therapeutics