Abstract
Human CYP3A4 metabolizes a majority of clinically important substrates at variable rates. Accounting for these unpredictable rates is the wide variation noted in expression of this enzyme that is due, in part, to xenobiotic exposure. We used primary cultures of human hepatocytes from 17 individuals to assess the inducibility of CYP3A4 mRNA by prototypical inducers, dietary flavonoids, and botanicals. Those agents producing the greatest mRNA accumulation were 10 μM RIF (699 ± 307% of control levels) 100 μM phenytoin (707 ± 188% of control), 1 mM phenobarbital (536 ± 207% of control), and 100 μM omeprazole (404 ± 8% of control). Various concentrations of RIF were found to exhibit a typical dose-response curve for CYP3A4 mRNA content. A reporter gene assay using the human pregnane X receptor (hPXR) and promoter regions of CYP3A4 transiently transfected into HepG2 cells, exhibited inductive properties by the aforementioned therapeutics that were similar to those observed in hepatocytes. Several flavonoids including quercetin, resveratrol, and curcumin were also examined for their ability to induce CYP3A4 in human hepatocytes. Only quercetin produced accumulation of CYP3A4 mRNA (230 ± 73% of control). When examined in a reporter gene assay, this flavonoid exhibited negligible increases in luciferase activity suggesting that quercetin induced CYP3A4 by mechanisms that may not involve PXR. We also examined the effects of herbals on CYP3A4 expression in human hepatocytes. Grapeseed extract, ginseng, silymarin, and kava-kava produced 270 ± 73, 155 ± 83, 100 ± 10, and 386 ± 185% of control CYP3A4 mRNA, respectively. Of these botanicals only kava-kava produced enhanced luciferase activity (11.6 ± 2.1 fold above DMSO treated cells). Such results indicate that kava-kava required PXR to mediate CYP3A4 induction. Collectively, results demonstrated that several botancials induce CYP3A4, suggesting the potential for drug-herbal interactions.
The human cytochrome P450 (P4501), CYP3A4, is responsible for approximately 60% of P450-mediated metabolism of drugs in therapeutic use today implicating this enzyme as important with respect to the action, duration, and disposition of drugs and their metabolites (Gibson et al., 2002). Because of the considerable role that CYP3A4 plays in drug metabolism, hepatic and intestinal expression of this P450 can mediate the therapeutic outcome of many agents. Wide variation in tissue concentrations of this enzyme has been found among individuals that ultimately affects drug disposition oftentimes making disposition difficult to predict (Wrighton et al., 2000). Variability in CYP3A4 expression can result from a variety of factors and is partially explained by the ability of various xenobiotics to increase the expression of this P450. Of these xenobiotics, many are therapeutic agents that enhance hepatic and/or intestinal CYP3A4 expression (Guengerich, 1999). At least five categories of agents are considered CYP3A inducers: steroid hormones having either glucocorticoid or anti-glucocorticoid activities, PB and PB-like agents such as PCBs and organochlorine pesticides, macrolide antibiotics, imidazole antifungal agents, and receptor and enzyme antagonists (e.g., nifedipine, troglitazone, lovastatin) [reviewed in (Quattrochi and Guzelian, 2001)]. The inducibility of CYP3A4 gene expression, coupled with the remarkable versatility of CYP3A catalytic activities, creates the potential for drug-drug interactions.
A major emphasis for pharmacotherapy is reducing the potential for drug interactions. Many adverse drug responses are caused by such interactions compounded by the massive consumption of over-the-counter medications, including botanicals/herbal products. Given that many herbals are oxidized by drug-metabolizing enzymes, it is logical to expect interactions of herbal compounds with conventional drugs. Herbal use in the United States has been experiencing unprecedented growth, accounting for $3.24 billion in sales in 1997, and it has been estimated that the annual increase (from 1997 to 1998) in sales of St. John's wort and green tea extracts was 15,000 and 5,000%, respectively (Miller, 1998). The use of medicinal herbs has particularly increased over the past few years among specific patient populations including HIV-infected patients. Individuals on retroviral therapy and consuming St. John's wort diplayed altered pharmacokinetics of HIV Type 1 protease inhibitors, including indinavir (Piscitelli et al., 2000). This is believed to be due to the inductive capacity of St. John's wort on CYP3A4 (Hennessy et al., 2002; Moore et al., 2000a). Moore et al. (2000a) identified hyperforin, a constituent of St. John's wort, as a potent ligand of the hPXR. That hyperforin is a ligand for this nuclear receptor, explains the mechanism by which St. John's wort induces CYP3A4 in primary cultures of human hepatocytes. Such findings suggest that drug interactions could occur from additional over-the-counter botanical supplements that possess the ability to enhance hepatic expression of CYP3A4. Indeed, decreased plasma concentrations of the HIV protease inhibitor, saquinavir, have been described in individuals exposed long-term to garlic supplements (Piscitelli et al., 2002). The increased clearance of saquinavir may be due to induction of hepatic and/or intestinal CYP3A4.
Despite recent public interest and wide spread consumption of botanicals, little is known about the ability of these supplements to induce P450 expression. We have previously shown that dietary flavonoids, including resveratrol, green tea extracts, kaempferol, and apigenin, induce the expression of CYP1A in primary cultures of human hepatocytes (Allen et al., 2001). The following investigation examined the effects of some of the most commonly consumed botanicals on the expression of CYP3A4. In comparison, several therapeutic agents known to induce CYP3A4 including clotrimazole, PB, and phenytoin were examined for their inductive properties toward CYP3A4 in primary cultures of human hepatocytes. To elucidate the mechanism by which these agents enhance CYP3A4 levels, we examined the effects of the aforementioned therapeutic agents and natural products in a reporter gene assay using the orphan nuclear receptor, hPXR. Results identify certain botanicals as CYP3A4 inducers and suggest a mechanism by which these agents may induce this P450.
Materials and Methods
Primary Human Hepatocyte Cultures.
Human hepatocytes plated on rat tail collagen-coated flasks (T-25) in serum-supplemented media were obtained from the Liver Tissue Procurement and Distribution System (University of Minnesota, Minneapolis, MN) (Runge et al., 2000). Characteristics of the hepatocyte donors are given in Table 1. Upon arrival at our laboratory, the hepatocytes had already been in primary culture for 24 h. The media was replaced with serum-free HMM (Cambrex Bio Science Walkersville, San Diego, CA) containing 10−7 M dexamethasone plus 10−7 M insulin, and the cells were maintained in an atmosphere of 95% air and 5% CO2 at 37°C for an additional 24 h. Hepatocytes were then treated with pharmaceutical agents including 0.1 to 50 μM RIF, 1000 μM PB, 10 μM mifepristone, 10 μM clotrimazole, 10 and 50 μM mevastatin, 10 and 100 μM phenytoin, or 100 and 200 μM omeprazole for 48 h. All inducers were dissolved in DMSO, which was added to the serum-free culture media at a final concentration of 0.1% (13.4 mM). Additionally, natural products including garlic (0.1 μg/ml media), quercetin (10 μM), resveratrol (5 μM), apigenin (5 μM), curcumin (5 μM), grapeseed extract (0.6 μg/ml), kava-kava (100 μg/ml), and ginseng (500 μg/ml) were prepared as previously described (Foster et al., 2001). Briefly, extracts were prepared from capsules or tablets obtained from a local vendor. Liquid capsules were opened and the contents emptied into a 1.5 ml microfuge tube, suspended in 3 vol/weight with 55% ethanol, and vortexed for 1 min. The suspension was then centrifuged for 18 min at 13,000 rpm and the ethanolic layer carefully removed. Tablets were crushed with a mortar and pestle and powders dissolved to 25 mg/ml in sterile water, vortexed on high for 1 min, centrifuged for 18 min at 13,000 rpm, and the supernatant carefully removed. The supernatant and the ethanolic layer were dissolved in DMSO at a stock concentration 1000-fold greater than that being tested in the cells. For the flavonoids (resveratrol, quercetin, curcumin, and apigenin) doses were either 5 or 10 μM, depending on the agent, and were dissolved in DMSO at stock concentrations 1000-fold greater than that being used in hepatocytes or HepG2 cells. With exception of quercetin, concentrations above 5 μM for the flavonoids were toxic to the cells (Shih et al., 2000; Williams et al., 2000). In the case of drug or natural product treatments, the media was removed daily and replaced with fresh media containing either inducers dissolved in vehicle or vehicle alone. After treatment, hepatocytes were harvested and microsomes and/or total RNA prepared (Carpenter et al., 1996; Raucy et al., 1997). Protein concentrations were determined with the bicinchoninic acid procedure using bovine serum albumin as the standard (Smith et al., 1985).
Clinical characteristics of human hepatocyte donors
RNA Isolation and Northern Blot Analysis.
Hepatocyte RNA was prepared using RNeasy kits, and quantitated by the absorbance at 260 nm; purity was assessed from the 260:280 nm absorbance ratio and by integrity of the 28s and 18s bands on agarose gels. Total RNA (10 μg) was subjected to electrophoresis on 1% agarose-2.2 M formaldehyde gels, followed by transfer to nylon membranes (Shih et al., 2000). RNA was bound to the membranes using a Stratalinker UV crosslinker (Stratagene, La Jolla, CA), after which the membranes were hybridized with random-primed32P-labeled cDNA probes encoding human CYP3A4 or 18s rRNA. The CYP3A4 probe (273 bp) was previously described (Raucy et al., 2002a) and spanned the CYP3A4 coding region between 110 to 383 bp. Hybridization conditions have been described elsewhere (Allen et al., 2001; Raucy et al., 2002a). DNA-RNA hybridization signals were measured on autoradiograms with a ScanMaker II flat bed scanner (Microtek, Redondo Beach, CA), and the signal intensities integrated using Un-Scan-It software (Silk Scientific, Orem, UT). Hybridization signals obtained with a human 18s rRNA probe (Ambion, Austin, TX) were used to normalize the amounts of RNA loaded onto the gels.
Protein Blot Analysis.
Western blotting of hepatocyte microsomal proteins to nitrocellulose, and subsequent immunochemical staining with 200 μg of anti-peptide CYP3A4 IgG was performed as described elsewhere (Lasker et al., 1998). The properties of the anti-peptide CYP3A4 polyclonal antibodies used for these studies have been reported earlier (Feierman and Lasker, 1996; Raucy et al., 2002a). Hepatocyte CYP3A4 enzyme levels were quantified by first scanning the blots with the ScanMaker II scanner and then integrating immunostaining intensities with Un-Scan-It software.
Construction of Plasmids for Transfections.
The full-length coding region of human PXR was obtained by reverse transcription-polymerase chain reaction as described (Raucy et al., 2002b). Briefly, the 1300 bp amplified product was cloned into pCR2.1 (Invitrogen, Carlsbad, CA) and subjected to sequence analysis. The sequences obtained agreed over the entire coding region with that previously described (Lehmann et al., 1998). The hPXR cDNA was then excised from pCR2.1 by digestion with BamHI andNotI and cloned into analogous sites of a pIRESneo vector (BD Biosciences Clontech, Palo Alto, CA). The CYP3A4 enhancer region containing the proximal and distal promoter integrated into a modified luciferase vector was a gift from Puracyp, Inc. (San Diego, CA). Briefly, the proximal promoter spanned a region from −560 to + 136 and the distal promoter included the region −7894 to −7131 from the transcription start site of the CYP3A4 gene.
Transient Transfections.
Plasmid DNA used in transfection experiments was prepared by purification on Qiagen (Valencia, CA) columns. Reporter constructs, pRL-TK (to control for transfection efficiency), hPXR in pIRES, and control vectors were introduced into HepG2 cells obtained from American Type Culture Collection (Manassas, VA). Hepatoma cells were transfected with lipofectamine 2000 reagent according to manufacturer's procedures. Briefly, HepG2 cells were seeded in 24-well Costar dishes at 3 × 106 cells per well in DMEM containing 10% FBS. Media was replaced with DMEM lacking FBS for transfection. HepG2 cells received 500 ng CYP3A4-reporter construct or 500 ng of control vector, 100 ng hPXR vector, and 100 ng pRL-TK. Five hours following transfection, 1 ml of DMEM containing 20% FBS was added to each well to create a final FBS concentration of 10%. The culture medium was removed after 24 h of incubation with the lipofectamine-DNA complexes, and fresh media containing 10% dextran-treated FBS containing drug or natural product was added. Control cells received media with 0.1% DMSO and cells were treated for 48 h with media changes at 24 h.
Following treatment, HepG2 cells were rinsed in PBS and harvested by adding 100 μl per well of 1X passive lysis buffer contained in a dual-luciferase reporter assay kit (Promega, Madison, WI). Luciferase activity of cell lysates was then determined using a Lumistar galaxy luminometer (BMG Labtechnologies, Offenburg, Germany) and the reporter assay system. Firefly luciferase activities were determined from two independent transfections and normalized against Renillaluciferase activities of the internal control pRL-tk vector obtained from the same culture. Results were expressed as relative light units or -fold increase above control (DMSO-treated cells) ± S.D.
Materials.
cDNA probes to human CYP3A4 were obtained from Puracyp, Inc., and the probe to human 18s rRNA was purchased from Ambion. Restriction enzymes were purchased from New England Biolabs (Beverly, MA), and bicinchoninic acid was obtained from Pierce Chemical Co. (Rockford, IL). RIF, PB, phenytoin, resveratrol, apigenin, curcumin, quercetin, and clotrimazole were purchased from Sigma-Aldrich (St. Louis, MO). Omeprazole was from AstraZeneca (Mölndal, Sweden), and mevastatin and mifepristone were obtained from BIOMOL Research Laboratories (Plymouth Meeting, PA). Natural products were purchased from TruNature (IVC Industries, Inc., Freehold, NJ). Culture dishes were from VWR (Westchester, PA), and FBS was from Hyclone Laboratories (Logan, UT). DMEM and lipofectamine 2000 were from Invitrogen. RNeasy and plasmid purification kits were obtained from Qiagen, and nylon membranes were purchased from Molecular Simulations, Inc. (Westboro, MA). All other reagents used were of the highest quality available.
Data Analysis.
Results are presented as the mean ± standard deviation in the case of two or more samples.
Results
Liver samples used to isolate hepatocytes were obtained from 17 different subjects, 10 of whom were males, ranging in age from 2 to 67 years (Table 1). Hepatocytes isolated from subjects shown in Table 1were placed into primary cultures under defined conditions, and were then used to assess the inductive effects of specific chemical agents on the CYP3A4 enzyme. Constitutive expression in DMSO-treated hepatocytes of CYP3A4 mRNA was 0.69 ± 0.6 AU/μg RNA and ranged from 0.16 to 2.02 AU/μg RNA of CY3A4 mRNA/18s rRNA, the highest expression was in hepatocytes from subject HH975 (2.02 AU/μg RNA) and the lowest from subject HH987 (0.16 AU/μg RNA). Among the 17 samples, CYP3A4 mRNA levels in DMSO-treated hepatocytes did not cosegregate (p < 0.01) with gender or age of the individual donors. Three subjects, HH984, HH977, and HH985, had been exposed to therapeutic agents known to be CYP3A4 inducers. CYP3A4 mRNA levels in DMSO-treated hepatocytes from these three subjects exhibited no significant differences (p < 0.01) in content when compared with CYP3A4 mRNA levels in hepatocytes from individuals that had not been previously exposed to such agents.
Culturing of hepatocytes also revealed that CYP3A4 protein was stable under the culture conditions employed here for at least the first 48 h in culture. Indeed, hepatocytes from three separate subjects (HH988, HH993, and HH984) exhibited CYP3A4 concentrations at 48 h that were 94 ± 6% of the content found in zero time cells (J. L. Raucy and J. M. Lasker, unpublished observations). At 72 and 96 h in culture, CYP3A4 protein levels were 65 ± 18% and 42 ± 33% of those at zero time, respectively. It was also noted that enzyme maintenance was dependent on the addition of low concentrations (10−7 M) of DEX to the culture medium. Deletion of this glucocorticoid resulted in CYP3A4 mRNA contents at 48 h that were 70% of that in cells exposed to 10−7 M DEX. However, if cells were treated with concentrations of DEX greater than 1 μM, notable increases in CYP3A4 mRNA levels were observed (Raucy et al., 2002a). Omission of 0.1 μM DEX from the hepatocyte culture medium also resulted in less dramatic chemical-mediated induction of CYP3A4. Indeed, only a 2-fold increase in CYP3A4 mRNA content upon RIF (10 μM) treatment occurred in the absence of DEX whereas in the presence of 0.1 μM DEX, a 5- to 8-fold increase was observed (Raucy et al., 2002a).
Initially, the effects of RIF treatment on CYP3A4 mRNA levels in primary hepatocyte cultures were examined. Hepatocytes from subject HH928 were treated with various concentrations of RIF ranging from 0.1 to 10 μM, and CYP3A4 mRNA levels assessed by Northern analysis (Fig.1A). CYP3A4 mRNA, when normalized to 18s rRNA, was accumulated to the greatest extent (970% of control) at 10 μM. However, enhanced expression in these hepatocytes was apparent at 0.1 μM (480% of control) followed by a gradual increase in accumulation up to 10 μM (Fig. 1A). Figure 1B represents results from additional hepatocyte samples (HH1002 and HH886). Concentrations ranging from 0.1 to 10 μM determined in HH1002 and HH928 produced a typical dose-response curve similar to that in Fig. 1A, with maximal induction of CYP3A4 mRNA occurring at 10 μM RIF (633 ± 275% of control levels). Doses above 10 μM including 25 and 50 μM, did not produce further increases in CYP3A4 mRNA expression (380 ± 201 and 239 ± 21% of control, respectively) in hepatocyte samples HH886 and HH1002.
A dose-response curve for RIF generated in human hepatocytes.
Panel A, human hepatocytes from subject HH928 were treated with various concentrations of RIF (0.1, 1, 2.5, 5, and 10 μM) for 48 h. Cells were then harvested and RNA isolated. Total RNA (10 μg) was subjected to Northern blot analysis as described under Materials andMethods. The upper blot is a representative Northern blot probed with CYP3A4 cDNA, and the lower panel was probed with 18s rRNA cDNA. Lane 1, RNA from hepatocytes treated with DMSO-treated cells; lane 2, RNA from hepatocytes treated with 10 μM RIF; lane 3, RNA from hepatocytes treated with 0.1 μM RIF; lane 4, RNA from cells treated with 1 μM RIF; lane 5, 2.5 μM RIF; lane 6, 5 μM RIF. Adjacent to the blot is a figure depicting the abundance of CYP3A4 mRNA, which was quantified by scanning signal intensities and normalizing to 18s rRNA. Results are expressed as percent of control. Panel B contains a graph depicting scanned and quantified Northern blots. Results from two separate human liver samples (HH928, HH1002) are represented in the graph for concentrations ranging from 0.1 to 10 μM, and for 25 and 50 μM liver samples were from subjects HH886 and HH1002. Values are expressed as percent of control (DMSO-treated) values ± S.D.
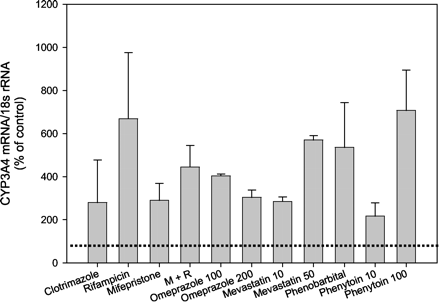
Induction of hepatocyte CYP3A4 by the anti-glucocorticoid, mifepristone, was examined. Cultures from subjects HH995, HH928, and HH997 were treated with 10 μM mifepristone and CYP3A4 mRNA content assessed by Northern analyses. At this concentration, mifepristone produced CYP3A4 levels that were 290 ± 79% of control (DMSO-treated cells) (Fig. 2). To determine whether a synergism or antagonism prevailed between mifepristone and RIF, hepatocytes from HH928 and HH995 were treated with 10 μM RIF and 10 μM mifepristone. The combination of both agents produced a 224% decrease in CYP3A4 mRNA accumulation from that generated by RIF and a 155% increase from that elicited by 10 μM mifepristone (Fig. 2). Such results suggest competition for the nuclear receptor, PXR, between these two agents. The inducibilty of hepatic CYP3A4 mRNA expression by an additional agent, phenytoin, was examined at two concentrations in hepatocytes from subjects HH924 and HH996. Treatment of cells with 10 μM phenytoin produced 217 ± 61% of control CYP3A4 mRNA (Fig. 2). Increasing the dose to 100 μM phenytoin produced 707 ± 188% accumulation of CYP3A4 mRNA over that in control cells. Additional agents were examined for their ability to enhance CYP3A4 mRNA in hepatocytes from HH928, HH995, HH997, HH996, HH921, HH924, HH977, HH975 and HH899. These therapeutics included 10 μM clotrimazole (HH921 and HH995), 10 and 50 μM mevastatin (HH995, HH928, HH997), 1 mM PB (HH996, HH977), and 100 and 200 μM omeprazole (HH996 and HH975) (Fig. 2). Without exception, all agents produced enhanced accumulation of CYP3A4 mRNA above DMSO-treated cells (control). For comparison, hepatocytes from the aforementioned subjects (HH928, HH995, HH997, HH996, HH921, HH924, HH977, HH975, and HH899) were treated with 10 μM RIF, which resulted in a mean increase in CYP3A4 mRNA for the nine samples of 669 ± 307% of DMSO-treated cells. Similar to the induction produced by RIF, omeprazole (100 μM) enhanced CYP3A4 mRNA to 404 ± 8% of control CYP3A4 mRNA levels. Mevastatin also enhanced CYP3A4 mRNA in hepatocytes from subjects HH924 and HH995. A concentration of 10 μM resulted in a modest increase in expression (285 ± 21% of control) whereas 50 μM mevastatin produced much greater induction (570% of control CYP3A4 mRNA) in these same hepatocytes. Despite the 536 ± 207% increase in CYP3A4 mRNA above control produced by 1 mM PB in cells from subjects HH977 and HH996, 100 μM phenytoin emerged as the better CYP3A4 inducer. Clotrimazole (10 μM) elevated CYP3A4 mRNA to 280 ± 197% of control levels in hepatocytes from subjects HH899, HH921, and HH995, suggesting that this antifungal is a weak CYP3A4 inducer when compared with RIF.
The effect of various pharmaceutical agents on CYP3A4 mRNA expression in human hepatocytes.
Hepatocytes were treated with various therapeutics including 10 μM clotrimazole (subjects HH921, HH995, and HH899), 10 μM RIF (HH975, HH977, HH997, HH996, HH928, HH995, HH899, HH921, HH924), 10 μM mifepristone (HH995, HH928, HH997), a combination of 10 μM each of mifepristone and RIF (M and R, HH928, HH995), 100 and 200 μM omeprazole (HH996, HH975), 10 and 50 μM mevastatin (HH995, HH924), 1 mM PB (HH996, HH977), and 10 and 100 μM phenytoin (HH924, HH996). After 48 h of exposure, cells were harvested and RNA isolated. Northern blot analysis was performed on total RNA (10 μg) as described under Materials andMethods. Results are expressed as percent of control (DMSO-treated) values and denote the mean ± S.D. of two to nine separate samples. The dotted line demarcates control (DMSO) values.
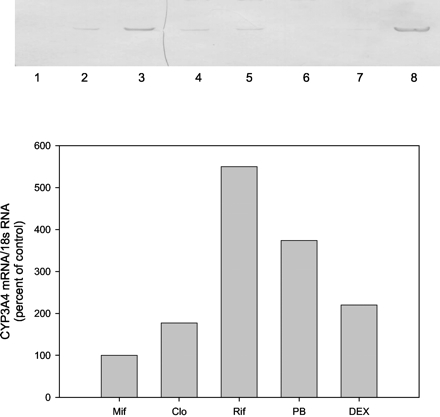
To determine whether CYP3A4 protein levels were also increased by several of the therapeutic agents described above, immunoblot analysis of hepatocytes from subject HH899 were treated with 10 μM each of RIF, clotrimazole, mifepristone, or 1 mM PB. Cells treated with PB, RIF, or clotrimazole exhibited enhanced CYP3A4 protein levels that were 374, 550, and 177% of control concentrations, respectively (Fig.3). Induction of CYP3A4 protein by these agents was reflective of the increases in mRNA. Conversely, CYP3A4 protein levels in hepatocytes treated with 10 μM mifepristone were equivalent to those in cells treated with DMSO; results inconsistent with the 380% enhancement of CYP3A4 mRNA.
Immunoblot analysis of CYP3A4 in microsomes derived from human hepatocytes.
In the top panel, hepatocytes from subject HH899, cultured in serum-free media, were treated with mifepristone (10 μM), clotrimazole (10 μM), RIF (10 μM), PB (1000 μM), and dexamethasone (10 μM) dissolved in DMSO or DMSO alone for 48 h. Treated cells were harvested, microsomes prepared, and microsomal proteins (25 μg) were subjected to Western blotting as described under Materials and Methods. The blot shown in the top panel was developed with previously characterized anti-peptide CYP3A4 IgG (Feierman and Lasker, 1996). Lane 1 contains microsomes from cells treated with mifepristone; lane 2 contains microsomes from hepatocytes treated with clotrimazole; lane 3 contains microsomes of cells treated with RIF; lane 4 represents hepatocytes treated with PB; lane 5 are microsomes from dexamethasone treated cells; lane 6 are hepatocytes cultured in the absence of the dexamethasone supplement and treated with 0.1% DMSO; and lane 7 are cells cultured in media containing 10−7M dexamethasone and treated with 0.1% DMSO. Microsomes (10 μg) prepared from a human liver sample are shown in lane 8. The bottom panel is the quantification of CYP3A4-staining intensity shown in the above immunoblot. Results are expressed as a percentage of control (DMSO-treated) cells.
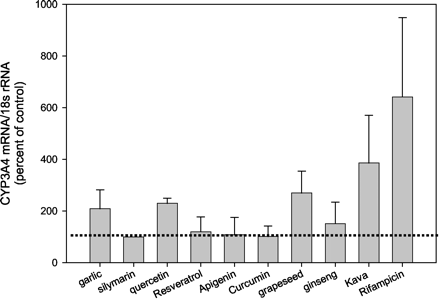
The capacity of flavonoids and natural products to modulate CYP3A4 gene expression was compared in hepatocyte cultures from various individuals (Fig. 4). Hepatocytes from subjects HH986, HH987, and HH977 were treated with quercetin whereas those from HH997 and HH977 were treated with resveratrol, apigenin, and curcumin. Of these agents, only quercetin (5 μM) produced increases (230 ± 73% of control) in CYP3A4 mRNA levels (Fig. 4). When compared with 10 μM RIF (642 ± 307% of control) quercetin was a much weaker inducer of this P450. In hepatocytes from subjects HH987 and HH996, grapeseed extract produced 270 ± 73% of control CYP3A4 mRNA at a concentration of 0.6 μg/ml. The major component of milk thistle, silymarin, did not exhibit inductive properties toward CYP3A4 at concentrations of 5 or 10 μM. Ginseng at a concentration of 500 μg/ml also did not enhance CYP3A4 mRNA content (155 ± 83% of control) in hepatocytes from subjects HH987 and HH997. In contrast, kava-kava caused a 386 ± 185% increase above control CYP3A4 mRNA in hepatocytes from subjects HH996 and HH987. Finally, the effects of garlic on CYP3A4 mRNA were examined in human hepatocytes from subjects HH987 and HH996. At a dose of 0.1 μg/ml, garlic elevated CYP3A4 mRNA to 209 ± 73% of control whereas higher doses produced a decline.
The effect of various botanicals on CYP3A4 mRNA expression in human hepatocytes.
Hepatocytes from various subjects were treated with botanicals or dietary flavonoids including 0.1 μg/ml garlic (HH987, HH996), 10 μM silymarin (HH986, HH987), 10 μM quercetin (HH986, HH987, HH977, HH997), 5 μM resveratrol (HH977, HH997), 5 μM apigenin (HH977, HH997), 5 μM curcumin (HH977, HH997), 0.6 μg/ml grapeseed extract (HH996, HH987), 500 μg/ml ginseng (HH987, HH997), 100 μg/ml kava-kava (HH996, HH987), and 10 μM RIF (HH986, HH987, HH977, HH997, HH996). After 48 h of exposure, cells were harvested and RNA isolated. Northern blot analyses were performed on total RNA (10 μg) as described under Materials andMethods.Results are expressed as percent of control (DMSO-treated) values ± S.D. and denote the mean of determinations from at least two hepatocyte samples. The dotted line demarcates control values.
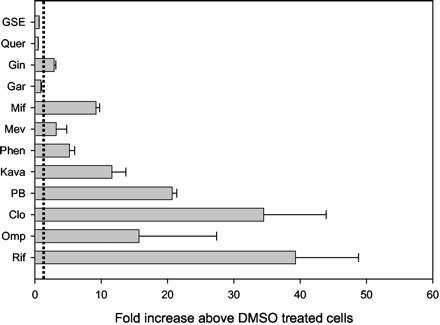
To determine whether the inductive responses observed in human hepatocytes and produced by the above agents were mediated by PXR, transient transfections of HepG2 cells were performed with plasmids containing the hPXR and the proximal and distal CYP3A4 promoters (Goodwin et al., 1999). These plasmids (hPXR and CYP3A4 promoters linked to luciferase) and that containing Renilla were transfected into HepG2 cells and subsequently treated with various inducers. After 48 h of exposure to each chemical, 10 μM RIF and clotrimazole produced the greatest -fold increase in firefly luciferase activity when normalized to Renilla expression (39 ± 9.5- and 34.5 ± 9.4-fold above DMSO treated cells) (Fig.5). When compared with RIF and clotrimazole, 200 μM omeprazole and 1 mM PB were slightly less effective inducers, exhibiting 15.7 ± 1.7- and 20.7 ± 0.7-fold increases in luciferase activity above DMSO-treated cells, respectively. Mifepristone (10 μM) and phenytoin (1 mM) produced luciferase values that were 9.2 ± 0.5- and 5.2 ± 0.8-fold above DMSO-treated cells, respectively, while mevastatin (10 μM) produced responses slightly above control cells (3.2 ± 1.6-fold). With the exception of clotrimazole and phenytoin, results were consistent with elevations in CYP3A4 mRNA evaluated in human hepatocytes. Of the natural products examined, only kava dramatically enhanced luciferase activity (11.6 ± 2.1-fold above DMSO-treated cells) in cells transformed with hPXR and the CYP3A4 enhancer. Dissimilar to elevated mRNA values observed in human hepatocytes treated with garlic, quercetin, and grapeseed extract, treatment of transiently transfected cells with these botanicals resulted in negligible induction (<3-fold above DMSO-treated cells).
The effect of various therapeutics and botanicals on CYP3A4-mediated luciferase activity.
HepG2 cells were transiently transfected with a luciferase plasmid containing the CYP3A4 proximal and distal promoters and an expression vector containing the hPXR cDNA as described under Materials and Methods. Transfected cells were then treated for 48 h with 0.1% DMSO, 10 μM RIF (Rif), 200 μM omeprazole (Omp), 10 μM clotrimazole (Clo), 1 mM PB (PB), 100 μg/ml kava kava (Kava), 1 mM phenytoin (Phen), 10 μM mevastatin (Mev), 10 μM mifepristone (Mif), 0.1 μg/ml garlic (Gar), 500 μg/ml ginseng (Gin), 10 μM quercetin (Quer), and 0.6 μg/ml grapeseed extract (GSE). Luciferase reporter gene expression was determined by normalizing firefly luciferase activity to that of Renilla(firefly/Renilla). Results are the mean ± S.D. of two individual transfections performed in duplicate and are expressed as -fold increase above control (DMSO-treated cells), denoted by the demarcation at 1-fold.
Discussion
Primary cultures of human hepatocytes were used to assess the effects of exemplary CYP3A4 inducers on hepatic expression of CYP3A4. We found that therapeutic agents including RIF, PB, phenytoin, mifepristone, omeprazole, clotrimazole and mevastatin were all capable of enhancing the expression of hepatocyte CYP3A4 mRNA. Of these inducers, RIF, phenytoin, and PB exhibited a dose-response effect. Previous reports (Schuetz et al., 1993; Lehmann et al., 1998) describe cholesterol-lowering agents such as lovastatin as inducers of CYP3A4. Here, we demonstrate for the first time induction of CYP3A4 by another therapeutic also capable of lowering cholesterol, mevastatin. Of the aforementioned inducers of hepatocyte CYP3A4, on a molar basis, RIF produced the greatest increase in CYP3A4 mRNA. The remaining agents at 10 μM produced either weak or moderate accumulation of CYP3A4 mRNA when compared with RIF. We also examined the effects of natural products including dietary flavonoids on hepatocyte CYP3A4 mRNA expression and found that of the flavonoids examined, only quercetin produced induction in human hepatocytes (Fig. 4). Natural products including garlic, grapeseed extract, and kava-kava produced between 200 to 400% increases in hepatocyte CYP3A4 mRNA above that in DMSO-treated cells.
Previous investigations (Li et al., 1995; Luo et al., 2002; Pascussi et al., 2000; Savas et al., 1999) examining CYP3A4 expression in primary hepatocytes have demonstrated increased transcription by RIF, clotrimazole, mifepristone, and omeprazole. Here we demonstrate similar increases by these agents but also show that dose dictates optimal induction. For example, 100 μM omeprazole exhibited a moderate increase (404 ± 8% of control) in CYP3A4 mRNA. However, at 200 μM, CYP3A4 mRNA was only 304 ± 34% of control, suggesting that higher doses of this agent may exhibit some toxicity in cultured cells. Clotrimazole exhibits similar properties in that higher doses result in decreased induction (data not shown). In contrast, RIF, phenytoin, and PB exhibited typical dose response curves. The later agent required a high concentration (1 mM) to produce optimal CYP3A4 mRNA accumulation. The high dose of PB may be necessary to initiate activation of PXR and ultimately enhance CYP3A4 gene expression (Jones et al., 2000; Moore et al., 2000b). Alternatively, it is possible that PB induces CYP3A4 through more than one pathway and thereby requires higher concentrations.
In studies described here using a PXR mediated-reporter gene assay, it was demonstrated that PXR was involved in the induction of CYP3A4 by mifepristone, mevastatin, phenytoin, clotrimazole, RIF, PB, and omeprazole. HepG2 cells transiently transfected with the CYP3A4 promoters but not with the nuclear receptor resulted in negligible increases in luciferase activity when treated with the aforementioned therapeutics. These results are similar to those previously reported for PB, mifepristone, clotrimazole, and RIF (Luo et al., 2002). However, this is the first report describing mevastatin and omeprazole as PXR activators and/or ligands. An advantage of the reporter assay is that such mechanisms of induction can be identified. Importantly, results generated with this assay were for the most part consistent with those obtained in human hepatocytes. The most potent inducers in both systems were RIF, PB, and omeprazole. Similarly, weak-to-moderate inducers in the reporter gene assay and hepatocytes were 10 μM each of mifepristone and mevastatin. Inconsistent with results generated in hepatocyte cultures were those produced by the reporter gene assay for phenytoin and clotrimazole. Clotrimazole exhibited marked induction of luciferase activity (35-fold above DMSO-treated cells) but elicited only 280 ± 197% of control CYP3A4 mRNA levels in hepatocytes. In contrast, phenytoin was a much better CYP3A4 inducer in human hepatocytes (707 ± 188% of control) than in the reporter gene assay (5.2-fold above control cells). Phenytoin may be increasing CYP3A4 content in hepatocytes through indirect PXR activation, which would explain the modest induction observed in the PXR-mediated reporter gene assay. However, the reason for clotrimazole exhibiting such potent increases in luciferase activity but causing only weak CYP3A4 mRNA accumulation in human hepatocytes is presently unclear. Regardless, these results suggest that the reporter gene assays can for the most part, be used to predict PXR-mediated induction of CYP3A4 in human hepatocytes.
In addition to therapeutic agents, we also examined the effects of natural products including dietary flavonoids on CYP3A4 expression. We previously demonstrated that certain flavonoids exhibited induction of CYP1A1 (Allen et al., 2001). These included resveratrol, kaempferol, and apigenin. Of these flavonoids, resveratrol produced the greatest increase in hepatocyte CYP1A1 mRNA whereas the other two agents produced weak-to-moderate increases in accumulation. Employing the CYP1A1 reporter gene assay described previously (Allen et al., 2001), these flavonoids were identified as Ah receptor ligands. In studies presented here, only quercetin enhanced expression of CYP3A4 mRNA in hepatocyte cultures when compared with other flavonoids. In contrast to the reporter gene assay for detecting CYP1A1 inducers, this flavonoid did not increase luciferase activity mediated by PXR and the CYP3A4 promoters, suggesting that quercetin does not produce CYP3A4 induction through the PXR. Alternatively, the lack of enhanced luciferase activity, but induction of CYP3A4 mRNA in hepatocytes by quercetin, may be due to a metabolite rather than quercetin itself. In HepG2 cells, expression of many enzymes is minimal. This low level of expression may limit metabolism of certain compounds including flavonoids and hence prohibit production of an active metabolite that possesses the ability to activate/ligand PXR and induce CYP3A4. Additionally, activation of PXR through indirect factors may also occur with flavonoids and these factors may be absent in HepG2 cells. Finally, the level of sensitivity of reporter gene assays may not be applicable to that in human hepatocytes.
With regards to the natural products examined for their ability to induce CYP3A4 in hepatocytes, grapeseed extract, garlic, and kava-kava-enhanced CYP3A4 mRNA accumulation. Based on studies with St. John's wort, it was anticipated that PXR might be involved (Moore et al., 2000a). However, only kava-kava increased gene expression in cells transiently transfected with PXR and the CYP3A4 promoters linked to luciferase (11-fold above DMSO-treated cells). Of the remaining herbals, grapeseed extract contains flavonoids and therefore may induce CYP3A4 by mechanism(s) similar to that of quercetin. The mechanism by which garlic enhances CYP3A4 was not obvious from experiments performed here but may involve activation of PXR through indirect mechanisms or require factors other than PXR. The optimal dose for induction by these botanicals may also be different from that in hepatocytes. That garlic enhances CYP3A4 mRNA expression clarifies clinical observations (Piscitelli et al., 2002) describing increased metabolic elimination of the HIV protease inhibitor, saquinavir, a CYP3A4 substrate, in patients consuming both the anti-retroviral and garlic simultaneously.
In summary, primary cultures of human hepatocytes were employed to demonstrate inducibility of CYP3A4 by both therapeutic agents and natural products. The natural products examined here consisted of several dietary flavonoids, garlic, silymarin, ginseng, kava-kava, and grapeseed extract. Of the flavonoids examined only quercetin and grapeseed extract increased CYP3A4 mRNA levels above 2-fold. Of the remaining natural products, all but silymarin and ginseng enhanced CYP3A4 mRNA accumulation in hepatocytes 2-fold or greater. Interestingly, while all of the therapeutic agents enhanced reporter gene activity mediated by CYP3A4 promoters and PXR, none of the flavonoids and only one of the natural products, kava-kava, increased luciferase expression. These findings suggest that induction by several herbals may occur through mechanisms other than PXR activation. Thus, the mechanism involved in CYP3A4 induction in hepatocytes by certain botanicals and dietary flavonoids remains to be determined. Because many botanicals increase expression of this important drug-metabolizing enzyme in human hepatocytes, caution is suggested when such products are administered simultaneously with therapeutic agents.
Footnotes
-
This research was supported by National Institutes of Health Grants GM49511 and AA08990 and by the Liver Transplant, Procurement, and Distribution System (DK62274).
- Abbreviations used are::
- P450
- cytochrome P450
- HIV
- human immunodeficiency virus
- PB
- phenobarbital
- PXR
- pregnane X receptor, which is also referred to as the steroid and xenobiotic receptor (SXR)
- HMM
- hepatocyte maintenance media
- RIF
- rifampicin
- DMSO
- dimethyl sulfoxide
- bp
- base pair(s)
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- DEX
- dexamethasone
- Received September 25, 2002.
- Accepted January 21, 2003.
- The American Society for Pharmacology and Experimental Therapeutics