Abstract
MRP2 (ABCC2) is an ATP-binding cassette (ABC)-type membrane protein involved in transport of conjugates of various drugs and endogenous compounds. MRP2 has been localized to the apical membrane of syncytiotrophoblasts and is assumed to be involved in diaplacental transfer of the above substances. It has been shown that both genetic and environmental factors can influence MRP2 expression. We therefore investigated whether gestational age, cellular differentiation, and genetic polymorphisms influence expression and localization of MRP2 in 58 human placenta samples. We detected a significant increase of transporter-mRNA with gestational age by quantitative real-time polymerase chain reaction (MRP2 mRNA/18S rRNA ratio × 1000 ± S.D.; 0.43 ± 0.13 in early preterms versus 1.18 ± 0.44 in late preterms versus 2.1 ± 0.63 in terms; p < 0.05). MRP2 protein followed the mRNA amount as shown by Western blotting (mean relative band intensity ± S.D.; 0.56 ± 0.1 versus 0.7 ± 0.18 versus 0.92 ± 0.19; early preterms versus terms p < 0.05). In cultured cytotrophoblasts, MRP2 expression increased with differentiation to syncytiotrophoblasts, with a peak on day 2 (MRP2 mRNA/18S rRNA ratio × 1000 ± S.D.; 0.06 ± 0.01 versus 0.88 ± 0.27 versus 0.24 ± 0.02 on days 0, 2, and 4). Moreover, we studied the effect of single nucleotide polymorphisms (C–24T; G1249A, and C3972T) in the MRP2 gene on placental expression. One of these polymorphisms (G1249A) resulted in a significantly reduced expression of MRP2 mRNA in preterms. In summary, the expression of MRP2 in human placenta is influenced by gestational age, cellular differentiation, and genetic factors.
Human placenta is the feto-maternal interface during pregnancy and functions as the main barrier between maternal and fetal circulation. The exchange of compounds between mother and child is facilitated by a single-layered syncytium, the syncytiotrophoblast. These polarized cells are attached to the outer layer of the terminal villi, consisting of fetal vessels and the villous stroma. During pregnancy, the functional multinuclear syncytiotrophoblast is formed from fusion of cytotrophoblasts. The latter cells loose their proliferative activity during the maturation process (Kingdom et al., 2000). Therefore, the fraction of cytotrophoblasts undergoing differentiation decreases with increasing gestational age. In vitro experiments using isolated cytotrophoblasts in cell culture showed spontaneous differentiation into syncytiotrophoblasts (Kliman et al., 1986).
The ATP-binding cassette (ABC) superfamily of membrane transporters is a family of proteins known to be involved in the organization of functional barriers in human organisms. The unidirectional ATP-dependent transport of substances of physiological and pharmacological relevance contributes to this function. The protective function of placenta and, especially, of the syncytiotrophoblasts against xenobiotics affecting fetal development is based at least in part on these transport proteins since they prohibit maternofetal transfer of potentially toxic compounds (Young et al., 2003).
Different members of the ABC superfamily are expressed in human placenta. These include proteins, such as the MDR1 (ABCB1) gene product P-glycoprotein, the breast cancer resistance protein (BCRP, ABCG2), and several members of the MRP (ABCC) subfamily, such as MRP5 and MRP2 (ABCC2) (MacFarland et al., 1994; Mylona et al., 1996; Nakamura et al., 1997; St Pierre et al., 2000; Maliepaard et al., 2001; Pascolo et al., 2003; Meyer zu Schwabedissen et al., 2005). The latter transporter had initially been designated as canalicular multispecific organic anion transporter (cMOAT). MRP2 is involved in the hepatobiliary excretion of conjugated bilirubin and conjugated drugs, which are metabolites of the phase II biotransformation. Moreover, MRP2 facilitates transport of anticancer agents, including cisplatin, vinblastin, and camptothecin derivatives (Borst et al., 2000).
In previous studies it has been shown that the Dubin-Johnson syndrome, an autosomal-recessive disorder, is linked to the absence of MRP2 in human liver resulting in symptomatic conjugated hyperbilirubinemia. Besides the known mutations in Dubin-Johnson syndrome, a number of single nucleotide polymorphisms have been recently reported, which do not cause the Dubin-Johnson phenotype (Gerk and Vore, 2002).
In summary, MRP2 can function as an important protective mechanism in human placenta. Whether environmental and/or genetic factors affect MRP2 expression in this tissue is unknown. We therefore investigated the influence of gestational age, genetic polymorphisms, and cellular differentiation on the expression and localization of MRP2 in placenta. In this paper we demonstrate a significant reduced expression in preterm placenta, increased expression during in vitro differentiation, and an influence of the G1249A polymorphism.
Materials and Methods
Materials. Chorionic villous tissues were obtained from women undergoing cesarean sections and normal birth. A total of 58 samples from preterm (n = 26) and term placentas (n = 32) were included in the present study. Written informed consent was obtained in each case. The study was approved by the local ethical committee. Following the World Health Organization definition, pregnancies with duration less than 37 weeks of gestation were defined as preterm. The group of preterms was divided into early (before 32 weeks gestation) (n = 10) and late preterm (32 to 37 weeks gestation) (n = 16). Demographic data are summarized in Table 1. Samples for isolation of cytotrophoblasts were taken from term placentas of normal deliveries (n = 4).
Demographic data
Data are expressed as mean ± S.D.
Cytotrophoblast Culture. Cytotrophoblasts were isolated as described by Kliman et al. (1986). After mechanical and enzymatic dissection of placental tissue of different term placentas (n = 4), cytotrophoblasts were separated using density gradient centrifugation at 1500g for 45 min on discontinuous Percoll-gradient (20–70%). Cells between the 40% and 50% Percoll bands were collected and plated onto 35-mm2 culture dishes at a density of 5 × 106 cells/dish. The cells were grown in M199 medium, supplemented with 10% fetal calf serum, and 100 units/ml penicillin/streptomycin and 5 ng/ml epidermal growth factor, in a 5% CO2 humidified atmosphere at 37°C. The cells were maintained in culture for 5 days. Every day one dish was harvested; the cells were scraped off the plates and collected by centrifugation at 800g at 4°C for 5 min.
Control of the Purity of the Isolated Cytotrophoblasts. The purity of the isolated cells was assessed using immunofluorescent staining of the cultured cells with an anti-cytokeratin 18 (Sigma-Aldrich, Taufkirchen, Germany) and an anti-vimentin antibody. Cells were cultured on coverslips for 3 days. After rinsing, the cells were fixed 15 min in 4% paraformaldehyde, washed three times with PBS (pH 7.4), and permeabilized with 0.1% Triton X-100. Cells were then washed several times with PBS and incubated in 5% fetal calf serum, followed by incubation over night at 4°C with the primary antibodies (anti-cytokeratin diluted 1:800 or anti-vimentin diluted 1:200). After several washing steps with PBS, the cells were incubated 1.5 h with the fluorescent-labeled anti-mouse and anti-goat antibodies (Molecular Probes, Eugene, OR), diluted 1:200. After that, the labeled cells were mounted on slides using DakoCytomation anti-fading mounting medium (DakoCytomation, Hamburg, Germany). Staining was detected using confocal laser scanning microscopy.
Real-Time Quantitative Reverse Transcription (RT)-PCR. RNeasy Mini extraction kit (QIAGEN, Frankfurt, Germany) was used for RNA extraction from 32 term and 26 preterm placentas and cytotrophoblasts of four different preparations. In brief, villous material was separated and frozen in liquid nitrogen. The tissue was mechanically homogenized using a microdismembranator (1 min, 2500 rpm). Subsequently, 60 mg of the tissue powder were incubated with guanidinium thiocyanate containing buffer and centrifuged through a membrane system. Binding at a silica gel membrane was performed according to the manufacturer's instructions. Finally, the integrity of the RNA was controlled by ethidium bromide staining in a formaldehyde-containing 1% agarose gel.
The isolated and purified polyA-RNA was reverse-transcribed using Random Hexamere primer and the TaqMan RT kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was used for establishing the mRNA amount. A predeveloped primer and probe mix was used for detection of 18S ribosomal RNA (Applied Biosystems). The MRP2 primer sequences were designed as intron-spanning based on the cDNA sequence published under GenBank accession number XM_083829. In detail, primer sequences for quantification of MRP2 were 5′-CTGGGAACATGATTAGGAAGC-3′ and 5′-GAGGATTTCCCAGAGCCGAC-3′, and the fluorescent-labeled probe sequence 5′-(FAM)CAFTCCGAGATGTGAACCTGGACAT-XTp. Calibration curves for MRP2 mRNA and 18S rRNA quantifications were prepared by several dilutions of cDNA-constructs ligated to pGEM-T-Easy (Promega GmbH, Mannheim, Germany) transformed to Escherichia coli KL-10-Gold (Stratagene, La Jolla, CA), with amounts ranging from 1 × 107 to 1 × 101 copies for MRP2 and 1 × 109 to 1 × 103 for 18S.
The RT-PCRs were set up in a reaction volume of 50 μl containing 15 ng or 0.15 ng of cDNA for amplification of the MRP2 mRNA or 18S rRNA, respectively. Amplification of the PCR products was performed using the ABI Prism 7700 sequence detector, a real-time PCR cycler (Applied Biosystems), and the Universal PCR mastermix supplied by Applied Biosystems. The fluorescence intensities of the probes were plotted against PCR cycle numbers. The amplification cycle displaying the first significant increase of the fluorescence signal was defined as threshold cycle (CT). The CT value of each sample was compared to the CT values of the standard series, which consisted of the cloned PCR fragment resulting in a quantification of copy numbers' mRNA. The fragment inserted in a common used vector was sequenced before using it as standard showing no differences from the published cDNA sequence (GenBank: accession number XM_083829) (data not shown).
Immunoblot Analysis of Crude Membrane Fractions of Term and Preterm Placentas. Crude membrane fractions were isolated from 29 placental homogenates using a subset of 17 term and 12 preterm placentas. Frozen tissue was homogenized using a Braun-Elvejhem homogenizer in homogenization buffer (10 mM Tris/HCl, 250 mM sucrose, and 1 mM EDTA) supplemented with protease inhibitors (0.1 mM phenylmethylsulfonyl fluoride, 0.3 μM aprotinin, and 0.1 μmol pepstatin). After gradual centrifugations at 9000g and 100,000g, the crude membrane fraction of placental tissue was resuspended in 50 μl of 5 mM Tris/HCl (pH 7.4). Aliquots were denatured in SDS-PAGE sample buffer at 37°C for 30 min. After incubation, the membrane proteins were separated by SDS-PAGE and electrotransferred onto nitrocellulose using a tank blotting system (Bio-Rad, München, Germany). Membrane preparation from human liver was used as positive control. For detection of MRP2-reactive bands, the primary anti-MRP2 antibody M2 I-4 (Alexis Corporation) and the secondary horseradish peroxidase-conjugated goat anti-mouse IgG antibody (Bio-Rad) were used. The antibodies were diluted in Tris-buffered saline containing 0.05% Tween 20 and 1% bovine serum albumin to a final concentration of 1:1000 for the MRP2 and 1:2000 for the secondary antibody. The immobilized antibodies were stained using an enhanced chemiluminescence (ECL) system (Amersham Biosciences, Freiburg, Germany) and exposed to X-ray films. After digitalization, band intensities were analyzed using Kodak ImageQuant Software (Eastman Kodak, Rochester, NY). The crude membrane fraction of a term placenta was used for relative quantification. The relative intensity of the samples to this internal standard represents the amount of protein.
Preparation of Apical and Basal Membrane Fractions. Apical and basal membrane fractions were isolated from two term and two late preterm placentas. First, placental cotyledons were washed several times with ice-cold PBS, after which, 20 g of the tissue were minced and washed again. Tissue was homogenized using a Braun-Elvejhem homogenizer (20 strokes, 1000 rpm) in incubation buffer (250 mM sucrose, 10 mM Tris/HCl, and 1 mM EDTA, pH 7.4) supplemented with protease inhibitors (0.1 mM phenylmethylsulfonyl fluoride, 0.3 μM aprotinin, and 0.1 μmol of pepstatin). After that, the homogenate was incubated for 1 h on ice under continuous stirring. After centrifugation at 9000g, the supernatant was centrifuged at 100,000g. The pellets containing the membranes were resuspended in incubation buffer and homogenized with a loose-fitting Dounce B homogenizer. For separation of basal and apical membranes, MgCl2 was added to a final concentration of 10 mM and centrifuged at 2000g after a 10-min incubation on ice. The separated membranes, apical fraction (supernatant) and the basal fraction (pellet), were resuspended in incubation buffer and homogenized with a tight-fitting Dounce B homogenizer (30 strokes). After centrifugation at 100,000g, the membranes were homogenized using the tight-fitting Dounce B homogenizer and centrifuged at 100,000g. After that, the pellets of both membrane fractions were resuspended in 1 ml of homogenization buffer, and frozen and stored in liquid nitrogen.
Laser Scanning Confocal Microscopy. Human chorionic villi of mature and premature placenta (41st and 26th week of gestation, respectively) were fixed in formalin after delivery and embedded in paraffin. Sections of 2 μm thickness were prepared on Superfrost Plus slides and dried overnight at 60°C. The paraffin-embedded sections were deparaffinized in two changes of xylene substitute for 10 min each. After that, the slides were incubated in ethanol of declining concentrations from 100% to 50% for 5 min and rinsed in distilled water two times. Heat-induced epitope retrieval was performed by boiling the tissue sections in citrate buffer (10 mM, pH 6.0) for 15 min. After several washing steps in cold PBS (pH 7.4), the sections were blocked in 2% BSA. Sections were incubated with primary anti-MRP2 antibody M2 III-6 (Alexis Corporation) overnight at room temperature in a humidified atmosphere. After several washing steps with PBS, the sections were incubated with a fluorescent-labeled secondary antibody against mouse IgG2α (Molecular Probes). After mounting in anti-fading mounting medium (DakoCytomation), the fluorescence was detected by laser scanning confocal microscopy. As control for background signals, sections were incubated with the secondary antibody only and scanned under the same conditions.
Restriction Fragment Length-Polymorphism. For studying the influence of single nucleotide polymorphisms (SNPs) on MRP2 mRNA expression, 58 samples of human placentas were screened for known polymorphisms. After mechanical tissue homogenization, genomic DNA was extracted using a QIAGEN Tissue Kit (QIAGEN), as described by the manufacturer. A total of 100 ng of the genomic DNA were amplified using primers (Table 2) flanking the regions of previously described SNPs (Ito et al., 2001). These primers were used for amplification of a 301-bp fragment in the 5′-flanking region containing the C–24T variant of a 260-bp fragment in exon 10 containing the G1249A variant, and of a 184-bp fragment in exon 28 containing the 3972 polymorphism. PCR was carried out in a volume of 25 μl containing 200 nM concentration of each primer, 10× PCR Buffer, 2.5 mM MgCl2, 1 mM deoxynucleoside-5′-triphosphate mix, and 1.25 to 2.5 U of TaqPolymerase. After amplification, the PCR products were digested using sequence-dependent restriction endonucleases. Restriction products were identified after electrophoretic separation in a 2% agarose gel and staining by ethidium bromide.
Primers used for amplification of fragments of the MRP2 gene containing previously described SNPs
Statistical Analysis. The amounts of mRNA and protein were compared using Student's t test for two independent groups. p < 0.05 was considered as statistically significant. In addition, the statistical difference was tested using the Kruskal-Wallis test of ranking, where indicated.
Results
MRP2 mRNA Expression in Total Tissue of Human Placenta. To study the influence of gestation on the expression of MRP2 in human placenta, we compared the MRP2 mRNA level in 32 term and 26 preterm placentas. The group of preterm placentas was divided into early (n = 10) and late (n = 16) preterms. The early preterm period was determined to be <32 weeks of gestation, since this gestational age is presumed to be clinically significant in preterm mortality.
The isolated and transcribed RNA was amplified by quantitative real-time PCR. The quantification of MRP2 mRNA and 18S rRNA was performed using cloned PCR fragments. The measured number of MRP2 mRNA copies was normalized to that of 18S rRNA. The normalized MRP2 mRNA amount in term placentas was significantly higher than those of the early and late preterms (Fig. 1). Term placentas showed about 1.8-fold higher mean expression than late preterm placentas and about 4.4-fold higher MRP2 expression than early preterms (mean MRP2 mRNA/18S rRNA ratio ± S.D.; 2.10 ± 0.24 versus 1.18 ± 0.35 versus 0.48 ± 0.17). We did not observe an influence of the gender of the fetus on the mRNA amount either in the group of preterm or the group of term placentas (data not shown).
Protein Expression of MRP2 in Human Term and Preterm Placenta. MRP2 protein was detected by immunoblot analysis in 13 term and 16 preterm placentas. For analysis, crude membrane fractions were prepared, separated by SDS-PAGE, and electrotransferred to nitrocellulose filters. Immunoblot analysis, performed using the monoclonal anti-MRP2 antibody (M2 I-4), yielded a double band at approximately 180 kDa (Fig. 2). These bands were detectable in every placental sample. As positive control, human liver was used. For quantification, an internal standard consisting of a term placenta crude membrane extract was loaded on every analytical gel; band intensity of the major band of other placental samples was related to this standard. The relative band intensity of MRP2 protein was significantly reduced in early preterm placentas (mean relative band intensity ± S.D.; 0.57 ± 0.1) compared with that of term placentas (mean relative band intensity ± S.D.; 0.92 ± 0.18; Student's t test, p < 0.05). The comparison of term and late preterm placentas showed no statistically significant difference (Fig. 2D).
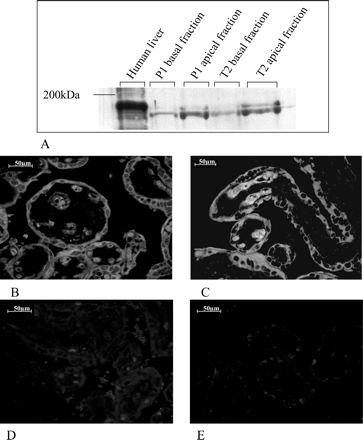
For determination of the localization of MRP2, immunoblot analysis was performed using membrane preparations enriched in apical or basal fraction of two term and two preterm placentas. In agreement with the data shown by St. Pierre et al. (2000), the abundant protein expression of MRP2 was found in the apical membrane fraction (Fig. 3A).
Immunofluorescent Staining of MRP2 in Human Placenta. To study whether the effect of lower protein expression is accompanied by a different localization of the MRP2 protein in human preterm placenta, we performed immunofluorescent staining of paraffin-embedded placenta sections. As shown in Fig. 3, B and C, the immunofluorescent staining was less pronounced in preterm placentas. Localization of MRP2 in the placental villi surrounding syncytiotrophoblasts was not changed by gestational age.
Expression of MRP2 as a function of gestational age. MRP2 mRNA amount was determined by real-time PCR and normalized to 18S rRNA amount. Preterm placentas of a gestational age lower than 32 weeks of pregnancy (left column) show a significantly (*1, p < 0.05, Student's t test) lower expression of the transport protein than preterm placentas of a gestational age between 32 and 37 weeks (middle column). Moreover, there is a statistically significant increase of the mean MRP2 mRNA expression in term placentas (right column) compared to early preterm placentas or late preterm placentas (*1, p < 0.05, and *3, p < 0.001, respectively). Data are expressed as MRP2 mRNA/18S rRNA ratio (× 1000) (mean values ± S.D.; early preterm placentas, n = 10; late preterm placentas, n = 16; term placentas, n = 32).
Immunoblot analysis of the MRP2 amount in placental samples. Crude membrane fractions were separated by SDS-PAGE and transferred to nitrocellulose filters. Detection was performed using an anti-MRP2 antibody M2 I-4 (Alexis Corporation) at a dilution of 1:1000. A shows staining of protein extract of early preterm placentas and B, that of term placentas. Moreover, C shows staining of preterm and term placentas. Band intensity was analyzed using Kodak ImageQuant Software and was normalized to the internal standard (IS) consisting of a crude membrane preparation of a term placenta. Human term placentas show a significantly (★, p < 0.05, Student's t test) higher expression of MRP2 than preterm placentas (D). Data are expressed as mean relative band intensity ± S.D. As positive control, human liver (HL) was used.
Detection of the localization of MRP2 in human placenta. A shows an immunoblot analysis of MRP2 in basal and apical membrane preparations of human term (T2) and late preterm (P1) placentas. Human liver was used as positive control. MRP2 is enriched in the fraction of apical membranes. Immunofluorescent staining of paraffin sections of placenta shows the localization in the syncytiotrophoblasts. Term placentas show an intensive staining, whereas the immunofluorescent signal (B) in human preterm placentas appears to be less intensive (C). Control staining with the secondary anti-mouse antibody was performed in term (D) and preterm (E) placenta.
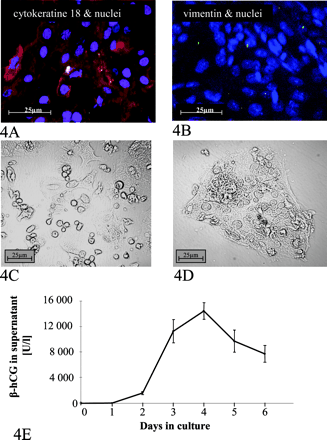
MRP2 mRNA Expression in Cultured Cytotrophoblasts. For determination of the influence of cellular differentiation of cytotrophoblasts on the expression of MRP2, the mRNA amount was determined in cultured cytotrophoblasts and normalized to the amount of 18S rRNA. MRP2 mRNA was detected in all cytotrophoblast samples. Spontaneous cellular differentiation to syncytiotrophoblasts was observed by light microscopy after the first day (Fig. 4, C and D). Purity of the isolate was controlled by staining with an anti-cytokeratin 18 antibody (Fig. 4A) and staining with an anti-vimentin antibody (Fig. 4B). Moreover, the maturation of these cells was determined by the biochemical marker β-hCG, a hormone that is secreted by the multinuclear syncytium. As shown in Fig. 4E, the cells secreted increasing amounts of this hormone during culture. With maturation, the MRP2 mRNA amount increased and decreased (Fig. 5) independently of the respective time course of the β-hCG level in supernatant. The peak of MRP2 mRNA at the second day of culture (MRP2 mRNA/18S rRNA ratio × 1000 ± S.D.; 0.88 ± 0.27) was 15.2-fold higher than that of uncultured cytotrophoblasts (MRP2 mRNA/18S rRNA ratio × 1000 of 0.06 ± 0.01; Student's t test, p < 0.05) and decreased from day 2 to day 5 (Fig. 5).
MRP2 Protein Amount in Cultured Cytotrophoblasts. Since cultured cytotrophoblasts showed an increase in MRP2 mRNA amount during differentiation, we studied whether this induction is accompanied by a change in the amount of MRP2 protein. Syncytiotrophoblasts were harvested from culture after 72 h and 144 h. The MRP2 protein amount of maturated cells was compared with cytotrophoblasts of the same individual by immunoblot analysis. MRP2 expression is increased in syncytiotrophoblasts compared to the cytotrophoblasts, the progenitor cells, which did not show a subsequent decrease parallel to the reduced amount of mRNA (Fig. 5, inset).
Purity of isolated and cultured cytotrophoblasts was controlled by staining of maturated cells with an anti-cytokeratin 18 antibody (A). Moreover, the cells were stained with an anti-vimentin antibody (B). Differentiation of the progenitor cells was established by light microscopy after 12 h (C) and 144 h (D) in culture, showing cell fusion and formation of a multinuclear syncytium (D). Maturation of cytotrophoblasts in culture was controlled by measuring the amount of β-hCG in the supernatant. Data are expressed as mean value ± S.D. from four different isolations. As shown in E, the level of this pregnancy hormone, a biochemical marker of the activity of syncytiotrophoblasts, increases with differentiation of these cells in vitro.
MRP2 Genotyping. Three of the five SNPs previously reported in a Japanese population were found in our placental samples of European individuals. Twenty-one placentas were heterozygous for the C to T transition 24 bases upstream from the initial codon (Table 3). Moreover, 5 homozygotes and 16 heterozygotes of the missense mutation G1249A (exon 10) were identified. This polymorphism causes a substitution of valine417 by isoleucine. Interestingly, the frequency of homozygote G 1249 carriers with 0.8 is higher in preterm placentas than in term placentas (identified frequency 0.5) (Table 3). The third SNP found in the examined samples was C3972T (exon 28). Nine homozygotes and 27 heterozygotes have been identified (Table 3). This variation at codon 1324, however, causes no missense since both triplets encode isoleucine.
C–24T G1249 (Val417Ile) and C3972T variants of the MRP2 gene in the population and in terms and preterms (preterm data in parentheses)
Genotype of Position –24 and MRP2 mRNA and Protein Amount. The amount of MRP2 mRNA expression established as described above and normalized to 18S rRNA showed no difference concerning –24C>T, regardless of gestational age of the placenta [MRP2/18S ratio × 1000 ± S.D.; terms: –24 CC 2.11 ± 0.34 (n = 20) and –24 CT 2.12 ± 0.35 (n = 12); Kruskal-Wallis test, χ2 = 0.133; df = 1; p = 0.715; preterms: –24 CC 1.00 ± 0.26 (n = 17) and –24 CT 0.54 ± 0.24 (n = 9); Kruskal-Wallis test: χ2 = 0.384; df = 1; p = 0.535]. Moreover, there was no effect of the –24C>T on the protein expression of MRP2 compared to the wild type [mean relative band intensity ± S.D.; terms, –24 CC 0.91 ± 0.08 (n = 8); and terms, –24 CT 0.91 ± 0.19 (n = 5); Kruskal-Wallis test: χ2 = 0.05; df = 1; p = 0.826; preterms, –24 CC 0.74 ± 0.08 (n = 10); and preterms, –24 CT 0.52 ± 0.15 (n = 6); Kruskal-Wallis test: χ2 = 1.428; df = 1; p = 0.232]. In summary, we did not find any influence of the genotype at position –24 in the 5′-untranslated region of the transporter gene on the expression of MRP2 in human placenta..
Detection of MRP2 expression in cultured cytotrophoblasts. Analysis of the amount of MRP2 mRNA normalized to 18S rRNA shows an increase of the transporter within the first 2 days of culture followed by a reduction (mean MRP2 mRNA/18S rRNA ratio × 1000 ± S.D.; n = 4 different isolations). In addition, the amount of MRP2 protein was determined by Western blot analysis. Comparing the expression at days 3 and 6 with that of cytotrophoblasts shows that the maturation of these progenitor cells is accompanied by increasing levels of MRP2 expressed in the membrane (inset).
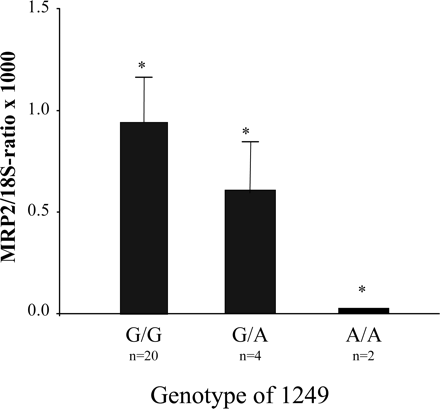
Influence of the Missense Mutation of Position 1249 on the Expression of MRP2. In addition, the missense SNP 1249 G>A in exon 10 was investigated in relation to the MRP2 mRNA expression. The amount of mRNA in preterm placentas is significantly reduced in carriers of the variant allele (1249 AA and 1249 GA) in comparison to those carrying GG at position 1249 [MRP2 mRNA/18S rRNA ratio × 1000 ± S.D.; preterms: 1249 GG, 0.03 ± 0.02 (n = 21); preterms: 1249 GA, 0.6 ± 0.28 (n = 3); preterms: 1249 AA, 0.95 (n = 2); Kruskal-Wallis test: χ2 = 4.08; df = 2; p = 0.044] (Fig. 6). In term placentas, there was no statistically significant difference in expression of the MRP2 mRNA in relation to genotype [MRP2 mRNA/18S rRNA ratio × 1000 ± S.D.; terms: 1249 GG, 1.62 ± 0.26 (n = 16); terms: 1249 GA, 2.5 ± 0.39 (n = 13); terms: 1249 AA, 2.92 ± 1.30 (n = 3); Kruskal-Wallis test: χ2 = 3.768; df = 2; p = 0.152].
Protein expression of MRP2, which has been established in a subset of the placental samples, did not differ in relation to the G1249A genotype [mean relative band intensity ± S.D.; terms: 1249 GG, 0.77 ± 0.28 (n = 6), and terms: 1249 GA, 1.00 ± 0.30 (n = 7); Kruskal-Wallis test: χ2 = 1.479; df = 1; p = 0.224]. However, homozygote AA carriers had a slightly lower protein amount in preterm placentas (mean related band intensity ± S.D.; preterms: 1249 GG, 0.67 ± 0.24 (n = 12) versus preterms: 1249 GA, 0.56 (n = 2) versus preterms: 1249 AA, 0.47 (n = 2); Kruskal-Wallis test: χ2 = 1.895; df = 2; p = 0.428].
Genotype of position 1249, which is located to exon 10 of the MRP2 gene, related to the normalized MRP2 mRNA amount in human preterm placentas. The amount of MRP2 mRNA is decreased in preterm placentas carrying the mutant allele (A) in position 1249 compared to those harboring the homozygous wild-type genotype (1249 GG). Data are expressed as mean MRP2 mRNA/18S rRNA ratio ± S.D.; *, p < 0.05 (Kruskal-Wallis test).
Influence of the C to T Genotype in Position 3972 on the Expression of MRP2. Testing the influence of C3972T on the MRP2 mRNA level showed no difference in preterm [MRP2 mRNA/18S rRNA ratio ± S.D.; preterms: 3972 CC, 0.90 ± 0.37 (n = 11) versus preterms: 3975 CT, 0.90 ± 0.25 (n = 11) versus preterms: 3972 TT, 0.48 ± 0.39 (n = 4); Kruskal-Wallis test: χ2 = 0.690; df = 2; p = 0.708] or term placentas (MRP2 mRNA/18S rRNA ratio ± S.D.; terms: 3972 CC, 2.39 ± 0.52 (n = 11) versus terms: 3972 CT, 2.08 ± 0.31 (n = 16) versus terms: 3972 TT, 1.63 ± 0.52 (n = 5); Kruskal-Wallis test: χ2 = 0.828; df = 2; p = 0.661]. Protein expression of MRP2 tended to be lower in those carrying the variant genotype. In term placentas, protein expression of MRP2 tended to be lower in 3972 TT carriers than in wild types [mean relative band intensity ± S.D.; terms: 3972 CC, 0.94 ± 0.1 (n = 4); terms: 3972 CT, 1.08 ± 0.10 (n = 6); terms: 3972 TT, 0.65 ± 0.08 (n = 3); Kruskal-Wallis test: χ2 = 3.912, df = 2, p = 0.141], similar to the trend in preterm placentas [mean band intensity ± S.D.; preterms: 3972 CC, 0.74 ± 0,08 (n = 7); preterms: 3972 CT, 0.72 ± 0.19 (n = 5); preterms: 3972 TT, 0.43 ± 0.14 (n = 4); Kruskal-Wallis test: χ2 = 2.694, df = 2, p = 0.260].
Discussion
In this study, we show a significant increase of MRP2 mRNA and protein amounts (Figs. 1 and 2) as a function of gestational age. Knowledge about variation of the transporter expression is important for developing strategies to deliver drugs to the mother with minimal risk for the fetus or for delivering drugs to the fetus. The present paper demonstrates lower expression of MRP2 in preterm placentas. Moreover, the expression of MRP2 in the apical membrane of the multinuclear syncytiotrophoblasts of term placenta, shown previously by St. Pierre et al. (2000) using immunohistochemistry, was confirmed by additional methods such as immunoblot analysis of separated apical and basal membrane fractions and by immunofluorescent microscopy staining (Fig. 3, A and B).
Modulation of MRP2 expression is of high interest since this protein is involved in transport of physiological and pharmacological compounds, and variable abundance may therefore be accompanied by a modulation of protective features of the placenta. Gestation implies an enrichment of the multinuclear syncytiotrophoblasts. This process is further actuated by subsequent maturation of cytotrophoblasts. Consequently, maturation of cytotrophoblasts is accompanied by higher expression of MRP2 as shown in cultured cytotrophoblasts (Fig. 4C).
Function and integrity of the multinuclear syncytium depends on fusion with cytotrophoblasts. It is assumed that expression of proteins is related to the capacity of absorbing cytotrophoblastic ribosomes and other structural proteins (Kingdom et al., 2000). Therefore, the primary induction of MRP2 mRNA on day 2 of culturing the progenitor cells could be related to the start of fusion. However, the following reduced amount of mRNA is not accompanied by a loss of protein as shown by Western blot analysis (Fig. 5).
Elimination of fetally produced toxic compounds and protection of the fetus against maternal or exogenous substances are two major functions of the placenta. MRP2, which is expressed at the apical membrane of the syncytiotrophoblasts, may contribute to both processes. In fact, MRP2 has been characterized to transport metabolites of phase II biotransformation, namely, conjugates of lipophilic substances with sulfate, glucuronate, and glutathione (Keppler et al., 1996, 1997; Koenig et al., 2003), including bilirubin glucuronides (Jedlitschky et al., 1997), thereby playing a pivotal role in elimination of hemoglobin metabolites.
To date, the in vivo regulation of MRP2 expression is poorly understood. There are different studies reporting an influence of xenobiotics on the expression level, including cycloheximidine, 2-acetylaminofluorene, and rifampin (Cole et al., 1992; Buchler et al., 1996; Kartenbeck et al., 1996; Fromm et al., 2000). Moreover, it has been reported that steroids, in particular, the glucocorticoid dexamethasone, induce expression of the transporter. This effect is presumed to be mediated by the pregnane X receptor (Courtois et al., 1999). Indeed, pregnane X receptor has been shown to regulate MRP2 in human liver (Kast et al., 2002).
Moreover, different endogenous molecules are reported to be involved in the regulation of MRP2 expression; for instance, a short-term regulation of the MRP2-mediated efflux is associated with protein kinase C induction by the intracellular signaling molecule cGMP. It has been reported that induction of cGMP via endothelin-1 receptor B represses the expression of mrp2 in proximal tubule of killifish (Masereeuw et al., 2000; Notenboom et al., 2004). Another mechanism that has been reported to reduce the expression of MRP2 is lipopolysaccharide-induced sepsis. It has been shown that inflammation due to an application of lipopolysaccharides causes a decreased expression of drug-metabolizing enzymes and eliminating transporters including mrp2 in rat intestine.
The change of plasma levels of hormones plays a major role in controlling the adaptation of the maternal organism to the pregnancy. In detail, mean plasma levels of free and conjugated estrogens increase significantly with gestational age, reaching a peak a few weeks before delivery. Estradiol is produced in large quantities from the fetoplacental unit. Placental pregnenolone is metabolized to dehydroepiandrosterone-3-sulfate (DHEAS) in the fetal adrenal glands; DHEAS returns to placenta. After uptake of DHEAS mediated by OATP-B and OAT-4 (Ugele et al., 2003), it is transformed to androstenedione and then to estradiol in the syncytiotrophoblasts.
It is possible that parts of the active estradiol can be conjugated in the syncytiotrophoblasts, since UDP-glucuronosyltransferase isoforms are expressed in these cells (Collier et al., 2002a,b; Syme et al., 2004). Metabolites of estradiol, including estradiol-17β-glucuronide and with a lower affinity estradiol-3β-glucuronide, are substrates of MRP2 (Gerk et al., 2004). MRP2-mediated transport is assumed to be one of the main mechanisms of eliminating estradiol-17β-glucuronide in liver, shown by comparison of transport activities in rat liver canalicular membrane vesicle and in vivo elimination of intravenously administrated substance (Morikawa et al., 2000). The changing expression of MRP2 in placenta as shown in this study can be associated with a higher transport activity of the placenta-mediated transport of cytotrophoblastic-produced estradiol metabolites. The level of estradiol-17β-glucuronide in peripheral maternal veins is higher than that of umbilical veins and arteries. However, it is assumed that this difference refers to a control of estradiol-17β-glucuronide synthesis located in the maternal organism (Okada et al., 1984).
The maternal mean plasma levels of estradiol-17β-glucuronide increase during pregnancy. Gerk et al. (2004) postulate that the increase of this cholestatic metabolite of estrogens is responsible for the reduction of maternal bile efflux during normal pregnancies. In fact, it has been reported that estradiol-17β-glucuronide induces a partial internalization of Mrp2 during the acute phase of cholestasis associated with a reduced bile flow. Pretreatment with dibuturyl-cAMP reduces the endocytotic internalization of the transporter and the cholestatic effect (Mottino et al., 2002). Recently, we reported decreasing levels of MRP5 in human term placentas (Meyer zu Schwabedissen et al., 2005). This transporter for cyclic nucleotides is assumed to be involved in controlling the intracellular level of signaling molecules by predominantly transporting cGMP, and with a lower affinity cAMP (Jedlitschky et al., 2000). Assuming that cAMP is protective against estradiol-17β-glucuronide-induced internalization, it can be assumed that this is one of the mechanisms involved in the higher expression of MRP2 in term placentas.
Drug therapy of the unborn child is important in the context of several diseases such as perinatal HIV infection. It has been shown that high active antiretroviral therapy is associated with the lowest rates of HIV transmission (Cooper et al., 2002). HIV protease inhibitors like saquinavir, ritonavir, and indinavir have been characterized as substrates for MRP2 (Gutmann et al., 1999; Huisman et al., 2002). High active antiretroviral therapy has been administered during the last weeks of pregnancy without achieving therapeutic drug concentrations in the fetal circulation (Forestier et al., 2001; Marzolini et al., 2002). This lack of transfer can be readily explained by the higher expression of MRP2 in later stages of pregnancy, as indicated by our study. It is noteworthy, however, that based on animal experiments, other ATP transporters like MDR-1 and BCRP are associated with reduced drug accumulation in the fetal circulation (Jonker et al., 2000; Pavek et al., 2001).
The effect of various genetic polymorphisms of ABC transporters on drug disposition has been addressed in several studies. It has been shown, for example, that the C3435T polymorphism in exon 26 of the MDR1 gene is associated with a lower P-glycoprotein mRNA expression and efflux activity (Hitzl et al., 2001). These results correlate with the steady-state concentrations of digoxin in white volunteers. Carriers of the 3435T showed higher area under the curve and Cmax values compared to those carrying the wild-type gene (Hoffmeyer et al., 2000; Johne et al., 2002).
Various polymorphisms in the MRP2 gene contribute to the etiology of the Dubin-Johnson syndrome (Paulusma et al., 1996; Wada et al., 1998; Tsujii et al., 1999; Mor-Cohen et al., 2001; Tate et al., 2002; Materna and Lage, 2003; Wakusawa et al., 2003). It was shown that single nucleotide changes like the 3517 A/T transition in exon 25 are associated with lower efflux of MRP2 substrates and altered distribution of the protein in transfected cells (Mor-Cohen et al., 2001; Keitel et al., 2003). Impaired protein maturation followed by proteasomal degradation of the transporter has been suggested as an explanation (Keitel et al., 2003). Similar results have been shown for the C2302T missense mutation (exon 18) (Toh et al., 1999; Hashimoto et al., 2002). Furthermore, different polymorphisms of MRP2 with unknown functional consequences have been described (Ito et al., 2001; Gerk and Vore, 2002).
In the present paper, we studied the effect of three single nucleotide polymorphisms on the mRNA expression in human placenta, namely, the C–24T, G1249A, and C3972T. The C–24T polymorphism in the 5′-untranslated region of MRP2 was not associated with significant changes in MRP2 mRNA or protein expression. These findings are in agreement with previously described results in duodenal samples of healthy Japanese subjects (Moriya et al., 2002). Accordingly, characterization of the 5′-flanking region of the human MRP2 gene showed no binding sequences for known vertebrate-encoded transcription factors in position –24 of the MRP2 gene (Stockel et al., 2000). Moreover, there was no statistically significant alteration of the MRP2 expression in term and preterm placentas as a function of the exon 28 polymorphism.
We found, however, a significant influence of the G1249A missense mutation on mRNA level in the present study. Our data suggest that 1249 AA is associated with a lower expression of MRP2 mRNA in human preterm placenta with, also, a trend in lower protein expression; however, there was no statistically significant difference observed in term placentas. In view of the small sample number, however, confirmation in a larger population is mandatory. The exact mechanism for different expression levels of MRP2 as a function of nonsynonymous genetic variants is unknown. A similar phenomenon has been described for MDR1, and the following mechanisms have been proposed: altered translation efficiency or allele-specific differences in RNA folding (Eichelbaum et al., 2004).
Taken together, our study indicates that MRP2 expression in human placenta is affected by gestational age, with increased protein concentrations at later stages of pregnancy. Therefore, the mature fetus shows a better protection against xenobiotics, which are substrates for MRP2. Likewise, cellular differentiation in vitro was accompanied by increased expression of MRP2. This phenomenon can also be of clinical relevance in the setting of drug treatment during pregnancy.
Acknowledgments
We thank M. Grube, K. May, N. Siebert, S. Kuno, and T. Brüggmann for technical and methodical support. Moreover, we thank Prof. W. Straube (Division of Gynecology) for the good collaboration.
Footnotes
-
Financial support: Karl & Lore Klein Stiftung, Oy-Mittelberg, Germany.
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.104.003335.
-
ABBREVIATIONS: ABC, ATP-binding cassette; BCRP, breast cancer resistance protein; PBS, phosphate-buffered saline; RT, reverse transcription; PCR, polymerase chain reaction; CT, threshold cycle; PAGE, polyacrylamide gel electrophoresis; SNP, single nucleotide polymorphism; base pair(s); β-hCG, β-human choriogonadotropin; DHEAS, dehydroepiandrosterone-3-sulfate.
- Received December 15, 2004.
- Accepted April 6, 2005.
- The American Society for Pharmacology and Experimental Therapeutics