Abstract
Mycophenolic acid (MPA) is the active metabolite of mycophenolate mofetil (MMF), a standard immunosuppressive drug approved for clinical use in the prevention of acute allograft rejection after organ transplantation. This study examines the role of the genetic variants of UDP-glucuronosyltransferase (UGT) 1A8 and 2B7 enzymes involved in the formation of the primary metabolite of MPA, the inactive phenolic glucuronide (MPAG), and the reactive acyl glucuronide (AcMPAG). The first exon of UGT1A8 was first resequenced in the region encoding for the substrate binding domain in 254 Caucasians and 41 African Americans. Eight nonsynonymous changes were observed and led to the following amino acid substitutions: S43L, H53N, S126G, A144V, A173G, A231T, T240A, and C277Y. Thirteen haplotypes were inferred, comprising only two previously described alleles, namely, UGT1A8*2 (A173G) and UGT1A8*3 (C277Y). Upon stable expression in human embryonic kidney 293 cells, the UGT1A8*3 (C277Y), *5 (G173A240), *7 (A231T), *8 (S43L), and *9 (N53G) proteins were associated with the most profound decreases in the formation of MPAG and AcMPAG, indicating that these amino acids are critical for substrate binding and enzyme function. Altogether, the low-activity UGT1A8 enzymes are carried by 2.8 to 4.8% of the population. The variant of the UGT2B7 protein (UGT2B7*2 Y268), the main enzyme involved in the formation of AcMPAG, demonstrated a catalytic efficiency comparable with that of UGT2B7*1 (H268). In conclusion, although the common UGT2B7*2 variant is predicted to have limited impact, several UGT1A8 variants identified may potentially account for the large interindividual variance in MMF pharmacokinetics and deserve further clinical investigations.
Mycophenolate mofetil (MMF; Cellcept, Hoffmann-La Roche, Nutley, NJ), an immunosuppressive drug, is approved for clinical use in the prevention of acute allograft rejection after organ transplantation, as well as hematopoietic stem cell transplantation (Sollinger, 1995; Bullingham et al., 1998; Cohn et al., 1999). Mycophenolic acid (MPA), its active metabolite, is a selective inhibitor of inosine monophosphate dehydrogenase (IMPDH). The metabolism of MPA involves mainly its conjugation by UDP-glucuronosyltransferase (UGT) enzymes, yielding two glucuronide conjugates; namely, the major derivative MPAG and the minor metabolite AcMPAG (Bullingham et al., 1998; Shipkova et al., 2001b). MPAG has no inhibitory effects on IMPDH and is the major urinary excretion product of MPA (Bullingham et al., 1996; Schutz et al., 1999). In contrast, AcMPAG may be biologically active by inhibiting IMPDH and leukocyte proliferation, and by inducing cytokine release (Schutz et al., 1999; Wieland et al., 2000; Shipkova et al., 2001b). A relationship between plasma levels of MPA and clinical outcomes in transplant patients has been demonstrated (Hale et al., 1998; van Gelder et al., 1999; Oellerich et al., 2000; Weber et al., 2002). Besides, AcMPAG has been suggested to be involved in some of the toxicities experienced by patients receiving MMF, including neutropenia and gastrointestinal disorders (Wieland et al., 2000; Maes et al., 2002). Therefore, factors affecting the extent of MPA glucuronidation are likely to be clinically significant.
Recently, UGT1A9 has been identified as the main enzyme involved in the hepatic formation of MPAG (Bernard and Guillemette, 2004). This enzyme was predicted to be the key determinant of MPAG formation in vivo because the metabolism of MPA takes place mainly in the liver (Bowalgaha and Miners, 2001; Shipkova et al., 2001a; Bernard and Guillemette, 2004). Functional genetic variants within the UGT1A9 gene have been uncovered recently by our group (Villeneuve et al., 2003; Girard et al., 2004). In human liver microsomes, the presence of the variants –275A>T and –2152T>C of the UGT1A9 promoter region were associated with a 2.3-fold higher hepatic expression of UGT1A9 and a 2.1-fold increased glucuronidation activity to generate MPAG (Girard et al., 2004). These in vitro observations were confirmed recently in a clinical setting by the group of Kuypers et al. (2005). In renal transplant recipients carrying these polymorphisms, a reduced MPA exposure and an increased MPA clearance were observed, demonstrating the clinical importance of genetic variability in the UGT genes involved in the in vivo metabolism of MPA.
MPAG is also produced by UGT1A8, which is expressed in the gastrointestinal tract and not in the liver (Cheng et al., 1998; Tukey and Strassburg, 2000; Zheng et al., 2002; Bernard and Guillemette, 2004). UGT1A8 has demonstrated the highest catalytic efficiency for MPAG formation in vitro (Bernard and Guillemette, 2004). Based on these metabolic studies, UGT1A8 could also play a role in the formation of AcMPAG, along with UGT2B7, which appears as the predominant enzyme responsible for its formation (Picard et al., 2005). Other extrahepatic UGTs, namely, UGT1A7 and UGT1A10, demonstrated a lower reactivity toward MPA glucuronidation and are predicted to play a minor role compared with UGT1A8, UGT1A9, and UGT2B7 (Basu et al., 2004; Bernard and Guillemette, 2004; Picard et al., 2005).
To this day, two coding region polymorphisms have been reported in the UGT1A8 gene, namely the variants A173G (UGT1A8*2) and C277Y (UGT1A8*3) (Huang et al., 2002). In vitro metabolic studies with heterologous expression of these variant allozymes revealed that the polymorphism at codon 277 induces a drastic reduction in the formation of MPAG, whereas no significant effect was observed for the codon 173 variation (Bernard and Guillemette, 2004). The effect of these variations on the formation of the AcMPAG remains to be determined. As for the UGT2B7 gene, a frequent polymorphism (UGT2B7*2; H268Y) has been reported in more than 50% of Caucasian individuals (Jin et al., 1993; Lampe et al., 2000). The functional impact of this polymorphism on the formation of AcMPAG has never been assessed.
The aim of this study was to further investigate genetic variations in the UGT1A8 gene by resequencing the first exon and assessing the functional impact of newly found and known variants on the formation of both MPAG and AcMPAG. As a secondary aim, we explored the role of known UGT2B7*1 (H268) and UGT2B7*2 (Y268) variant enzymes in the formation of AcMPAG. Together, results of this study identify new genetic factors resulting in structural changes in the UGT1A8 protein that could potentially alter MPA metabolism in extrahepatic tissues. In contrast, the UGT2B7*2 common variant allozyme is predicted to have a modest influence on drug metabolism in vivo.
Materials and Methods
Reagents and Chemicals. MPA was obtained from Sigma Diagnostics Canada (Mississauga, ON, Canada). MPAG and AcMPAG were generous gifts from Hoffmann-La Roche (Mississauga, ON, Canada). All other chemicals and reagents were of the highest grade and commercially available.
Genomic DNA Samples. DNA samples from 254 healthy unrelated Caucasian subjects were obtained from the Quebec Family Study for UGT1A8 single-nucleotide polymorphism (SNP) genotyping (Simonen et al., 2002). Additional random DNA samples from African-American subjects (n = 41) used in a previous study were sequenced (Butler et al., 2005). Subject identifiers for these samples had been removed before their reception in our laboratory. All subjects provided written consent for experimental purposes and the present study was reviewed and approved by the Institutional Review Boards (CHUL Research Center and Laval University).
Resequencing of theUGT1A8Gene and Genotyping. The first exon of UGT1A8 (–34/+935) was amplified using a previously described strategy (Thibaudeau et al., 2006). In brief, three pairs of primers were designed to amplify overlapping fragments covering the coding region of the first exon, a small portion of the 5′-flanking region, and the intron-exon junction. PCR conditions for the amplification primers were 3 min at 95°C for denaturation, followed by 35 cycles at 95°C for 30 s, a 30-s annealing period, and 72°C for 30 s, with a final extension at 72°C for 7 min. PCR products were sequenced using an ABI 3700 automated sequencer (Applied Biosystems, Foster City, CA). Samples with ambiguous sequencing chromatograms and samples with SNPs were subjected to a second independent amplification, followed by DNA sequencing. Sequences were analyzed with the Staden preGap4 and Gap4 programs (Staden, Cambridge, UK). Allelic and genotype frequencies were calculated for all alleles. Haplotypes and their respective frequencies were inferred using the Phase 1.0.1 software (Stephens et al., 2001).
UGT-HEK293 Microsomal Preparations. The UGT1A8*1, UGT2B7*1, and UGT2B7*2 constructions were kindly provided by Dr. Thomas Tephly (Cheng et al., 1998). The UGT1A8*2 and UGT1A8*3 variants were prepared as described previously (Bernard and Guillemette, 2004). All other UGT1A8 variants were generated by PCR site-directed mutagenesis using the primers presented in Table 1 and inserted in the pcDNA3 vector. Before enzymatic assays, Western blot analyses and catalytic activities on known substrates were performed for each preparation. Stable HEK293 cells transfection with variant pcDNA3-UGT expression plasmids, preparation of microsomes by differential centrifugation, and determination of UGT protein levels by Western blot has been described previously (Villeneuve et al., 2003). Protein expression levels for UGT1A8 alleles were determined by Western blot using a polyclonal anti-UGT1A antibody (in-house; RC-71) and were *1 (1.0), *2 (0.7), *3 (0.5), *4 (0.4), *5 (0.5), *6 (0.5), *7 (0.2), *8 (1.4), *9 (1.5), H53N (1.1), A144V (0.4), and T240A (0.4). Protein expression levels for UGT2B7 allozymes were determined previously (Thibaudeau et al., 2006).
Site-directed mutagenesis primers sequences for UGT1A8 variants
Variant positions are in bold.
Analytical Procedures for MPA, MPAG, and AcMPAG Detection. Detection of MPA, MPAG, and AcMPAG was supported by a previously published high-performance liquid chromatography coupled with mass spectrometry protocol used with slight modifications (Bernard and Guillemette, 2004). In brief, the analysis system consisted of a high-performance liquid chromatography module (Alliance model 2690; Waters Corporation, Milford, MA) and a triple-quadrupole mass spectrometer (API 3000). Acidified assays were centrifuged for 6 min at 14,000g, and 250 μl of supernatant was collected. Ten-microliter samples, maintained at 4°C, were injected on a 100 × 4.6 mm (4.0-μm diameter) Synergic RP-Hydro C-18 reversed-phase column (Phenomenex, Torrance, CA). The mobile phase consisted of solution A (MeOH + 3 mM ammonium formate) and solution B (H2O + 3 mM ammonium formate) using the following gradient: 62% A (0–4.5 min), 95% A (4.5–6.5 min), and 62% A (6.5–9.5 min). The flow rate was 0.9 ml/min. MS detection of MPA was followed in the multiple reactions monitoring positive ion mode with mass fragmentation of 321.1 > 207.2 (MPA) and 514.3 > 321.1 (MPAG and AcMPAG). Under these conditions, retention times for MPAG, AcMPAG, and MPA were 1.66, 2.47, and 4.41 min, respectively. The signals were found to be linear from 10 to 5000 ng/ml for MPAG, AcMPAG, and MPA. The limit of quantification was 10 ng/ml using a signal-to-noise ratio of 3. The within-day precision was <5.0%, and the between-day precision was <10%.
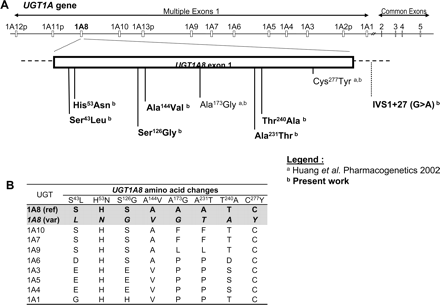
UGT1A8 genetic variants. A, schematic representation of polymorphisms located in UGT1A8. Amino acid position is relative to UGT1A8 according to the reference sequence (AF297093). B, amino acid alignment of UGT1A proteins at UGT1A8 polymorphic sites. ref, reference sequence; var, variant sequence.
Enzymatic Assays and Kinetic Parameters Determination. The procedure for enzymatic assays was described previously (Bernard and Guillemette, 2004). In brief, incubations were performed for 1 h at 37°C with 50 μg of UGT protein, 50 mM Tris-HCl, pH 6.8, 10 mM MgCl2, 2 mM UDP-glucuronic acid, pepstatin, and phosphatidylcholine. Determination of Vmax and Km was performed for all UGT1A8 and UGT2B7 variants with MPA ranging from 25 to 1250 μM. Absolute velocity values were adjusted according to protein expression levels relative to the corresponding UGT*1 allele determined by Western blot. Visual inspection of fitted functions (V as a function of [S]) and Eadie-Hofstee plots (V as a function of V/[S]) was used to select the best-fit enzyme kinetic model (Venkatakrishnan et al., 2001). Kinetic parameters calculations were performed with the SigmaPlot 8.0 software assisted by the Enzyme Kinetics 1.1 software (SPSS, Chicago, IL). Values were expressed as the mean of two to five experiments performed in duplicate. Means comparisons were performed with the JMP 4.0.2 software (SAS Institute, Cary, NC) using Student's t test with a statistical significance threshold of p < 0.05.
Results
Identification of Novel Missense Mutations in theUGT1A8First Exon. The resequencing of the first exon of UGT1A8 led to the discovery of four novel missense mutations in the Caucasian population (n = 254) at nucleotides 376 (A>G), 431 (C>T), 691 (G>A), and 718 (A>G) relative to the start codon and at nucleotides 128 (C>T) and 157 (C>A) in the African-American population (n = 41). These nonsynonymous changes led to the following amino acid substitutions: S43L, H53N, S126G, A144V, A231T, and T240A (Fig. 1). The allelic frequencies of these variants were 0.2 to 1.4% (Table 2). The two previously reported single-nucleotide polymorphisms (SNPs) of UGT1A8 at codons 173 and 277 were confirmed with genotypic frequencies of 0.238 and 0.012, respectively. In addition to these, four synonymous variations were found at nucleotides 90 (G>A; V30V), 441 (T>G; L147L), 765 (A>G; T255T), and 804 (T>C; N268N), and one intronic missense mutation was found at nucleotide 883 relative to the start codon, which is 27 base pairs downstream of the end of exon 1 (IVS1+27). All SNP frequencies were found to follow the Hardy-Weinberg equilibrium (data not shown).
Frequency of UGT1A8 variants in the Caucasian and African-American populations
Newly identified SNPs are in bold.
Eleven haplotypes were inferred in the Caucasian population (n = 508 chromosomes). In contrast, five haplotypes, of which two were not encountered in the Caucasians, were observed in the African Americans (n = 82 chromosomes) (Table 3). These haplotypes generated ten different diplotypes (Table 4). The UGT1A8*1a, UGT1A8*1b (T255T), and UGT1A8*2a (A173G) alleles were found to be the most frequent at 59, 13, and 22%, respectively. The other haplotypes with nonsynonymous variations were found at frequencies of 0.2 to 1.4%. The subjects with the A144V variant also presented the A173G variant, resulting in the UGT1A8*4 allele. The T240A variant was found only once and in the presence of the A173G variant, representing the UGT1A8*5 allele. The S126G and A231T variants were found alone generating the UGT1A8*6 and UGT1A8*7 alleles, respectively. The UGT1A8*1, *2, and *4 alleles were also found in the African-American population at frequencies of 92.7, 3.7, and 1.2%, respectively. The S43L polymorphism (UGT1A8*8) was found only in one individual, whereas the H53N polymorphism was found in combination with the A173G variant, generating the UGT1A8*9 allele.
UGT1A8 haplotypes in Caucasian and African-American subjects
Haplotypes (H) and their respective frequencies were inferred with the Phase 1.00.1. Newly identified SNPs and nucleotide changes are in bold.
UGT1A8 diplotypes in Caucasian and African-American subjects
Kinetic Analyses of UGT1A8 Variant Allozymes on MPAG and AcMPAG Formation. To assess the impact of nonsynonymous UGT1A8 polymorphisms on the glucuronidation of MPA, kinetic analyses were performed on all variants for the determination of Km, Vmax, and CLint values. Kinetic estimates are presented in Table 5. A novel finding of this study is the observation that UGT1A8 has the capability to generate both MPAG and AcMPAG. The UGT1A8*3, *7, *8, and *9 enzymes were associated with the most profound effects on the level of MPAG formation, with 3.3 to 82.5-fold reduced CLint values. This decrease was mostly explained by an altered velocity of the enzyme, except for the codon 277 variation (*3), which affects both the affinity and the velocity of the protein, consistent with our previous observations (Bernard and Guillemette, 2004). In contrast, the UGT1A8*4 protein appears as a high-activity enzyme for the formation of MPAG with a 1.8-fold higher CLint value, explained by both a significantly better affinity and an increased velocity caused by the V144G173 mutations compared with the reference *1 protein. In the case of the UGT1A8*5 protein, despite an enhanced affinity for the formation of MPAG, the velocity of the enzyme was drastically reduced, leading to a clearance value similar to the *1 protein. In turn, the UGT1A8*2 and *6 proteins demonstrated modest modifications of their kinetic parameters compared with the reference *1 protein.
Kinetic estimates for MPAG and AcMPAG formation by UGT1A8 and UGT2B7 variant allozymes
Kinetic parameter determination was performed by incubating MPA (25 to 1250 μM) with HEK293 cells stably expressing UGT1A8 and UGT2B7 variants. Velocity values were adjusted according to protein expression levels relative to the *1 allele determined by Western blot. All values are expressed as the mean ± S.E.M. of two to five experiments performed in duplicate.
With regard to AcMPAG, its formation was undetectable for the *3 and *7 proteins and severely reduced for the *5 protein (20-fold reduction) because of an altered velocity. Velocities were also significantly reduced for the *2, *4, *6, and *8 enzymes by 2- to 4-fold compared with the UGT1A8*1 protein. The affinity of variant proteins *2, *4, *5, *6, *8, and *9 was not significantly altered compared with UGT1A8*1. In the individuals tested, 2.8% of Caucasians and 4.8% of African Americans carry at least one of the low-activity alleles (*3, *7, *8, and *9).
To gain insight into the nucleic acid positions responsible for critical changes in the kinetic parameters of the UGT1A8 protein, few mutations were tested alone. Results indicate that the replacement of a threonine by an alanine at codon 240 abolishes the ability of the UGT1A8 protein to form the acyl glucuronide and drastically compromised the formation of the phenolic glucuronide. In turn, the H53N change acts only on the velocity of the protein, whereas the A144V mutation alters specifically the formation of the MPAG (Km and Vmax) with no significant detectable effect on the kinetic parameters for the formation of the acyl.
Kinetic Analyses of the UGT2B7*1 and *2 Allele AcMPAG Formation. The UGT2B7 enzyme was found to generate high levels of AcMPAG with no detectable formation of MPAG. Both the affinity and the capacity of the UGT2B7*1 protein were higher for the formation of the acyl glucuronide compared with the UGT1A8*1 protein, with a 31-fold higher Clint value. No significant changes in the kinetic parameters were associated with the UGT2B7*2 protein, with Km and Vmax values similar to those observed for the UGT2B7*1 protein.
Discussion
In a recent study, we revealed the existence of common variations in the upstream region of the UGT1A9 gene (–275T>A and –2152C>T) associated with higher protein expression and higher glucuronidation activity in human liver samples (Girard et al., 2004). These genetic variants were further shown to influence the pharmacokinetics of MMF in transplant recipients (Kuypers et al., 2005). Based on in vitro data, two additional UGTs, UGT1A8 and UGT2B7, are proposed to play a critical role in the metabolism of MPA (Basu et al., 2004; Bernard and Guillemette, 2004; Picard et al., 2005). In this study, polymorphisms conferring a low-activity phenotype were identified in UGT1A8, the extrahepatic MPA-metabolizing enzyme that demonstrates the highest catalytic efficiency for MPAG formation. It is thus predicted that specific UGT1A8 variants identified here may have an impact on the pharmacokinetics of MMF and potentially on the metabolism of other UGT1A8 substrates.
Six novel nonsynonymous variations in the coding region of UGT1A8 (S43L, H53N S126G, A144V, A231T, and T240A) were identified, and the two previously described variants *2 (A173G) and *3 (C277Y) were also observed. The A173G and C277Y variants were initially reported with frequencies of 14.5 and 2.2%, respectively (Huang et al., 2002). Although the C277Y variant is reported in this study with a similar frequency, a 2-fold higher frequency was observed for the A173G and could be attributed to several differences in the population studied (254 healthy subjects of French-Canadian origin versus 69 individuals with lung cancer patients, their family members, and other volunteers).
Among the variants found, UGT1A8*3 (C277Y), *5 (G173A240), *7 (A231T), *8 (S43L), and *9 (N531G) were associated with the most profound decreases in the formation of MPA glucuronides in vitro. The formation of MPAG in the gastrointestinal tract is predicted to be reduced in the presence of those alleles, occurring in 2.8% of Caucasians and 4.8% of African Americans. As for AcMPAG, although UGT2B7 is the most active UGT compared with UGT1A8 (CLint = 12 versus 0.39 μl/min/mg), it is predicted that UGT1A8 variants would have a limited impact on the formation of the acyl glucuronide in vivo. Before this study, UGT1A8*3 had been identified as a low-activity protein on various substrates, including a dramatic reduction in MPAG formation, but its impact on AcMPAG formation was not assessed (Huang et al., 2002; Bernard and Guillemette, 2004). Our study further shows that a similar effect can be observed for AcMPAG. Such an impact could be explained by the fact that this amino acid variation involves the substitution of a highly conserved cysteine for a tyrosine. Likewise, the T240A variant (not encountered alone in the population studied) seems to be responsible for the reduced capacity observed for the UGT1A8*5 protein (G173A240) to generate MPA glucuronides, as the activity of the *5 (G173A240) and T240A variants are similar. This also suggests a negligible role of the A173G variation (*2 allele) on UGT1A8 protein activity, as seen previously (Huang et al., 2002; Bernard and Guillemette, 2004). The dramatic activity reduction associated with the UGT1A8*7 (A231T) protein is somehow surprising because the alanine-threonine substitution represents a fairly conservative change. Nevertheless, the velocity of the UGT1A8*7 (A231T) protein was reduced by 281-fold for MPAG compared with UGT1A8*1, indicating a critical role of this amino acid for enzyme function. As for the UGT1A8*8 (S43L) and *9 (N53G173) proteins, they were both associated with similar decreases in velocity, with 2.8- and 3.3-fold reduced Vmax values, respectively. The H53N variation involves the substitution of a highly conserved histidine for an asparagine, which could explain the reduced activity observed with the variant protein.
One of the UGT1A8 variant allozymes, UGT1A8*4 (V144G173), demonstrated an effect specific to the glucuronide product formed. The combination of variations at codons 144 and 173 led to an enhanced activity of the protein specifically for the formation of MPAG whereas the formation of AcMPAG was lowered. According to the kinetic properties of the V144 alone and the G173 alone (UGT1A8*2), the effect on AcMPAG formation would be a consequence of the amino acid substitution at codon 173, whereas the increased capacity of MPAG formation would mostly be caused by the change at codon 144.
UGT2B7 has been identified as the main enzyme involved in the formation of AcMPAG (Picard et al., 2005) and is expressed in the liver and the intestine (Jin et al., 1993; Radominska-Pandya et al., 1998). The common UGT2B7*2 (Y268) allele, carried by 27% of Asians and up to 54% of Caucasians (Jin et al., 1993; Guillemette et al., 2000; Lampe et al., 2000), demonstrated a catalytic efficiency comparable with that of UGT2B7*1 in most studies for various substrates, including opioids, androgens, 3′-azido-3′-deoxythimidine, and morphine (Coffman et al., 1998; Barbier et al., 2000; Bhasker et al., 2000; Innocenti et al., 2001). Thus, it is predicted that the in vivo formation of AcMPAG in the liver and the gastrointestinal tract is probably not significantly modulated by UGT2B7*2. Other variants of UGT2B7, namely, SNPs of the 5′-regulatory region, could affect the levels of expression of the gene and would deserve further consideration with regards to AcMPAG formation. One example is the –79G>A polymorphism in the UGT2B7 gene, in complete linkage disequilibrium with UGT2B7*2, that results in a reduction of 2.5- to 7-fold lower promoter activity and found in approximately 5% of the population (Duguay et al., 2004).
The pharmacokinetics of MPA and its metabolites have been shown to be highly variable in various transplant subpopulations (Bullingham et al., 1998; Ensom et al., 2002; Jacobson et al., 2005; Srinivas et al., 2005). One of the factors likely involved is the genetic diversity of UGT genes, such as the UGT1A9 –275/–2152 variants recently associated with significantly lower MPA exposure in renal transplant patients (Kuypers et al., 2005). As proposed by the authors, the altered pharmacokinetics related to the presence of these common polymorphisms is believed to be at least partially caused by a reduction of enterohepatic recirculation, a process accounting for up to 40% of the plasma MPA area under the concentration-time curve (Seifeldin, 1995; van Gelder et al., 2001). Given the important role of this process in the pharmacokinetics of MMF, the intestinal conjugation of MPA certainly deserves further attention (Bullingham et al., 1998). UGT1A8 is one of few UGT enzymes to be specifically expressed in the gastrointestinal tract (Cheng et al., 1998; Tukey and Strassburg, 2000; Zheng et al., 2002), along with UGT1A9 and 2B7 (Tukey and Strassburg, 2000; Turgeon et al., 2001). This isoform could significantly contribute to the intestinal formation of the inactive glucuronide MPAG, but also the reactive and potentially toxic metabolite AcMPAG, yet to a much lesser degree than UGT2B7 (31-fold lower CLint value). In conclusion, although the common variant of UGT2B7 at codon 268 is predicted to have limited impact in vivo, several UGT1A8 variants were identified and could contribute to the interindividual variability in MMF pharmacokinetics and deserve further clinical investigation.
Acknowledgments
We thank Patrick Caron and Lyne Villeneuve, Pharmacogenomics Laboratory, Canada Research Chair in Pharmacogenomics, Laval University, Quebec, Canada, for technical assistance.
Footnotes
-
This work was supported by the Canadian Institutes of Health Research (MOP-42392) and Canada Research Chair Program (C.G.). O.B. is the recipient of a studentship award from the Fonds de la Recherche en Santé du Québec. C.G. is the chairholder of the Canada Research Chair in Pharmacogenomics.
-
Part of this work has been presented at the 13th North American Meeting of the International Society for the Study of Xenobiotics, 2005 October 23–27th, Maui, HI.
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.106.010553.
-
ABBREVIATIONS: MMF, mycophenolate mofetil; MPA, mycophenolic acid; IMPDH, inosine monosphospate dehydrogenase; UGT, UDP-glucuronosyltransferase; MPAG, mycophenolic acid phenolic glucuronide; AcMPAG, mycophenolic acid acyl glucuronide; SNP, single-nucleotide polymorphism; PCR, polymerase chain reaction; HEK, human embryonic kidney; CLint, intrinsic clearance.
- Received April 19, 2006.
- Accepted June 20, 2006.
- The American Society for Pharmacology and Experimental Therapeutics