Abstract
Fresh hepatocytes cultured in a sandwich configuration allow for the development of intact bile canaliculi and the ability to measure hepatic uptake and biliary clearance. A disadvantage of this model is its dependence upon hepatocytes from fresh tissue. Therefore, the ability to use cryopreserved human hepatocytes in this model would be a great advantage. Multiple variables were tested, and the recommended conditions for culturing cryopreserved human hepatocytes in a sandwich configuration in 24-well plates are as follows: BioCoat plates, a cell density of 0.35 × 106 cells/well in 500 μl, an overlay of Matrigel and InVitroGRO media. These conditions resulted in good hepatocyte morphology and the formation of distinct bile canaliculi. The function of multiple uptake and efflux transporters was tested in multiple lots of cryopreserved and fresh human hepatocytes. For taurocholate [Na+ taurocholate cotransporting polypeptide/organic anion transporting polypeptide (OATP) uptake/bile salt export pump efflux], the average apparent uptake, apparent intrinsic biliary clearance, and biliary excretion index among five cryopreserved hepatocyte lots was high, ranging from 11 to 17 pmol/min/mg protein, 5.8 to 10 μl/min/mg protein, and 41 to 63%, respectively. The corresponding values for digoxin (OATP-8 uptake/multidrug resistance protein 1 efflux) were 0.69 to 1.5 pmol/min/mg protein, 0.60 to 1.5 μl/min/mg protein, and 37 to 63%. Both substrates exhibited similar results when fresh human hepatocytes were used. In addition, substrates of breast cancer resistance protein and multidrug resistance-associated protein 2 were also tested in this model, and all cryopreserved lots showed functional transport of these substrates. The use of cryopreserved human hepatocytes in 24-well sandwich culture to form intact bile canaliculi and to exhibit functional uptake and efflux transport has been successfully demonstrated.
Biliary excretion of therapeutic agents is often an important component of total drug clearance in vivo (Siegers and Bumann, 1991). Likewise, compounds that undergo little metabolism in vivo nevertheless can exhibit a significant degree of biliary elimination (Corsini et al., 1999; Martin et al., 2003). In the pharmaceutical industry, within particular discovery therapeutic areas (i.e., cardiovascular, cancer, anti-infectives), one or more series of compounds may suffer from extensive biliary excretion. Therefore, being able to address this issue would help to understand mechanisms of clearance and help in the drug discovery process. Use of cannulated animals to assess biliary clearance is possible; however, the throughput is low, and species differences in biliary clearance are known (Pahlman et al., 1998). Because the ultimate species of concern is human, it is necessary to use an in vitro human model to assess biliary elimination. Ideally, this model should allow for at least a moderate number of compounds to be easily tested.
Use of fresh hepatocytes in culture to assess hepatic transport has gained much support in recent years. Initial work by LeCluyse et al. (1994) showed that culturing hepatocytes in a sandwich configuration resulted in the repolarization of hepatocytes over time and that this therefore was a good model to assess hepatic function, including that associated with the establishment of intact bile canaliculi. Subsequent refinement of the culture conditions led to the development of a valuable in vitro model to assess hepatic transport using fresh rat hepatocytes cultured in a sandwich configuration (Liu et al., 1999b,c) In contrast to a typical culture with no overlay, the sandwich culture model allows the hepatocytes to assume a more three-dimensional orientation and to form intact bile canaliculi and proper localization of efflux transporters more easily (Hoffmaster et al., 2004). Brouwer and coworkers (Liu et al., 1999a) have shown a good correlation exists in rats between in vitro biliary excretion and in vivo biliary excretion, as well as in vitro predicted biliary clearance and in vivo biliary clearance. The sandwich culture technique has been applied to human hepatocytes with some success as well, and to date, the plate format has been in 60-mm dishes or six-well plates. However, the dependence on commercially available fresh human tissue is a significant limitation of this model.
The commercial availability of good-quality cryopreserved human hepatocytes has increased in recent years such that they are now readily available. Multiple vendors offer cryopreserved human hepatocytes, and plateable lots are also available. Cryopreserved hepatocytes have been successfully used for metabolism studies (Lau et al., 2002), for uptake studies in suspension (Nakai et al., 2001; Houle et al., 2003; Shitara et al., 2003), and for induction studies (Garcia et al., 2003). The ability to culture cryopreserved human hepatocytes in a sandwich configuration to assess hepatic uptake and biliary elimination would be a great advantage because this would allow for studies on demand, and this would also allow for the establishment of routine studies or screens. Therefore, the purpose of the present study was to determine whether cryopreserved human hepatocytes could be cultured in a sandwich configuration in a small-well format to form intact bile canaliculi and to characterize the transport of well studied compounds. The results indicate that human cryopreserved hepatocytes can be used in a 24-well plate sandwich culture format to assess uptake and efflux transport.
Materials and Methods
Chemicals and Reagents. InVitroGro-HI, InVitroGro-CP, and InVitro-Gro-HT hepatocyte media were purchased from In Vitro Technologies, Inc. (IVT) (Baltimore, MD). Hepatocyte maintenance media (HMM) was purchased from Cambrex Corporation (Baltimore, MD). Williams' media E (WME), Dulbecco's modified Eagle's media (DMEM), glutamine, penicillin, streptomycin, and Hanks' balanced salt solution (HBSS) were purchased from Invitrogen (Carlsbad, CA). BioCoat 24-well plates, Matrigel, and ITS+ culture supplement [human recombinant insulin, human transferrin (12.5 mg each), selenous acid (12.5 μg), bovine serum albumin (2.5 g), and linoleic acid (10.7 mg)] were purchased from BD Biosciences (Bedford, MA). Mouse anti-MRP2 (M2 III-6) monoclonal antibodies were obtained from IQ Products (Groningen, The Netherlands). Mouse anti-MDR1/3 (C219) and mouse anti-BCRP (BXP-21) monoclonal antibodies were obtained from ID Labs, Inc. (London, ON, Canada). 5-(and-6)-Carboxy-2′,7′-dichloro-fluorescein diacetate (CDFDA) was obtained from Molecular Probes (Eugene, OR). Triton X-100 was purchased from Bio-Rad Laboratories (Hercules, CA). Dulbecco's phosphate-buffered saline, taurocholate, digoxin, estradiol-17β-d-glucuronide (EG), sulfobromophthalein (BSP), and salicylate were purchased from Sigma-Aldrich (St. Louis, MO). [3H]Taurocholate (1.19 Ci/mmol), [3H]digoxin (21.8 Ci/mmol), [3H]estradiol-17β-d-glucuronide (53.0 Ci/mmol), and [14C]salicylate (55.5 mCi/mmol) were purchased from PerkinElmer Life Sciences (Boston, MA). [3H]BSP (17.5 Ci/mmol) was obtained from Hartmann Analytic (Köln, Germany).
Human Hepatocytes. Cryopreserved human hepatocytes were purchased from IVT. Fresh hepatocytes in a BioCoat/Matrigel sandwich configuration in 24-well plates were purchased from IVT and from CellzDirect (Pittsboro, NC). Characteristics of the hepatocyte lots are shown in Table 1.
Characteristics of fresh and cryopreserved hepatocytes obtained from commercial sources
The cryopreserved lots (130, FEP, LOF, NLR, and QKR) and the fresh lot F00927 were purchased from In Vitro Technologies, Inc. Fresh lot Hu156 was purchased from CellzDirect.
Hepatocyte Sandwich Culture. InVitroGRO-HT (thawing), InVitro-GRO-CP (plating), and InVitroGRO-HI (incubation) media were supplemented with Torpedo Antibiotic Mix (IVT), per the manufacturer's instructions. HMM was supplemented with 10–7 M dexamethasone, 10–7 M insulin, 100 units/ml penicillin G, and 100 μg/ml streptomycin. WME and DMEM were supplemented with 10–7 M dexamethasone, 2 mM glutamine, 100 units/ml penicillin G, 100 μg/ml streptomycin, and ITS+. For HMM, WME, and DMEM, 10% and 2% fetal bovine serum (FBS) were added for thawing and plating, respectively. There was no FBS in the incubation medium. Cryopreserved human hepatocytes were thawed based upon IVT's standard method: hepatocytes were thawed at 37°C then placed in ice, after which the cells were poured into 37°C InVitroGRO-HT thawing medium at a ratio of one vial (∼5 million cells)/50 ml in a conical tube. The cells were then centrifuged at 50g for 3 min and resuspended to 0.7 × 106 cells/ml in InVitroGRO-CP plating medium. Cell viability was determined by trypan blue exclusion. On day 1, hepatocyte suspensions were plated in BioCoat 24-well plates at a density of 0.35 × 106 cells/well in a volume of 0.5 ml/well. After 18 to 24 h of incubation at 37°C, cells were overlaid with ice-cold 0.25 mg/ml Matrigel in incubation medium at 0.5 ml/well. Cultures were maintained in FBS-free media, which was replaced every 24 h.
Observation of Bile Canaliculi Formation and Substrate Function Assay. CDFDA was used for detecting the formation of bile canaliculi. On day 5, the cells were rinsed with regular HBSS, and 0.5 ml of 5 μM CDFDA in HBSS was added. (Unless indicated, all rinse solutions were at 37°C.) After a 20-min incubation, the cell cultures were rinsed three times with HBSS. Sandwich-cultured hepatocyte morphology and 5-(and-6)-carboxy-2′,7′-dichloro-fluorescein (CDF) accumulation in bile canaliculi were assessed by phase-contrast microscopy and fluorescent microscopy, respectively, using a Nikon TE-300 or TE-2000U microscope (Nikon, Melville, NY) with a 20× objective and a Retiga 1300 or EXi digital CCD camera (QImaging, Burnaby, BC, Canada). Digital images were analyzed by MetaVue software (Molecular Devices Corporation, Downingtown, PA) or Clemex Vision PE software (Clemex Technologies, Inc., Longueuil, QC, Canada). For transporter function assays, on day 5, parallel cell cultures were rinsed twice with 0.5 ml of either regular HBSS or Ca2+/Mg2+-free HBSS containing 1 mM EGTA and then incubated in the same buffers for 10 min. Substrates in regular HBSS were then added to both sets of cultures. At various time points, cells were rinsed three times with ice-cold regular HBSS. For radiolabeled compounds, cells were lysed with 0.5 ml of 0.5% Triton-100 in phosphate-buffered saline. For unlabeled compounds, cells were lysed with 0.5 ml of methanol. The samples were analyzed by liquid scintillation spectroscopy or by LC/MS/MS.
LC/MS/MS Conditions. Samples were concentrated on an EVX-192 Apricot Evaporex apparatus (Apricot Designs, Monrovia, CA). The residue was reconstituted to half the original volume in aqueous mobile phase before LC/MS/MS analysis. A Gilson 215 Multiprobe autosampler (Gilson Instruments, Middleton, WI) was used to deliver 25-μl injection volumes. Further sample concentration and on-line sample clean up was achieved using a dual-column/column-switching system (Janiszewski et al., 2001) that consisted of a pair of 1.5 × 5-mm cartridge style columns (Optimize Technologies, Inc., Oregon City, OR), custom packed with Showa Denko (Tokyo, Japan) ODP polymeric material (13-μm particle size), and plumbed using a 10-port two-position switching valve (Valco Instruments, Houston, TX). Two high-performance liquid chromatography pumps (model PU-1580; Jasco Inc., Easton, MA) delivered flow rates of 2 ml/min. The aqueous mobile phase (load step) was 98% 2 mM ammonium acetate/2% methanol. The organic mobile phase (elute step) was 10% 2 mM ammonium acetate/90% acetonitrile/methanol (50:50). Mass spectrometry analysis was performed on a SCIEX API 4000 triple quadruple mass spectrometer (PE Sciex, Ontario, ON, Canada) equipped with a turbo ion spray interface. Data were acquired in positive ion mode with an electrospray ionization probe voltage of 5.5 kV. Selected reaction monitoring was used to simultaneously monitor for analyte and internal standard. The following selected reaction monitoring transitions were used for the detection of analytes: 422 m/z → 377 m/z for topotecan, 482.2 m/z → 258 m/z for rosuvastatin, 393.2 m/z → 349.4 m/z for SN-38, and 687.3 m/z → 319.7 m/z for internal standard. A Q2 offset voltage of 5 V was used, and collision energy was set to be 40 eV for rosuvastatin and 25 eV for topotecan and SN-38.
Data Analysis. The equations used to calculate apparent uptake rate (Uptakeapp), apparent intrinsic biliary clearance (CLbile,int,app) (Liu et al., 1999a), and biliary excretion index [(BEI); BEI was determined using B-CLEAR technology (Qualyst, Inc., Raleigh, NC)] (Liu et al., 1999b) over a 10-min interval are shown below. In the presence of Ca2+/Mg2+, the bile canaliculi remain intact (closed), whereas in the absence of Ca2+/Mg2+, the bile canaliculi tight junctions are disrupted (opened). Therefore, quantifying the accumulation of a test compound in the presence and absence of divalent cations allows one to determine the amount of test compound in the bile canaliculi. 
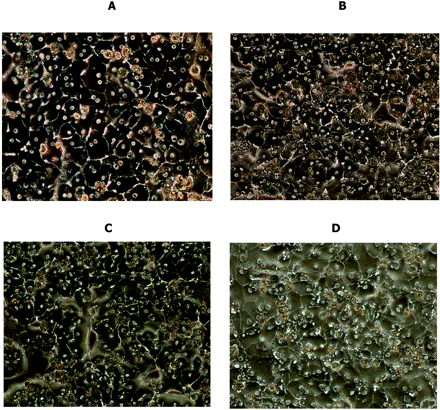
Effect of various plate coatings on the distribution of sandwich-cultured cryopreserved human hepatocytes. Sandwich cell cultures were prepared in 24-well plates using various plate coatings followed by an overlay of RC, GC, or Matrigel (M) and maintained using InVitroGRO media. The phase-contrast photos were taken on day 5. A, hepatocyte lot QKR, RC coating/RC overlay; B, hepatocyte lot QKR, GC coating/GC overlay; C, hepatocyte lot 130, Matrigel coating/Matrigel overlay; and D, hepatocyte lot 130, BioCoat plate/Matrigel overlay.
Results
Cell Culture Conditions. To determine cell culture conditions, various plate coatings, cell densities, overlay matrices, and media were examined using 24-well plates. Four separate plate coatings were tested, and it was found that hepatocytes were generally more uniformly distributed on rigid collagen (RC) and BioCoat plates compared with gelled collagen (GC) or Matrigel plates (Fig. 1). Cell densities of 0.2 × 106, 0.35 × 106, and/or 0.5 × 106 cells/well (in 500 μl) were also tested on rigid collagen-coated plates and BioCoat plates. The lowest density of cells exhibited poor plate coverage and poor cell-cell contact resulting in little or no bile canaliculi formation. Use of a cell density of 0.35 × 106 cells/well consistently resulted in near confluent distribution of cells, whereas use of 0.5 × 106 cells/well exhibited a similar degree of confluence and a similar BEI for taurocholate relative to that observed with 0.35 × 106 cells/well (data not shown). Regarding the overlay matrices, a gelled collagen overlay was tested on gelled collagen-coated plates; a Matrigel overlay was tested on Matrigel-coated plates (Fig. 1) and BioCoat plates; and a rigid collagen overlay was tested on rigid collagen-coated plates and BioCoat plates. Cell morphology was well maintained with GC/GC and similar to that observed with BioCoat/Matrigel (Fig. 1); however, using GC is difficult in a 24-well format and therefore not desirable for use in a routine assay. Both the RC/RC and BioCoat/RC configurations exhibited similar cell morphology and bile canaliculi formation. However, bile canaliculi formation seemed less extensive compared with the BioCoat/Matrigel configuration (Fig. 2), which is consistent with a single study in which the BEI of 1 μM taurocholate in both the RC/RC and BioCoat/RC configurations (34 and 39%, respectively) was significantly lower than that observed using the BioCoat/Matrigel configuration (63 ± 15%, N = 8). An additional disadvantage of using a collagen overlay is that extra care is needed when rinsing the cells, because the collagen overlay is easily dislodged, whereas this is not the case with a Matrigel overlay.
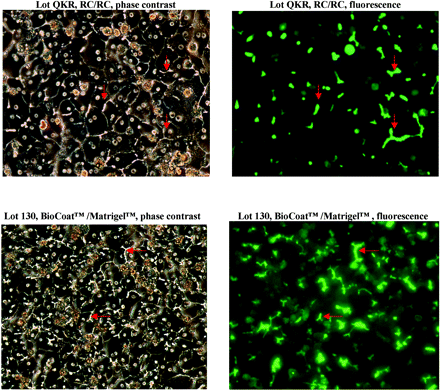
Formation of bile canaliculi in sandwich-cultured cryopreserved human hepatocytes. Sandwich cell cultures were prepared using RC or BioCoat/Matrigel in 24-well plates with InVitroGRO media. On day 5, cultures were incubated with 5 μM CDFDA for 20 min. CDFDA is hydrolyzed to fluorescent CDF inside the hepatocytes, which is then transported into bile canaliculi via MRP2. Panels on the left were obtained by phase-contrast microscopy, and panels on the right were obtained by fluorescent microscopy. Red arrows show examples of bile canaliculi.
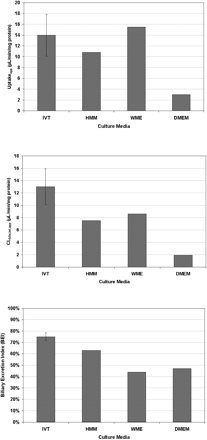
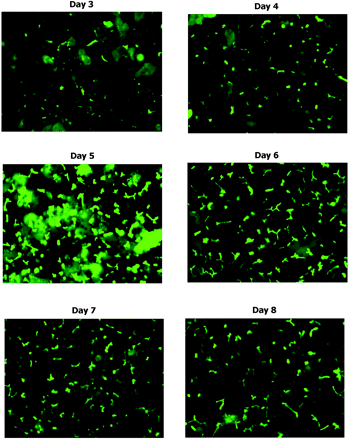
Using a BioCoat/Matrigel sandwich configuration, various cell culture media—InVitroGRO, HMM, WME, and DMEM—were examined and evaluated based upon transporter substrate function assays. Uptakeapp of taurocholate was highest with InVitroGRO and WME, but use of InVitroGRO resulted in the highest CLbile,int,app and BEI for taurocholate, whereas use of DMEM resulted in the lowest values for uptakeapp and CLbile,int,app (Fig. 3). Overall, the recommended conditions (based on experimental results and ease of use) for sandwich culture of cryopreserved human hepatocytes in a 24-well format are: BioCoat plates, a cell density of 0.35 × 106 cells/well in 500 μl (In Vitro Technologies, Inc. recommends this cell density and volume), an overlay of Matrigel and InVitroGRO media. As shown in Fig. 2, using these conditions sandwich-cultured cryopreserved human hepatocytes maintained good morphology and formed distinct bile canaliculi. In addition, a time course study showed that bile canaliculi were well formed by day 5 and that the extent of formation began to decline after day 6 (Fig. 4).
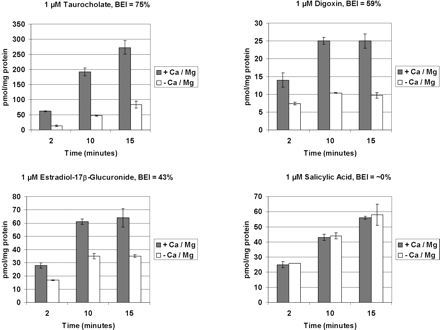
Substrate Efflux. Several compounds were chosen to assess the function of multiple uptake and efflux transporters in cryopreserved human hepatocytes cultured in a sandwich configuration. Representative plots of accumulation of compound over time are shown in Fig. 5. In this example, [3H]taurocholate, a substrate of the uptake transporters NTCP and OATPs and the efflux transporter BSEP, was rapidly taken up into human hepatocytes and effluxed with a BEI of 75%. Digoxin, a substrate for the uptake transporter OATP-8 and the efflux transporter MDR1, showed a slower uptakeapp but a significant degree of efflux as evidenced by a BEI of 59%. [3H]Estradiol-17β-d-glucuronide, a substrate of the uptake transporters OATPs and the efflux transporter MRP2, exhibited a BEI of 43%. In contrast, [14C]salicylate exhibited essentially no efflux into bile canaliculi, consistent with this compound not being excreted into bile in vivo (Laznicek and Laznickova, 1994). In addition to transport activity, reverse transcription-polymerase chain reaction analysis of day 5 samples from hepatocyte lots 130 and QKR showed expression of all tested transporters (OATP-B, OATP-C, BCRP, BSEP, MDR1, and MRP2) and Western blotting of day 5 samples from hepatocyte lots 130, QKR, or FEP using antibodies against MDR1/3, MRP2, or BCRP, respectively, showed positive bands (data not shown).
Effect of culture media on the uptake, biliary clearance, and biliary excretion of 1 μM [3H]taurocholate in sandwich-cultured human hepatocyte lot QKR. Hepatocytes were cultured in a sandwich configuration using various media and assayed for taurocholate efflux on day 5. Data are expressed as the mean ± S.D. (N = 3) for InVitroGRO media or as the average of two separate studies for HMM, WME, and DMEM.
Formation of bile canaliculi in sandwich-cultured cryopreserved human hepatocyte lot FEP over multiple days. Sandwich cell cultures were prepared using BioCoat/Matrigel in 24-well plates with InVitroGRO media. On each day from days 3 to 8, cultures were incubated with 5 μM CDFDA for 20 min. CDFDA is hydrolyzed to fluorescent CDF inside the hepatocytes, which is then transported into bile canaliculi via MRP2.
The uptake and efflux of taurocholate and digoxin were assessed in multiple lots of cryopreserved human hepatocytes as well as two lots of fresh human hepatocytes. As shown in Fig. 6, across five lots of cryopreserved hepatocytes, average taurocholate uptakeapp was high and ranged from 11 to 17 pmol/min/mg protein. Likewise, taurocholate uptakeapp in the two fresh hepatocyte lots was also high at 11 and 17 pmol/min/mg protein. The range of values for taurocholate CLbile,int,app among cryopreserved hepatocyte lots was 5.8 to 10 μl/min/mg protein, whereas the two fresh lots exhibited CLbile,int,app values of 6.9 and 9.9 μl/min/mg protein. Average BEI for taurocholate ranged from 41 to 63%, whereas the BEI for this substrate in each of two fresh human hepatocyte lots was 50 and 59%. Similar to the results presented in Fig. 5, the time course of accumulation of taurocholate in fresh and cryopreserved human hepatocytes in sandwich culture was similar (Fig. 7). As shown in Fig. 8, average uptakeapp for digoxin ranged from 0.69 to 1.5 pmol/min/mg protein, whereas uptakeapp in the two fresh hepatocytes lots were 0.75 and 1.8 pmol/min/mg protein. The range of values for digoxin CLbile,int,app among cryopreserved hepatocyte lots was 0.60 to 1.5 μl/min/mg protein, whereas the two fresh lots exhibited CLbile,int,app values of 0.5 and 2.4 μl/min/mg protein. The average BEI for digoxin in the six cryopreserved lots ranged from 37 to 63%, whereas the BEI in each of two fresh human hepatocyte lots was 33 and 56%.
Representative accumulation plots for various radiolabeled substrates using human cryopreserved hepatocytes in sandwich culture. Studies were conducted as described under Materials and Methods. Each plot represents the mean ± S.D. of three replicates at each time point generated during a single experiment. BEI was determined at 10 min.
Substrates of BCRP and MRP2 were also tested in this model. As seen in Table 2, all BCRP substrates tested showed uptake and efflux. Both rosuvastatin and mitoxantrone exhibited high uptakeapp in two separate lots of cryopreserved hepatocytes. In all lots tested, CLbile,int,app and BEI for rosuvastatin were relatively high. Cryopreserved hepatocytes also exhibited functional MRP2 activity as shown in Table 3. BSP exhibited very rapid uptakeapp in both lots of hepatocytes (35 and 37 pmol/min/mg protein) and high CLbile,int,app; however, BEI values were low (11–23%). Estradiol-17β-d-glucuronide was taken up readily into cryopreserved hepatocyte lot 130, whereas uptakeapp for lot QKR was ∼6-fold lower. This compound exhibited an CLbile,int,app of 3.8 and 1.8 μl/min/mg protein and a BEI of 36 and 37% in hepatocyte lots 130 and QKR, respectively.
Uptake, biliary clearance, and biliary excretion of BCRP substrates in sandwich-cultured cryopreserved human hepatocytes
All compounds were tested as described under Materials and Methods. Compounds were tested at 2 μM, except for mitoxantrone (1 μM). Results are presented as the average of two separate experiments, except for lot 130 rosuvastatin (mean ± S.D. of three separate experiments).
Uptake, biliary clearance, and biliary excretion of MRP2 substrates in sandwich-cultured cryopreserved human hepatocytes
All compounds were tested at 1 μM as described under Materials and Methods. Results are presented as the average of two separate experiments.
Discussion
The use of sandwich-cultured fresh hepatocytes to study hepatic uptake, metabolism, and efflux has previously been reported. However, to date, this model has only been used with fresh hepatocytes. This is a significant disadvantage, particularly with regard to human hepatocytes, because the availability of fresh human tissue of high quality is unpredictable, and when it is available, it is often at odd times. The ability to circumvent this limitation by using cryopreserved human hepatocytes is now possible because of the increased commercial availability of cryopreserved human hepatocytes. Equally important, being able to conduct sandwich culture with cryopreserved hepatocytes will allow investigators to conduct multiple studies on multiple days using hepatocytes from a single donor to assess intraassay variability and to study multiple endpoints (i.e., transport and metabolism).
The results described in this study demonstrate that cryopreserved human hepatocytes can be used in a sandwich configuration to repolarize and form intact bile canaliculi and that this model can successfully be used to measure hepatic uptake and efflux transport. The substrates tested show that there is functional NTCP- and OATP-mediated uptake and that there is functional efflux as a result of MDR1, MRP2, BSEP, and BCRP. Importantly, the uptakeapp and efflux of digoxin and taurocholate in multiple lots of cryopreserved hepatocytes were similar to that observed with two lots of fresh human hepatocytes. The ability to conduct these studies in 24-well plates has also been demonstrated, which reduces the number of hepatocytes needed per test compound and allows for more compounds to be tested in a given study. The results also show that LC/MS/MS can routinely be used to quantify the transport of unlabeled test compounds at low micromolar concentrations.
In addition to an absolute determination of uptake or efflux transport for a given substrate, this model may be used to rank compounds. For example, as seen in Figs. 6 and 8, uptakeapp and CLbile,int,app for taurocholate is much higher (∼10-fold) compared with that observed for digoxin. This is consistent with a previous in vivo study showing high clearance of taurocholate (Cowen et al., 1975) and previous in vitro and in vivo observations showing that digoxin has a relatively slow uptake into hepatocytes and its in vivo biliary clearance is low (Angelin et al., 1987; Olinga et al., 1998). It is interesting to note with taurocholate that uptakeapp and CLbile,int,app across all lots of cryopreserved and fresh hepatocytes varied by <2-fold. In contrast, uptakeapp and CLbile,int,app of digoxin across all hepatocyte lots tested ranged from 2.6- to 5-fold, respectively. For digoxin, the larger variability is primarily due to the high uptakeapp and CLbile,int,app observed for the fresh hepatocyte lot F00927. Nevertheless, to better understand the source of this variability, one would need to determine the expression of the uptake and efflux transporters in the various lots of hepatocytes in this model. Variability was also observed for estradiol glucuronide uptakeapp between hepatocyte lots 130 and QKR (Table 3). The 6-fold variability observed is similar to the 4- to 5-fold variability observed for uptake of this compound in suspension cultures of cryopreserved or fresh hepatocytes, respectively (Shitara et al., 2003).
Uptake, biliary clearance, and biliary excretion of 1 μM[3H]taurocholate in various lots of sandwich-cultured fresh or cryopreserved human hepatocytes. Data represent the average of separate studies (indicated in brackets). For lots 130, FEP, and QKR, the data represent the mean ± S.D. Fresh hepatocyte lots are indicated with an asterisk.
Comparison of [3H]taurocholate accumulation between fresh and cryopreserved hepatocytes cultured in a sandwich configuration on day 5. Results are expressed as the mean ± S.D. of four separate experiments run in triplicate (cryopreserved lot QKR) or the mean of four replicates from a single experiment (fresh, IVT lot F00927). A BioCoat/Matrigel sandwich configuration was used. The taurocholate concentration was 1 μM.
A common criticism of the sandwich culture model is that it is potentially cholestatic because the model does not currently allow for the constant draining of the bile canalicular tree that occurs in vivo. Therefore, this may result in altered regulation/expression of transporters, especially NTCP. In fact, Brouwer and coworkers (Liu et al., 1998) have shown that sandwich culturing of fresh rat hepatocytes results in a significant down-regulation of Ntcp expression. Although the temporal relationship of basolateral and apical transporter expression levels has not yet been completed with cryopreserved human hepatocytes, data from a recent publication suggest a trend toward better maintenance of NTCP expression in fresh human hepatocyte cultures compared with fresh rat hepatocyte cultures—after 3 days in culture NTCP mRNA declines <40% in human compared with >95% in rat. Likewise, NTCP-mediated activity in human hepatocyte cultures is somewhat maintained (although quite variable) after 5 days in culture (Jigorel et al., 2005). This needs to be further investigated using our current conditions with cryopreserved hepatocytes, but it nevertheless highlights the potential for species differences in this model and therefore reemphasizes the advantage of being able to conduct studies, as needed, with cryopreserved human hepatocytes.
In this article, we have used two parameters to assess biliary elimination—CLbile,int,app and BEI. It is important to note that BEI is not the in vitro equivalent of the percentage of an i.v. dose excreted in bile in vivo. BEI is the percentage of drug within the hepatocytes that has been excreted into bile canaliculi—it is primarily dependent on the transport of drug across the apical membrane. In contrast, in vivo, the amount of an i.v. administered drug recovered in the bile is dependent upon transport across the hepatic basolateral membrane and transport into the bile canaliculi, as well as any nonhepatic clearance mechanisms. Evidence has been given that, generally, BEI can be used to rank compounds as those that will exhibit a low or high percentage of biliary excretion in vivo, and it may be expected that a compound that exhibits a high biliary clearance in vivo, should exhibit a high BEI (>∼50%) in vitro (Liu et al., 1999a). However, this is not always the case as evidenced by the data with BSP (Table 3). Historically, BSP was used extensively to monitor liver function because it is rapidly taken up into the liver and readily excreted into the bile. In fact, early work with BSP showed that it accumulates in the liver to levels much higher than that in plasma because of its very high uptake rate and that biliary clearance, although high, quickly becomes saturated (Wheeler et al., 1960). Our data indeed show a very rapid uptakeapp of BSP (Table 3). This uptakeapp is much higher than even that of the endogenous bile acid taurocholate (Fig. 6). CLbile,int,app is also high and similar to that of taurocholate; however, the BEI for BSP is low and not consistent with in vivo observations. The likely reason for this discrepancy is that BSP is taken up very rapidly, accumulates in the hepatocyte, and quickly saturates efflux, as observed in vivo. The level of drug is so high initially that during the short interval of the experiment (15 min), the amount excreted in the bile is small. Therefore, this highlights the need to carefully interpret BEI data when dealing with drugs that exhibit very high uptake rates. This example also highlights the utility and preference for determining CLbile,int,app instead of BEI.
Uptake, biliary clearance, and biliary excretion of 1 μM [3H]digoxin in various lots of sandwich-cultured fresh or cryopreserved human hepatocytes. Data represent the average of separate studies (indicated in brackets). For lot FEP, the data represent the mean ± S.D. Fresh hepatocyte lots are indicated with an asterisk.
In summary, the ability to use cryopreserved human hepatocytes in sandwich culture to form intact bile canaliculi and to exhibit functional uptake and efflux transport has been successfully demonstrated. The higher capacity afforded by the use of 24-well plates and the ability to quantify test compounds via LC/MS/MS make this an attractive model within the pharmaceutical industry. Use of cryopreserved hepatocytes allows studies to be conducted as needed. In addition, the use of cryopreserved hepatocytes could allow for pools of multiple donors to be tested in this model if desired. Studies are ongoing to further characterize hepatobiliary disposition using cryopreserved human hepatocytes in this model.
Footnotes
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.105.009118.
-
ABBREVIATIONS: IVT, In Vitro Technologies, Inc.; HMM, hepatocyte maintenance media; WME, Williams' media E; DMEM, Dulbecco's modified Eagle's media; HBSS, Hanks' balanced salt solution; MRP (ABCC), human multidrug resistance-associated protein; MDR (ABCB), human multidrug resistance; BCRP, human breast cancer resistance protein; CDFDA, 5-(and-6)-carboxy-2′,7′-dichloro-fluorescein diacetate; EG, estradiol-17β-d-glucuronide; BSP, sulfobromo-phthalein; CDF, 5-(and-6)-carboxy-2′,7′-dichloro-fluorescein; FBS, fetal bovine serum; LC/MS/MS, liquid chromatography/tandem mass spectrometry; SN-38, 7-ethyl-10-hydroxycamptothecin; uptakeapp, apparent uptake rate; CLbile,int,app, apparent intrinsic biliary clearance; BEI, biliary excretion index; RC, rigid collagen; GC, gelled collagen; NTCP (SLC10A1), human Na+ taurocholate cotransporting polypeptide; OATP (SLCO), human organic anion transporting polypeptide; BSEP (ABCB11), human bile salt export pump.
- Received December 23, 2005.
- Accepted June 14, 2006.
- The American Society for Pharmacology and Experimental Therapeutics