Abstract
Inflammatory bowel disease (IBD) is an inflammatory condition that affects the gastrointestinal tract. The solute carrier (SLC) superfamily of transporters comprise proteins involved in the uptake of drugs, hormones, and other biologically active compounds. The purpose of this study was to determine the mRNA expression levels of 15 solute carrier transporters in two regions of the intestine in IBD patients. Endoscopic biopsy specimens were taken from two locations (terminal ileum and colon) for histological examination and RNA extraction. We quantitatively measured the mRNA expression of 15 SLC transporters in 107 IBD patients (53 with Crohn's disease and 54 with ulcerative colitis) and 23 control subjects. mRNA expression was evaluated using the quantitative reverse transcription-polymerase chain reaction technique. We observed that in the ileum of IBD patients, mRNA levels for serotonin transporter, equilibrative nucleoside transporter (ENT) 1, ENT2, and organic anion-transporting polypeptide (OATP) 2B1 were significantly elevated, whereas levels for apical sodium-dependent bile acid transporter (ASBT) and organic zwitterion/cation transporter (OCTN) 2 were significantly lower. In colon, mRNA levels for ENT1, ENT2, concentrative nucleoside transporter (CNT) 2, OATP2B1, and OATP4A1 were significantly higher, whereas mRNA levels for OCTN2 were significantly decreased. In inflamed colon of IBD patients the mRNA expression levels of ENT1, ENT2, CNT2, OATP2B1, OATP4A1, and peptide transporter 1 were significantly higher. We conclude that intestinal SLC mRNA levels are dysregulated in IBD patients, which may be linked to the inflammation of the tissue and provides an indication about the role of inflammatory signaling in regulation of SLC expression.
The solute carrier (SLC) superfamily of transporters consists of more than 300 members subdivided into 47 families. They are expressed in most tissues, but primarily in the liver, lung, kidney, and intestine. Most solute carrier transporters are localized at either the basolateral or apical plasma membrane of polarized cells, but some are expressed in mitochondria and other organelles. The expression levels of SLCs are modulated by cytokines, hormones, growth factors, extracellular signals, and changes in the metabolic state of the cell. Typical SLC transporters consist of several transmembrane α-helices connected by intra- and extracellular loops and function as either monomers or hetero- or homodimers. SLC transporters are responsible for the uptake of amino acids, peptides, ions, xenobiotics, drugs, and other biologically active compounds (Koepsell et al., 2007). The driving force of SLCs is mostly ion-dependent, but some of them function as equilibrative transporters.
Inflammatory bowel disease (IBD) is a disease affecting the inner lining of the gut and can be divided into two entities: Crohn's disease (CD) and ulcerative colitis (UC). Until now, there have been only a few studies implicating the involvement of SLC family members in the pathophysiology of IBD and/or other inflammatory conditions. For example, the expression and activity of the peptide transporter 1 (PEPT1) (gene symbol SLC15A1) has been shown to be increased by proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interferon-γ (Vavricka et al., 2006). This observation was proposed to be linked with the PEPT1-mediated uptake of muramyl dipeptide, a degradation product of the bacterial outer wall, followed by activation of the intracellular receptor NOD1 and consequently of the transcription factor nuclear factor-κB. PEPT1 was also reported to be involved in the suppression of inflammation in mouse models of colitis because of uptake of the therapeutic tripeptide KPV (Dalmasso et al., 2008). Another SLC transporter implicated in IBD is the organic cation/zwitterion transporter 2 (OCTN2) (gene symbol SLC22A5). It has been reported that SLC22A genes are high susceptibility genes in IBD, located at the so-called IBD locus 5 (IBD5) and that genetic polymorphisms within this region contribute to the inflammatory phenotype (Waller et al., 2006; Cucchiara et al., 2007; Silverberg et al., 2007). These observations are consistent with a mouse study showing that homozygous inactivation of Octn2 resulted in spontaneous development of colitis (Shekhawat et al., 2007). Decreased expression levels of Octn2 and another carnitine transporter Atb0+ were also shown to be associated with development of an inflammatory condition in rats (D'Argenio et al., 2006). More recently, the mRNA expression levels of OCTN1 and OCTN2 were reported to be significantly decreased in patients with UC (Noble et al., 2008). Another SLC superfamily member implicated in the pathogenesis of IBD is the apical sodium-dependent bile acid transporter (ASBT) (gene symbol SLC10A2). It has been shown that the protein levels of this transporter are lower in patients with CD than in control individuals (Jung et al., 2004). It has been proposed that ASBT mRNA levels are decreased by proinflammatory cytokines via a mechanism that involves the c-Fos transcription factor in human, rat, and mouse intestinal epithelium (Neimark et al., 2006). In addition, the expression and function of another SLC superfamily member, serotonin transporter (SERT) (gene symbol SLC6A4), is affected by 2,4,6-trinitrobenzenesulfonic acid-induced inflammation in mice (Linden et al., 2005). In concert with this finding, it has been reported that function and expression of SERT are decreased by the proinflammatory cytokines TNF-α and interferon-γ in human intestine-derived cells (Foley et al., 2007). These observations suggest that certain SLC transporters are affected in inflammatory conditions, such as IBD. Given the physiological function of SLCs, they may contribute to the pathology of this disease, or their function may be affected as a consequence of inflammation.
We have recently described the distribution of 15 SLC transporters along the human intestinal tract (Meier et al., 2007). This group included PEPT1, OCTN1, and OCTN2, the concentrative nucleoside transporters (CNTs) (gene family SLC28), ASBT, the organic anion-transporting polypeptides (OATPs) (gene family SLCO), the equilibrative nucleoside transporters (ENTs) (gene family SLC29), the organic cation transporter 1 (OCT1) (gene symbol SLC22A1), the organic anion transporter 2 (OAT2) (gene symbol SLC22A7), and SERT. In the present study, we have determined the expression levels of these 15 SLC transporters in terminal ileum and colon of patients with IBD in comparison with non-IBD control patients. Localization, function, and implicated association with IBD of these transporters are summarized in Table 1.
Characteristics of 15 solute carrier transporters investigated in this study
Materials and Methods
Patients and Colonoscopy. In this study, 53 patients with UC and 49 patients with CD as well as 23 control subjects were enrolled after having given their informed consent. Diagnosis of patients with UC and CD was based on clinical history, laboratory findings, and endoscopic and histological criteria. Tissue biopsy sampling was performed by experienced gastroenterologists. For patients with active disease (patients with newly diagnosed disease and patients with refractory disease) biopsy specimens were taken both from the inflamed and the unaffected region (paired biopsies). Unaffected areas were defined as mucosa regions without any macroscopic/endoscopic signs of inflammation (ulceration, edema, discoloration, hemorrhagic appearance, or mucinous/fibrinous coating); these biopsies were obtained at least 10 to 15 cm distant from the inflamed area. Control subjects had an indication for a gastrointestinal tract endoscopy for reasons not related to IBD. Biopsy specimens were obtained during routine endoscopy, submerged in RNAlater solution (Ambion, Austin, TX), and stored at –80°C until further processing. The summary of patients' characteristics is presented in Table 2. Additional patient information is shown in Supplemental Tables 1 and 2.
Summary of patients included in the study
Isolation of RNA from Biopsy Specimens. For RNA isolation from biopsy specimens obtained from IBD patients, intestinal biopsy specimens were homogenized for 30 s (Polytron PT 2100; Kinematika AG, Littau, Switzerland), and RNA was extracted using the RNeasy Mini Kit (QIAGEN GmbH, Hilden, Germany) following the instructions provided by the manufacturer. Control biopsy specimens were syringed in TRIzol reagent, and RNA was extracted according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). RNA was quantified with a GeneQuant spectrophotometer (GE Healthcare, Little Chalfont, Buckinghamshire, UK) and an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE).
Synthesis of cDNA and Quantitative Real-Time PCR. cDNA was produced using the reverse transcription kit (Promega, Madison, WI) with 1.2 μg of RNA as a template and random hexamers as primers. The product was diluted with H2O to a total volume of 120 μl. For each PCR reaction 2 μl of the cDNA was used in the final reaction volume of 10 μl. SLC expression levels were determined by TaqMan real-time PCR and absolute quantification. The cycling conditions were identical to those used in our previous study: stage 1 (50°C, 2 min); stage 2 (95°C, 10 min); and stage 3: 40 repeats (95°C, 15 s; 60°C, 1 min). Based on our previous observations, for detectable targets each PCR was performed in duplicate; for targets below the detection limit PCR was performed as a single measurement. PCR for villin was performed in triplicate. For each assay, a dilution series (12, 120, 1200, 12,000, 120,000, and 12,000,000 copies) of the cloned PCR product was quantified in parallel. The expression levels of the target genes in each sample were determined by the ratio of cDNA copies of a target gene versus cDNA copies of villin. The samples below the cutoff limit (<0.01 ratio of target cDNA copies versus villin cDNA copies) were considered undetectable. All of the TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) used in this study were the same as those used in our previous study (Meier et al., 2007).
Statistical Analysis. Basic statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA). Statistical significance of the differences in mRNA expression levels between the patient subgroups was determined by performing an analysis of variance (Kruskal-Wallis test) with Dunn's multicomparison test. For paired biopsy analysis, statistical significance was determined using a paired t test. The effect of steroid therapy, age, gender, and smoking on expression levels was analyzed using mixed models of analysis of variance with patients as random factors. Here, the values of mRNA expression levels were logarithmically transformed before analysis was performed using SPSS 13 (SPSS Inc., Chicago, IL) (Supplemental Table 3).
Ethical Considerations. The study protocol and consent forms were approved by the State Ethical Committee of Basel and University Hospital Zurich before the start of the study. The sample collection was carried out at University Hospital Basel and University Hospital Zurich between 2001 and 2007.
Results
mRNA Expression of SLC Transporters in Terminal Ileum of IBD Patients. Despite considerable individual variation between patients within each subgroup, we observed clear and significant differences in SLC mRNA expression levels in IBD patients compared with the control group. We found that in terminal ileum, mRNA expression levels of ASBT and OCTN2 were significantly decreased in IBD patients (Table 3). In addition, the mRNA expression levels of CNT2 were decreased in IBD patients, but the decrease was not statistically significant. The elevation of mRNA levels of SERT, ENT1, ENT2, and OATP2B1 in the ileum of IBD patients was statistically significant, but the increase in mRNA levels of PEPT1 and OCTN1 was not. The increase in the expression levels of CNT1 was only significant in CD patients. We observed statistically significant elevation in mRNA expression of OATP4A1, but its levels in IBD patients remained on the border of the detection limit. The mRNA levels of CNT3, OAT2, OATP1A2, and OCT1 were below the detection limit.
Relative mRNA expression levels of 15 SLC transporters
The expression level of the target genes in each sample was evaluated based on the ratio of cDNA copies of a target gene vs. cDNA copies of villin. Numbers represent mean values (± S.D.). Targets with detectable mRNA expression levels and statistically significant changes are indicated in boldface.
mRNA Expression of SLC Transporters in Colon of IBD Patients. In colon, we observed that OCTN2 mRNA levels were significantly decreased in IBD patients (Table 3). The mRNA levels of ENT1, ENT2, OATP2B1, OATP4A1, and CNT2 were significantly increased, whereas the increase in the mRNA expression of OCTN1 was statistically significant only in UC patients, and the increase in mRNA expression of PEPT1 was significant only in CD patients. The mRNA levels of ASBT, SERT, CNT1, CNT3, OAT2, and OATP1A2 in colon of IBD patients were below the detection limit. OCT1 mRNA was detected, but its levels were not altered in IBD patients. Among 15 targets tested, only 2 exhibited significant differences between CD and UC patients, namely OATP2B1 and OATP4A1. Both OATP2B1 and OATP4A1 expression levels were higher in UC patients.
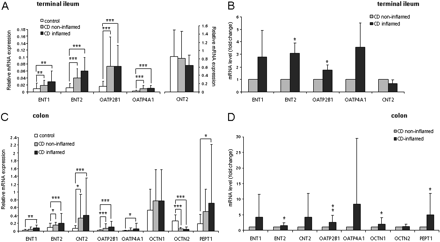
Changes in mRNA Expression Levels of SLC Transporters in Inflamed Tissues of CD Patients. Analysis of the biopsy specimens based on the inflammatory status of the tissue revealed that changes in mRNA expression levels of certain SLC transporters in CD patients were correlated with the inflammation (Fig. 1). In terminal ileum, expression levels of ENT1 and ENT2 in the inflamed tissues were higher than those in noninflamed tissues, but this difference was not statistically significant (Fig. 1A). The mRNA expression levels of OATP2B1 and OATP4A1 were not changed, whereas the expression of CNT2 was decreased, but not significantly. To exclude the influence of individual variations in basal mRNA levels of SLC transporters we next analyzed only paired biopsies from terminal ileum of CD patients. We observed that the increase in mRNA expression levels of ENT2 and OATP2B1 between the noninflamed and inflamed tissues became significant (Fig. 1B). The changes in mRNA expression levels of ENT1, OATP4A1, and CNT2 were still apparent but did not reach statistical significance in paired samples. Likewise, compared with levels in noninflamed tissues, mRNA levels of ENT1, ENT2, CNT2, OATP2B1, OATP4A1, and PEPT1 were higher, but not significantly, in the inflamed colonic tissues of all CD patients (Fig. 1C). The expression levels of OCTN1 and OCTN2 were not changed upon inflammation. When only paired biopsies were analyzed, the mRNA levels of ENT2, OATP2B1, and PEPT1 were significantly increased in colon of CD patients. Interestingly, we observed a significant increase in mRNA levels of OCTN1, but not OCTN2, when only paired biopsies were included in the analysis. The mRNA levels of ENT1, CNT2, and OATP4A1 were increased, but not significantly (Fig. 1D).
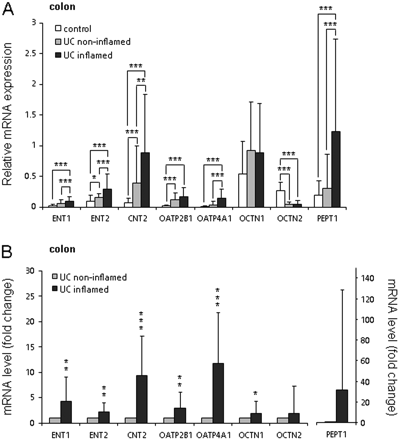
Changes in mRNA Expression Levels of SLC Transporters in Inflamed Colon of UC Patients. In colon of UC patients, the inflamed tissues expressed significantly higher mRNA levels of ENT1, ENT2, CNT2, OATP4A1, and PEPT1. Similarly to colon of CD patients, the levels of OCTN1 and OCTN2 in UC patients were not changed upon inflammation (Fig. 2A). When paired biopsies were analyzed, the mRNA levels of ENT1, ENT2, CNT2, OATP2B1, OATP4A1, and OCTN1 were significantly higher. Despite the strong tendency for an increase, the elevation of the mRNA levels of PEPT1 in inflamed tissue did not reach statistical significance, when paired biopsies were analyzed (Fig. 2B).
Changes in mRNA expression levels of SLCs in the inflamed tissues of CD patients. A, mRNA expression levels of ENT1, ENT2, but not of OATP2B1 and OATP4A1, are elevated in inflamed tissues of terminal ileum of all CD patients in the study. Mean values of mRNA expression levels are presented. Error bars indicate S.D. values. B, paired analysis of inflamed and noninflamed tissues from the same patients shows a significant increase in ENT2 and OATP2B1 in terminal ileum. The mRNA expression levels in noninflamed tissues were set to 1. C, mRNA expression levels of ENT1, ENT2, CNT2, OATP2B1, OATP4A1, and PEPT1 are increased in inflamed colon of CD patients. Averages of mRNA expression levels are presented. D, paired comparison of inflamed and noninflamed tissues shows a significant increase in ENT2, CNT2, OATP2B1, OCTN1, and PEPT1 mRNA levels. The mRNA expression levels in noninflamed tissues were set to 1. *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
Changes in mRNA Expression Levels of SLC Transporters in IBD Patients Receiving Steroid Therapy. Next, we examined whether SLC mRNA levels in IBD patients were influenced by any medication taken. We found a strong correlation between colonic mRNA expression levels of OCTN2 and steroid therapy in male patients (Table 4): an approximately 2.5-fold increase in OCTN2 expression in male patients with both CD (from 0.02 to 0.05) and UC (from 0.04 to 0.09). In addition, in male CD patients, steroid therapy decreased mRNA levels of ASBT in terminal ileum, but this increase was not statistically significant. The mRNA levels of OATP4A1 in UC patients taking steroids were increased 2-fold, but this change was not statistically significant. All of the changes in mRNA expression levels of SLCs in IBD patients taking steroids are summarized in Table 4.
Changes in the mRNA expression levels of SLCs in IBD patients receiving steroid therapy
Statistical significance was estimated using an unpaired t test with two-tailed p values.
Discussion
SLC transporters play an important role in the absorption of drugs and biologically active compounds in the intestine. Determining expression levels of SLC transporters in IBD intestine provides an indication about the role of inflammatory signaling cascades on regulation of SLC expression. Because we did not observe any significant gender- or age-dependent changes in mRNA expression levels in either of two intestinal regions of IBD patients (Supplemental Table 3), we propose that these differences in mRNA expression levels may be attributed to inflammation and/or therapy. We observed certain small, but significant, differences in the expression levels of some SLCs in both regions of the control group, compared with the control levels reported in our previous study (Meier et al., 2007). Significant differences in mRNA levels were detected for four of nine detectable target genes in terminal ileum (PEPT1, ASBT, CNT1, and CNT2) and for three of nine detectable target genes in colon (OCT1, CNT2, and ENT2). These differences may derive from individual variations in mRNA levels of these transporters among the patients in each subgroup and be dependent on variables such as individual differences in medication.
We observed significantly decreased ASBT mRNA levels in IBD patients—an observation that has previously been reported for CD patients at the ASBT protein level (Jung et al., 2004). Although we did not observe any changes in ASBT mRNA levels in the inflamed tissues, steroid therapy seems to be associated with a tendency for decreased ASBT mRNA levels in terminal ileum of male CD patients. This finding is in apparent disagreement with the previous observation that CD patients undergoing steroid treatment exhibit increased levels of ASBT (Jung et al., 2004). This contradiction may be explained by the small numbers of patients used in the previous study and larger heterogeneity of the dose- and time-dependent treatment of IBD population tested in the present study.
We also observed a significant decrease in mRNA levels of the carnitine transporter OCTN2 in IBD patients. The OCTN2 protein is encoded by the IBD5 locus and polymorphisms in the SLC22A5 coding regions have been linked to IBD pathology (Russell et al., 2006; Waller et al., 2006; Cucchiara et al., 2007; Silverberg et al., 2007). In rats, a decrease in Octn2 expression levels triggers inflammation of the gastrointestinal tract (Shekhawat et al., 2007), possibly due to an aberration of carnitine transport and fatty acid metabolism (D'Argenio et al., 2006). It is interesting to note that a decrease in mRNA expression of OCTN2 was recently reported in the sigmoid region of UC colon (Noble et al., 2008), although to a lesser extent than to the decrease observed here in colon and terminal ileum (Table 3). Low levels of OCTN2 even in noninflamed tissues from IBD patients could result from either a specific genetic background, e.g., polymorphisms/mutations in the OCTN2 gene and/or its promoter sequence, or it could be due to suppression by circulating proinflammatory cytokines. Interestingly, we observed a slight but not statistically significant decrease in OCTN2 mRNA levels in all inflamed tissues compared with all biopsy specimens from noninflamed tissue (Figs. 1C and 2A). Moreover, a closer examination of paired biopsies reveals that 50% of CD patients and 82% of UC patients express lower mRNA levels of OCTN2 in paired inflamed tissues (data not shown). In addition, we demonstrated higher mRNA expression levels of OCTN2 in patients taking steroids (Table 4). Moreover, in patients in remission of the disease, colonic OCTN2 mRNA levels were slightly but not significantly higher than those in CD patients not in remission (data not shown), suggesting that elevation of the mRNA levels of OCTN2 might be correlated with an overall therapeutic effect. All of these observations support the idea of OCTN2 being associated with IBD and/or inflammation. The exact mechanisms by which the OCTN2 protein contributes to the pathogenesis of IBD remain unclear.
Changes in mRNA expression levels of SLCs in the inflamed colon of UC patients. A, mRNA expression levels of ENT1, ENT2, CNT2, and OATP4A1, and PEPT1 are significantly higher in the inflamed tissues. mRNA levels of OCTN1 and OCTN2 are not significantly changed. Mean values of mRNA expression levels are presented. Error bars indicate SD values. B, paired comparison of inflamed and noninflamed tissues from the same patients shows a significant increase in mRNA levels of ENT1, ENT2, CNT2, OATP2B1, OATP4A1, and OCTN1. Elevation of mRNA levels of OCTN2 and PEPT1 is not significant. The mRNA expression levels in noninflamed tissues were set to 1. *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
We observed an increase in mRNA levels of most intestinal nucleoside transporters in colon and in terminal ileum. It has been reported that altered expression levels of certain nucleoside transporters are associated with pathological states, such as ampullary cancer (Santini et al., 2008) and human immunodeficiency infection (Guallar et al., 2007). The relevance of the observed increase in nucleoside transporter expression in IBD intestine remains unclear. Elevated levels of nucleoside transporters may trigger an influx of nucleosides, such as adenosine, and may be responsible for inflammation of the tissue, similarly to the mechanism proposed for inflammatory lung diseases (Hirsh et al., 2007). In support of this finding, mRNA levels of nucleoside transporters are elevated in the inflamed tissues of IBD patients (Figs. 1 and 2). This observation may also imply a role for proinflammatory cytokines in transcriptional regulation of nucleoside transporter genes in intestinal cells, as already reported in the context of liver parenchymal cells (Fernández-Veledo et al., 2004) and macrophages (Soler et al., 2001). Whereas we did not observe any changes in nucleoside transporter mRNA levels in patients receiving standard therapies, it would be interesting to measure mRNA levels of nucleoside transporters in IBD patients taking anti-TNF-α medications, such as infliximab or adalimumab.
Another group of target genes exhibiting increased mRNA levels in IBD patients is the family of organic anion-transporting polypeptides. In both terminal ileum and colon, we observed increased mRNA expression levels of OATP2B1 and OATP4A1 in IBD patients. This elevation is correlated with the inflammatory status of the tissue in both intestinal regions and is most significant in colon of UC patients (Fig. 2). It has been reported that the mRNA levels of murine hepatic Oatp2 are sensitive to proinflammatory signaling (Hartmann et al., 2002), but until now there has been no clear indication that OATPs may be involved in the pathology of IBD in humans.
We also observed increased mRNA expression of PEPT1 in both regions of the intestine in IBD patients. This elevation was statistically significant in colon of both CD and UC patients and was correlated with the inflammation of the tissue. Elevated expression of PEPT1 has been previously implicated in inflammation and the pathology of IBD (Vavricka et al., 2006).
The intestinal SLC mRNA levels are dysregulated in IBD patients, which may be linked to the inflammatory status of the affected tissue. Determining expression levels of SLC transporters in inflamed and noninflamed intestine provides an indication about the role of inflammatory signaling cascades on regulation of SLC expression. Although it is important to bear in mind the possibility that changes in mRNA levels may not fully correlate with such alterations in the corresponding protein levels, we believe that the expression levels of SLCs may help to assess the severity of the disease and/or serve as a prognostic factor for patients taking certain medications and that this information may help to optimize drug therapies for IBD patients.
Acknowledgments
We acknowledge Christian Hiller and Dr. Christa Wenger for providing additional cDNA of control samples and for helping with the analysis of patients' medication. We also thank Martin Moser for helping with the data collection, Ursula Behrens for helping with the transfer of the samples, and Prof. Burkhardt Seifert for performing additional statistical analysis. In addition, we acknowledge Dr. Sarah Haile for helping with the choice of a suitable statistical analysis.
Footnotes
-
This work was supported by the Swiss National Science Foundation [Project Grants 320000-114009/1, 32-120463/1, 3347CO-108792 (Swiss IBD Cohort), and PASMA-114623]; and by the Zurich Center of Integrative Human Physiology.
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.109.027367.
-
ABBREVIATIONS: SLC, solute carrier; IBD, inflammatory bowel disease; CD, Crohn's disease; UC, ulcerative colitis; PEPT, peptide transporter; TNF-α, tumor necrosis factor-α; OCTN/Octn, organic zwitterion/cation transporter; ASBT, apical sodium-dependent bile acid transporter; SERT, serotonin transporter; CNT, concentrative nucleoside transporter; OATP/Oatp, organic anion-transporting polypeptide; ENT, equilibrative nucleoside transporter; OCT, organic cation transporter; OAT, organic anion transporter; PCR, polymerase chain reaction.
-
↵
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material. - Accepted May 28, 2009.
- Received March 3, 2009.
- The American Society for Pharmacology and Experimental Therapeutics