Abstract
Interleukin (IL) 1β is a proinflammatory cytokine known to markedly alter expression of major organic anion transporters in rodent hepatocytes. However, its effects toward human hepatic transporters remain poorly characterized. Therefore, the present study was aimed at determining IL-1β effects on expression of organic anion transporters in primary human hepatocytes and highly differentiated human hepatoma HepaRG cells. Exposure to 1 ng/ml IL-1β was first shown to markedly repress mRNA expression of sodium-taurocholate cotransporting polypeptide (NTCP), a major sinusoidal transporter handling bile acids, in both human hepatocytes and HepaRG cells. It concomitantly reduced NTCP protein levels and NTCP-mediated cellular uptake of taurocholate in HepaRG cells. Other transporters such as the influx transporters organic anion transporting polypeptide (OATP)-B, OATP-C, and OATP8 and the efflux pumps multidrug resistance-associated protein (MRP) 2, MRP3, MRP4, and breast cancer resistance protein were also down-regulated at mRNA levels in human hepatocytes treated by IL-1β for 24 h, and most of these transporters were similarly repressed in IL-1β-exposed HepaRG cells; the cytokine also reduced bile salt export pump (BSEP) and OATP-C protein expression in human hepatocytes. IL-1β was further shown to activate the extracellular signal-regulated protein kinase (ERK) in human hepatocytes and HepaRG cells; however, chemical inhibition of this kinase failed to counteract repressing effects of IL-1β toward NTCP, BSEP, OATP-B, and OATP-C. Taken together, these data indicate that IL-1β treatment reduced expression of major organic anion transporters in human hepatic cells in an ERK-independent manner. Such IL-1β effects may likely participate in both cholestasis and alterations of hepatic detoxification pathways caused by inflammation in humans.
Interleukin (IL) 1β, a major proinflammatory cytokine contributing to endotoxin- or sepsis-induced cholestasis in liver, impairs hepatic detoxification pathways (Prandota, 2005; Aitken et al., 2006). Thus, IL-1β decreases expression of various drug-metabolizing enzymes, including cytochromes P450 and glutathione S-transferases, in human and rat hepatocytes (Abdel-Razzak et al., 1993; Maheo et al., 1997). In addition, it interferes with drug-related regulation of detoxifying proteins (Assenat et al., 2004).
Organic anion transporters handling bile acids or drugs also constitute important targets of IL-1β in rodents (Geier et al., 2007; Petrovic et al., 2007). Indeed, IL-1β-treated mice display reduced expression of various hepatic transporters, including solute carrier (SLC) proteins like sinusoidal sodium-dependent taurocholate transporter Ntcp (Slc10a1) and organic anion transporting polypeptides (Oatp) 1 (Slco1a1) and Oatp2 (Slco1a2), and ATP binding cassette (ABC) transporters like bile salt export pump (Bsep) (Abcb11) and multidrug resistance-associated protein (Mrp) 2 (Abcc2) (Hartmann et al., 2002; Geier et al., 2005). In addition, Ntcp and Mrp2 are also down-regulated in primary rat hepatocytes exposed to IL-1β (Denson et al., 2002; Li et al., 2002). However, IL-1β-mediated regulation of hepatic organic anion transporters remains much less characterized in human hepatocytes than in their rodent counterparts, even if MRP2 (ABCC2) and MRP3 (ABCC3) have been shown to constitute targets of IL-1β in certain human hepatoma cell lines (Lee and Piquette-Miller, 2003; Hisaeda et al., 2004). In this context, it is noteworthy that an inverse correlation between sodium-taurocholate co-transporting polypeptide (NTCP) (SLC10A1) mRNA levels and IL-1β secretion has been recently reported in human liver slices exposed to lipopolysaccharide (LPS) (Elferink et al., 2004), suggesting that at least some human organic anion transporters, especially NTCP, may be down-regulated by IL-1β, as their rodent counterparts. The present study was designed to investigate this hypothesis. Using human primary hepatocytes and human highly differentiated hepatoma HepaRG cells, well recognized as convenient models for studying regulation of transporters (Jigorel et al., 2006; Le Vee et al., 2006), we report that IL-1β treatment markedly reduced functional expression of NTCP in human hepatocytes; mRNA levels of other major organic anion transporters, especially BSEP (ABCB11) and OATPs (SLCOs), were also decreased.
Materials and Methods
Chemicals and Reagents. Recombinant human IL-1β was provided by R&D Systems (Minneapolis, MN). [3H(G)]taurocholic acid (sp. act. 1.19 Ci/mmol) and [6, 7-3H(N)]estrone-3-sulfate (sp. act. 57.3 Ci/mmol) were purchased from PerkinElmer Life Sciences (Boston, MA). Probenecid and the extracellular signal-regulated protein kinase (ERK) inhibitor U0126 were from Sigma Aldrich (Saint-Quentin Fallavier, France). All the other compounds and reagents were commercial products of the highest purity available. Vehicles for IL-1β and U0126 were phosphate-buffered saline and dimethylsulfoxide, respectively; control cultures received the same dose of vehicles as treated counterparts.
Cell Isolation and Culture. Human hepatocytes were obtained from adult donors undergoing hepatic resection for primary and secondary tumors via the Biological Resource Center (Rennes, France). Cells were prepared by perfusion of histologically normal liver fragments using a collagenase solution (Jigorel et al., 2005). They were primary cultured on plastic dishes in Williams' E medium, as already reported (Chouteau et al., 2001; Jigorel et al., 2005). All the experimental procedures complied with French laws and regulations and were approved by the National Ethics Committee. Human hepatoma HepaRG cells were cultured in Williams' E medium supplemented with 10% fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 5 μg/ml insulin, and 5 × 10–5 M hydrocortisone hemisuccinate; their hepatocytic differentiation was induced by addition of 2% dimethylsulfoxide for 2 weeks as previously described (Gripon et al., 2002).
RNA Isolation and Analysis. Total RNA was isolated from cells using the TRIzol reagent (Invitrogen, Cergy-Pontoise, France). RNA was then subjected to reverse transcription-real time quantitative polymerase chain reaction (RT-qPCR) using the fluorescent dye SYBR green methodology and an ABI Prism 7000 detector (Applied Biosystems, Foster City, CA) (Jigorel et al., 2005). Most of the primers were exactly as previously described (Jigorel et al., 2006). Other primers were MRP4 sense, GCTCAGGTTGCCTATGTGCT, MRP4 antisense, CGGTTACATTTCCTCCTCCA; C-reactive protein (CRP) sense, GAACTTTCAGCCGAATACATCTTTT, CRP antisense, CCTTCCTCGACATGTCTGTCT; IL-8 sense, AAGAAACCACCGGAAGGAAC, IL-8 antisense, AAATTTGGGGTGGAAAGGTT; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense, GGCATGGACTGTGGTCATGAG, GAPDH antisense, TGCACCACCAACTGCTTAGC. Relative quantification of the steady-state target mRNA levels was calculated after normalization of the total amount of cDNA tested to a GAPDH endogenous reference.
Western Blot Analysis. Total cellular protein and crude membrane extracts were prepared from HepaRG cells and primary hepatocytes as previously described (Jigorel et al., 2006). Proteins were then separated on polyacrylamide gels and electrophoretically transferred to nitrocellulose membranes. After blocking in Tris-buffered saline containing 4% bovine serum albumin, membranes were incubated overnight at 4°C with primary antibodies directed against NTCP (Kullak-Ublick et al., 1997), OATP-C (Zollner et al., 2003), BSEP (Alexis Biochemicals, Lausen, Switzerland), or phospho-ERK (Cell Signaling Technology, Danvers, MA). Peroxidase-conjugated monoclonal antibodies were thereafter used as secondary antibodies. After washing, immunolabeled proteins were visualized by chemiluminescence. Gel loading and transfer were checked by staining membranes with ponceau red.
Transport Assays. Transport activity caused by NTCP or OATPs was analyzed through measuring sodium-dependent intracellular accumulation of the NTCP substrate taurocholate or probenecid-sensitive uptake of the OATP substrate estrone-3-sulfate, as previously described (Jigorel et al., 2005). Briefly, cells were incubated at 37°C for 30 min with 0.17 μM[3H]taurocholate in the presence or absence of sodium or with 3.4 nM [3H]estrone-3-sulfate in the presence or absence of the OATP inhibitor probenecid used at 2 mM. After washing in phosphate-buffered saline, cells were lysed in distilled water, and accumulation of radiolabeled substrates was determined through scintillation counting. Taurocholate accumulation values in the presence of sodium minus accumulation values in the absence of sodium and estrone-3-sulfate uptake values in the absence of probenecid minus uptake values in the presence of probenecid are thought to represent NTCP and OATP activities, respectively (Jigorel et al., 2005; Le Vee et al., 2006).
Statistical Analysis. Quantitative data were usually expressed as mean ± S.E.M. They were statistically analyzed using the Student's t test or by analysis of variance followed by the Newman-Keuls test. The criterion of significance was p < 0.05.
Results and Discussion
We first determined whether the cellular models retained for our study, i.e., primary human hepatocytes and highly differentiated hepatoma HepaRG cells, were convenient for analyzing the effects of IL-1β when used at a low concentration (1 ng/ml) close to those observed in physiological situations and which was noncytotoxic as shown by light microscopic examination of the cultures and measurement of cell viability using the colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (data not shown). For this purpose, we analyzed expression of referent inflammation markers such as CRP and IL-8, well known targets of IL-1β (Rowell et al., 1997; Kleemann et al., 2003). As shown in Table 1, IL-1β treatment for 8 or 24 h was able to highly induce mRNA expression of CRP and IL-8 in both primary hepatocytes and HepaRG cells. This indicated that these cells were fully responsive to the cytokine and thus were suitable for investigating IL-1β effects toward organic anion transporter expression.
Effects of IL-1β treatment on expression of referent inflammation markers
We next analyzed NTCP expression in IL-1β-treated human hepatic cells because Ntcp is a major and well established target of IL-1β in rodent hepatocytes (Hartmann et al., 2002; Li et al., 2002; Geier et al., 2003). Moreover, analysis of sodium-dependent and -independent taurocholate uptake in hepatic cells (Fig. 1A) indicated that sodium-dependent accumulation of taurocholate represented 94 and 91% of taurocholate uptake in human hepatocytes and HepaRG cells, respectively, fully supporting that NTCP is the main transporter involved in bile acid uptake in human hepatic cells. Treatment by IL-1β was found to down-regulate NTCP mRNA expression in both human hepatocytes and HepaRG cells and in an exposure time-dependent manner (Fig. 1B). Thus, mRNA NTCP expression was markedly repressed in response to a 24-h exposure to IL-1β, by an 11.1- and a 21.2-fold factor in primary human hepatocytes and HepaRG cells, respectively, whereas a shorter IL-1β treatment (8 h) had a more limited effect (repression by a 2.1- and 1.8-fold factor in hepatocytes and HepaRG cells, respectively). Moreover, IL-1β was found to reduce NTCP expression at protein level in HepaRG cells, as assessed by Western blot analysis (Fig. 1C). The cytokine also markedly inhibited sodium-dependent cellular uptake of taurocholate (Fig. 1D), indicating that NTCP activity was down-regulated. Taken together, these data establish that NTCP expression is markedly impaired in response to IL-1β in human hepatocytes as in their rodent counterparts (Green et al., 1996; Geier et al., 2005). Such a regulation likely contributes to LPS-related alteration of NTCP levels in human liver slices, as already suggested (Elferink et al., 2004), and is probably important to consider because NTCP is the main transporter responsible for bile acid uptake in human hepatocytes (Fig. 1A).
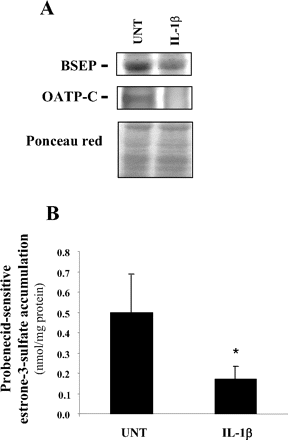
In addition to NTCP, several organic anion transporters were found to be markedly down-regulated in response to IL-1β treatment in human hepatic cells. It was especially the case for the SLC transporters OATP-B (SLCO2B1) and OATP-C (SLCO1B1) and for the ABC transporter BSEP, whose mRNA expressions were repressed by at least a 5-fold factor after a 24-h exposure to IL-1β in both primary human hepatocytes and HepaRG cells (Table 2). In parallel, BSEP and OATP-C protein levels were found to be decreased in IL-1β-exposed primary hepatocytes (Fig. 2A); moreover, IL-1β reduced intracellular uptake of the OATP substrate estrone-3-sulfate in HepaRG cells (Fig. 2B), likely indicating that at least some OATPs were targeted by IL-1β at mRNA, protein, and activity levels. Transporters belonging to the MRP subfamily, namely, MRP2 and MRP3, and the ABC transporter breast cancer resistance protein (ABCG2), which mediates transport of organic anions such as bilirubin diglucuronide, antibiotics, and bile acids (Janvilisri et al., 2005), were also down-regulated, although in a more moderate manner because the factors of repression ranged from 1.7- to 4.1-fold in primary hepatocytes and HepaRG cells (Table 2). In a similar manner, a 24-h exposure to IL-1β only moderately repressed OATP8 (SLCO1B3) mRNA levels in HepaRG cells by a 1.9-fold factor, whereas a shorter treatment time (8 h) had no effect. In contrast, OATP8 expression was much more decreased in IL-1β-treated primary human hepatocytes because it was repressed by a 3.4- and 8.1-fold factor after an 8- and 24-h exposure to the cytokine, respectively (Table 2). This discrepancy between human primary hepatocytes and HepaRG cell responsiveness may be because of the constitutive very low expression of OATP8 in differentiated HepaRG cells (Le Vee et al., 2006). Similarly to OATP8, MRP4 (ABCC4) was also partly differently regulated in response to IL-1β in primary human hepatocytes versus HepaRG cells; indeed, a 24-h exposure to the proinflammatory cytokine induced expression of this transporter by a 1.7-fold factor in the latter cells, whereas it decreased it by a 1.8-fold factor in the former cells (Table 2). Moreover, a shorter exposure to IL-1β (8 h) also induced MRP4 expression in HepaRG cells by a 2-fold factor, whereas it only very slightly up-regulated it by a 1.3-fold factor in human hepatocytes. However, it is noteworthy that most of the transporters analyzed in the present study were similarly regulated in both human hepatocytes and HepaRG cells in response to IL-1β, which lends weight to the data concerning these transporters. This also suggests that the differences of response between primary hepatocytes and HepaRG cells, only observed for OATP8 and MRP4, likely do not lead to reconsider the interest of HepaRG cells as an important convenient in vitro model for studying hepatic drug-detoxifying pathways (Guillouzo et al., 2007).
Effects of IL-1β treatment on expression of transporters
Down-regulation of functional NTCP expression in human hepatic cells exposed to IL-1β. A, intracellular accumulation of taurocholate was determined in primary human hepatocytes and HepaRG cells in the absence or presence of sodium as described under Materials and Methods. Data shown are the mean ± S.E.M. of three independent experiments in triplicate. B, primary human hepatocytes and human hepatoma HepaRG cells were either untreated (UNT) or exposed to 1 ng/ml IL-1β for 8 or 24 h. NTCP mRNA expression was then determined by RT-qPCR as described under Materials and Methods. Data are expressed as percentage of NTCP mRNA levels found in untreated cells, arbitrarily set at the value of 100%. They are the mean ± S.E.M. of values from six independent hepatocyte populations (primary human hepatocytes) or from four independent experiments (HepaRG cells). *, p < 0.05 when compared with IL-1β-untreated counterparts. C, HepaRG cells were either untreated (UNT) or exposed to 1 ng/ml IL-1β for 24 h. NTCP protein content was then determined by Western blot analysis. Data shown are representative of three independents experiments. The loading and transfer of equal amounts of proteins were checked by ponceau red staining. D, HepaRG cells were either untreated (UNT) or exposed to 1 ng/ml IL-1β for 24 or 48 h. NTCP-mediated uptake of taurocholate was then determined as described under Materials and Methods. Data are expressed as sodium-dependent accumulation of taurocholate, i.e., accumulation values in the presence of sodium minus accumulation values in the absence of sodium. They are the mean ± S.E.M. of three independent experiments in triplicate. *, p < 0.05 when compared with sodium-dependent taurocholate accumulation in untreated cells.
Owing to the well known links between inflammation and IL-1β (Ramadori and Christ, 1999), IL-1β-mediated down-regulation of several major human hepatic organic anion transporters implicated in both sinusoidal uptake (OATPs) or canalicular secretion (MRP2, breast cancer resistance protein) of drugs likely contributes to the well established alteration of hepatic detoxification pathways observed during inflammation in humans (Aitken et al., 2006). In a similar manner, dramatic decrease in hepatic concentrations of Ntcp, Bsep, Oatp1, Oatp2, Oatp4, and Mrp2 mRNA levels has been observed in LPS-treated rats (Cherrington et al., 2004) and probably participates in the impaired hepatic uptake and secretion of drugs in endotoxemic rats (Bolder et al., 1997). Moreover, this similarity between human and rodent transporter responses to inflammation was also supported by the fact that the canalicular efflux pump P-glycoprotein/MDR1 (ABCB1) and the sinusoidal influx organic cation transporter OCT1 (SLC22A1), known to be repressed in LPS-treated rats (Petrovic et al., 2007), were also down-regulated at mRNA levels in primary human hepatocytes exposed to IL-1β for 24 h by a 1.7- and 4.9-fold factor, respectively (data not shown).
The fact that hepatic transporters such as NTCP and BSEP, primarily handling bile acids at sinusoidal and canalicular pole, respectively, were hugely repressed by IL-1β suggests that this cytokine likely impairs hepatic secretion of bile acids from human hepatocytes; this may contribute to reduced bile flow and cholestasis, which frequently occurs as a complication in patients with sepsis (Moseley, 1997). Reduction of intracellular uptake of taurocholate into IL-1β-exposed HepaRG cells fully argues in favor of this hypothesis. However, it should be kept in mind that other regulatory pathways linked to production of cytokines, such as tumor necrosis factor α or IL-6, or to retention of bile acids can also affect transporter expression (Geier et al., 2007) and thereby may contribute to the development and the maintenance of inflammation-related cholestasis in humans.
Down-regulation of BSEP and OATP-C protein expression (A) and of estrone-3-sulfate accumulation (B) in response to IL-1β. Primary human hepatocytes and human hepatoma HepaRG cells were either untreated (UNT) or exposed to 1 ng/ml IL-1β for 48 h. A, BSEP and OATP-C protein content in primary human hepatocytes were then determined by Western blot analysis. Data shown are representative of the analysis of two independent hepatocyte populations. The loading and transfer of equal amounts of proteins were checked by ponceau red staining. B, probenecid-sensitive intracellular accumulation of the OATP substrate estrone-3-sulfate was determined in HepaRG cells as described under Materials and Methods. Data are the mean ± S.E.M. of three independent experiments in triplicate. *, p < 0.05 when compared with estrone-3-accumulation in untreated cells.
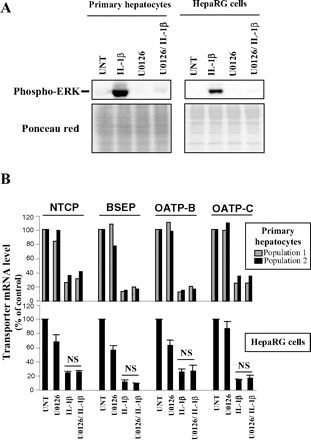
Activation of the mitogen-activated protein kinase (MAPK) ERK is known to play a key-role in many biological effects of IL-1β (Ogata et al., 2007). This led us to finally analyze its involvement in regulation of four human organic anion transporters repressed in a major way by IL-1β, i.e., NTCP, BSEP, OATP-B, and OATP-C. We first verified that IL-1β exposure elicited a marked activation of ERK in human hepatocytes and HepaRG cells through Western blotting using antibodies recognizing the active phosphorylated form of this kinase (Fig. 3A). We next analyzed the effects of the ERK inhibitor U0126 on IL-1β-mediated transporter repression. For this purpose, cells were exposed to IL-1β or U0126 or coexposed to IL-1β and U0126 for 12 h to avoid some toxicity occurring for longer treatment time in U0126-treated cells (data not shown). U0126 was fully active in our hands as shown by its inhibitory role on ERK activation in both human hepatocytes and HepaRG cells (Fig. 3A). It did not affect constitutive transporter mRNA levels in human hepatocytes and basal OATP-C mRNA levels in HepaRG cells (Fig. 3B). It only moderately decreased constitutive expression of NTCP, BSEP, and OATP-B in HepaRG cells comparatively to IL-1β-mediated down-regulations of these transporters (Fig. 3B). The ERK inhibitor did not prevent down-regulation of NTCP, BSEP, OATP-B, and OATP-C in two independent populations of IL-1β-exposed human hepatocytes (Fig. 3B). U0126 similarly failed to significantly counteract IL-1β-mediated repression of these transporters in HepaRG cells, i.e., mRNA levels of transporters were not statistically different in IL-1β-exposed and IL-1β/U0126-treated HepaRG cells (Fig. 3B). Taken together, these data do not support a major role of ERK in IL-1β-mediated down-regulation of transporters. Besides ERK, other MAPKs such as the p38 MAPKs and c-Jun N-terminal kinase contribute to IL-1β effects (Liang et al., 2007; Ogata et al., 2007). Interestingly, we have found that these two MAPKs were activated by IL-1β in both human hepatocytes and HepaRG cells (data not shown). However, whether these two kinases may play a role in IL-1β-mediated down-regulation of transporters remains to be determined. In addition, other pathways related to transcription factors or nuclear receptors, involved in inflammation-related regulation of rodent transporters (Teng and Piquette-Miller, 2005; Geier et al., 2007; Ho and Piquette-Miller, 2007), may also be implicated.
Lack of ERK inhibition effect on IL-1β-mediated down-regulation of NTCP, BSEP, OATP-B, and OATP-C mRNA expression. A, human hepatocytes and HepaRG cells were either untreated (UNT) or exposed to 1 ng/ml IL-1β for 30 min in the absence or the presence of the ERK inhibitor U0126 used at 5 μM. Expression of phospho-ERK was then determined by Western blot analysis as described under Materials and Methods. Data shown are representative of one human hepatocyte population or of three independent experiments (HepaRG cells). The loading and transfer of equal amounts of proteins were checked by ponceau red staining. B, human hepatocytes from two independent donors and HepaRG cells were either untreated (UNT), exposed to 1 ng/ml IL-1β or 5 μM U0126, or coexposed to IL-1β + U0126 for 12 h. NTCP, BSEP, OATP-B, and OATP-C mRNA expressions were then determined by RT-qPCR. Data are expressed as percentage of transporter mRNA levels found in untreated cells, arbitrarily set at the value of 100%. Results for the two independent hepatocyte populations are shown, whereas data shown for HepaRG cells are the mean ± S.E.M. from four independent experiments. NS, not significant.
In summary, we have shown that exposure to the proinflammatory cytokine IL-1β down-regulates expression of major organic anion transporters in human hepatic cells. Such a repression may contribute to the known alterations of pharmacokinetic features of drugs caused by inflammation in humans.
Footnotes
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.107.016907.
-
ABBREVIATIONS: IL, interleukin; SLC, solute carrier; OATP, organic anion transporting polypeptide; ABC, ATP binding cassette; BSEP, bile salt export pump; MRP, multidrug resistance-associated protein; NTCP, sodium-taurocholate cotransporting polypeptide; LPS, lipopolysaccharide; ERK, extracellular signal-regulated protein kinase; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; CRP, C-reactive protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MAPK, mitogen-activated protein kinase.
- Received May 28, 2007.
- Accepted November 6, 2007.
- The American Society for Pharmacology and Experimental Therapeutics